Academic Editor: Francesco Onorati
Background: Iron deficiency leads to health problems. Conversely, iron overload induces the generation of reactive oxygen species and health problems. Body iron status contributes to the development of various diseases, including aortic disease. Indeed, several clinical studies have reported that iron status can be linked to the pathogenesis of aortic disease. At the cellular level, iron uptake is regulated by the cellular iron transporter, transferrin receptor 1, while systemic iron homeostasis is regulated by hepcidin. As body iron status is regulated to maintain cellular and systemic iron homeostasis, iron metabolism in aortic disease is puzzling and not well understood. Methods: Perspective and short communication. Conclusions: This review provides an overview of the relevant research investigating the association between cellular iron metabolism and aortic disease.
Iron deficiency contributes to health problems such as anemia [1]. On the other
hand, iron overload induces the generation of reactive oxygen species via the
Fenton reaction (Fe
Clinical studies have proposed iron status is harmful or beneficial for aortic disease (Table 1, Ref. [4, 5, 6, 7, 8, 10, 11]). A number of clinical studies have reported an association between iron overload and aortic disease [4, 5, 6, 7, 8, 9]. Most of these reports have used serum ferritin levels as a marker of iron levels [4, 5, 6]. In contrast to these reports, a previous study has reported that higher iron status lowers the risk of coronary artery disease [10]. The Ludwigshafen Risk and Cardiovascular Health Study showed that iron depletion is associated with coronary artery disease [11]. Taken together, these findings indicate that iron is associated with the pathogenesis of aortic disease (Fig. 1). However, optimal systemic iron levels in aortic disease are still controversial. When understanding the pathogenesis of aortic disease, it is important to consider cellular iron levels and iron metabolism in the aortic region, in addition to systemic iron levels.
| Harmful effects of iron on aortic disease | Beneficial effects of iron on aortic disease |
| An association between high stored iron levels and a risk of myocardial infarction [4] | An association between increased body iron and reduced coronary artery disease risk [10] |
| An association between body iron stores and the progression of carotid atherosclerosis [5] | An association between iron depletion and coronary artery disease [11] |
| An association between increased serum ferritin levels and the presence of coronary artery calcium score [6] | |
| An association between iron chelation and endothelial function in patients with coronary artery disease [7] | |
| An association between serum ferritin levels and peripheral arterial disease [8] |
 Fig. 1.
Fig. 1.Iron is associated with the pathogenesis of aortic disease. Iron is associated with the pathogenesis of aortic disease, such as abdominal aortic aneurysm and atherosclerosis. In addition to systemic iron levels, local iron levels and iron metabolism in the aortic region should be considered.
Although there have been several clinical studies on iron and aortic disease, the role of cellular iron metabolism in the pathogenesis of aortic disease remains largely unknown. Cellular iron uptake is regulated by the cellular iron transporter, transferrin receptor 1 (TfR1) [12]. TfR1 is ubiquitously expressed in each tissue and has been shown to play a role in several diseases.
Regarding aortic disease, we have shown the role of TfR1 in the pathogenesis of
abdominal aortic aneurysm (AAA). First, we found iron deposition in human AAA
walls by Berlin blue staining. In addition, we found that the Berlin
blue-positive area was similar to the positive areas of a macrophage marker, CD68
and the oxidative stress marker, 8-Hydroxy-2
In these studies, immunohistochemical analysis demonstrated that TfR1 was expressed in AAA walls. Of note, increased aortic TfR1 expression was observed in murine and human AAA walls, and the TfR1 positive area was consistent to areas where a macrophage marker, F4/80, and iron accumulation occurred in murine AAA walls. TfR1 is upregulated under cellular iron-deficient conditions and accelerates cellular iron uptake. Conversely, TfR1 is downregulated under cellular iron overload conditions and inhibits cellular iron uptake [12]. Since increased iron deposition and TfR1 expression were observed in murine and human AAA walls, these results indicate that dysregulated TfR1 may contribute to aortic iron overload in AAA walls (Fig. 2). As regard to TfR1 in aortic disease, we have also shown increased aortic TfR1 expression in hypertensive model rats [14, 15]. Taken together, these results suggest the involvement of aortic TfR1 in the pathogenesis of aortic disease.
