1. Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia,
occurring in 2%–4% of the general population with increasing prevalence among
the elderly [1]. It is predicted that 10%–15% AF patients will require
percutaneous coronary intervention (PCI) for coronary artery disease (CAD) during
their life, while patients with AF and acute coronary syndrome (ACS) will be more
likely to experience adverse outcomes than ACS patients without AF [2, 3].
Concomitant risks of subsequent ischemic events, in-stent thrombosis and
treatment-related bleeding need to be carefully considered before initiating
appropriate antithrombotic therapy.
Several risk clinical scores have been proven to enhance the assessment of
thrombo-embolic risk in AF. CHADS-VASc score [congestive heart
failure (CHF), hypertension, age 75 years, diabetes mellitus, stroke,
vascular disease, age 65–74 years, sex category (female)] is a recognized tool
to stratify stroke risk and recommended by guidelines [4, 5]. Several studies have
showed that a