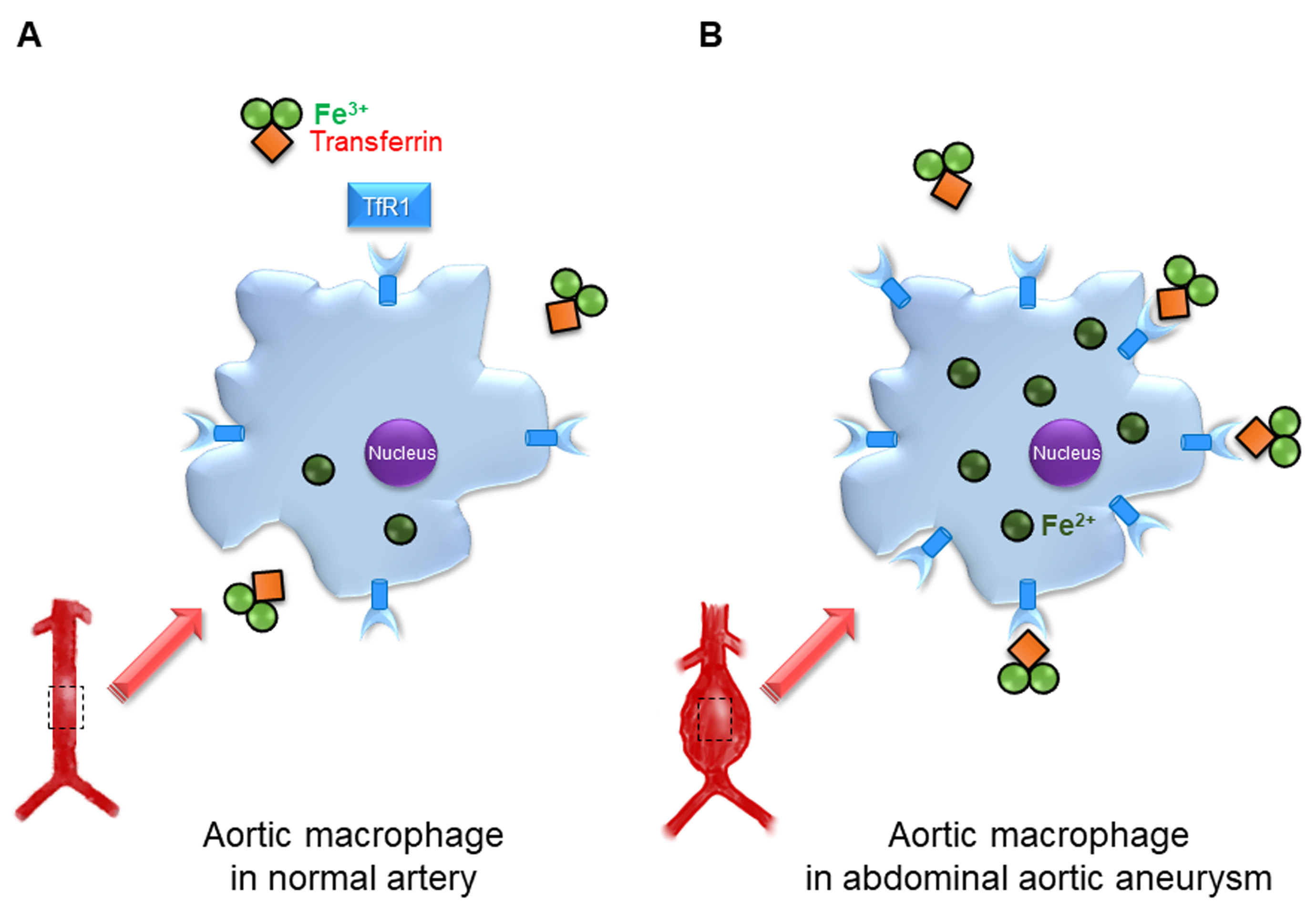 Fig. 2.
Fig. 2.Transferrin receptor 1 in the pathogenesis of abdominal aortic aneurysm. (A) Transferrin receptor 1 (TfR1) is expressed in aortic macrophages of normal artery. (B) In abdominal aortic aneurysm (AAA) walls, increased TfR1 expression in aortic macrophages induces intracellular iron accumulation, and this may promote AAA progression.
Systemic iron metabolism is regulated by hepatic hormone hepcidin and is kept by duodenal iron absorption. Hepcidin is upregulated in response to iron overload or inflammation, and is downregulated by iron deficiency [16]. Dietary reducted ferrous iron is exported to the blood by the iron transporter, ferroportin [17].
Hepcidin is reported to be a master iron regulator in the pathogenesis of aortic disease. The Nijmegen Biomedical Study showed that serum hepcidin levels were associated with the presence of aortic plaques in postmenopausal women in the general population. This study also reported that the hepcidin/ferritin ratio was associated with the ankle-brachial index in men and women [18].
Hepcidin regulates ferroportin expression [17]. Hepcidin binds to ferroportin, inducing its degradation, then inhibiting cellular iron export from duodenocytes, hepatocytes, and macrophages. If hepcidin is absent, ferroportin in the cell membrane results in the export of cellular iron from these cells. Malhotra et al. [19] reported the role of hepcidin in aortic disease using hepcidin gene-deficient mice. Crossing hepcidin gene-deficient mice with low-density lipoprotein receptor-deficient mice was associated with a decrease in atherosclerotic lesions compared to control mice. These mice showed reduced atherosclerosis with reduced aortic macrophage iron and macrophage proinflammatory phenotype, compared to control mice after a high-fat diet. Hepcidin inhibits cellular iron export from macrophages by downregulating ferroportin expression and enhancing intracellular iron accumulation. Intracellular iron accumulation leads to the composition of oxidized low-density lipoprotein cholesterol and proinflammatory intermediates and intracellular reactive oxygen species generation, which may promote atherosclerotic plaque progression. Collectively, these results suggest that decreased aortic macrophage proinflammatory phenotypes might contribute to reduced atherosclerosis with decreased hepcidin levels (Fig. 3). However, it is unknown whether decreased hepcidin levels could affect aortic disease in humans.
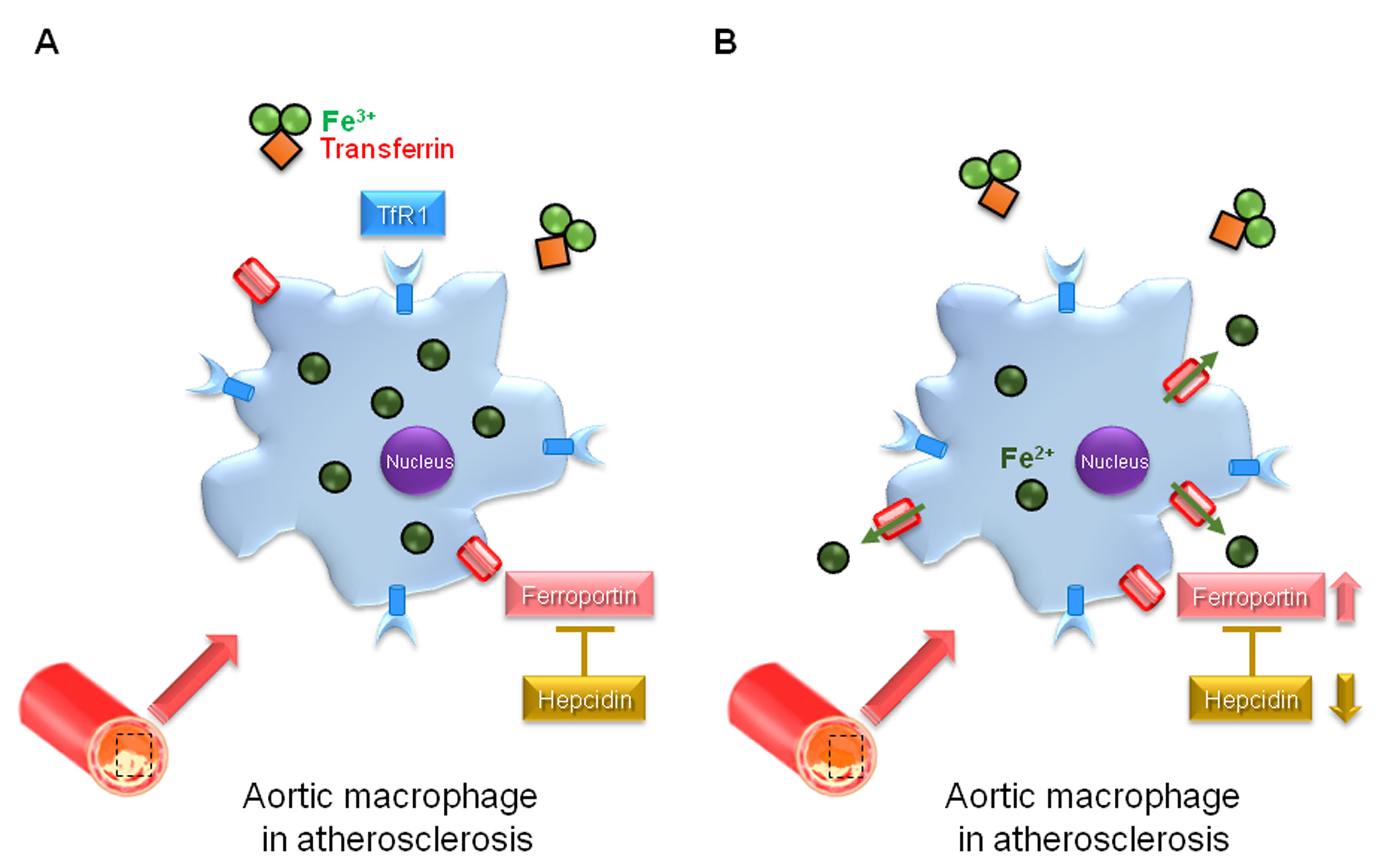 Fig. 3.
Fig. 3.Hepcidin in the pathogenesis of atherosclerosis. (A) In atherosclerotic lesion, hepcidin suppresses cellular iron export from aortic macrophages with downregulation of ferroportin expression and enhances intracellular iron accumulation, and this may promote atherosclerosis. (B) In hepcidin gene-deficient mice with low-density lipoprotein receptor deficient mice, cellular iron export is induced from aortic macrophages with upregulation of ferroportin expression and decreases intracellular iron accumulation, this may suppress atherosclerosis.
Similar to our AAA study, several experimental studies have shown iron accumulation in atherosclerotic lesions [20, 21]. For instance, iron accumulation was reported in the endothelium, smooth muscle cells, and intima enriched foam cells in the aorta of apolipoprotein E-deficient mice [20]. In addition, hereditary hemochromatosis mice crossed with apolipoprotein E-deficient mice showed iron deposition in the media layer of the aorta and enhanced atherosclerotic lesions [21]. Collectively, an investigation into cellular iron metabolism could reveal the pathogenesis of aortic disease such as AAA and atherosclerosis, and the causal link between iron and aortic disease.
Iron is associated with the pathogenesis of aortic disease, such as AAA and atherocclerosis. The investigation of cellular iron metabolism could help to clarifying the complex association between iron and aortic disease. Although further studies are necessary, a focus on cellular iron metabolism in aortic disease may result in new therapeutic strategies for the condition.
AAA, abdominal aortic aneurysm; TfR1, transferrin receptor 1.
Conceptualization—YN and MI; literature review and resources—YN; writing—original draft preparation—YN; writing—review and editing—YN and MI; figure preparation and editing—YN; supervision—MI. All authors have read and agreed to the published version of the manuscript.
Not applicable.
Not applicable.
This work was supported by a Grant-in-Aid for Scientific Research (C) JSPS KAKENHI Grant No. 25460919, 16K09273, and 19K07950 and The Salt Science Research Foundation (No. 1544, 1641, 1826, and 2134) (to Yoshiro Naito).
The authors declare no conflict of interest.
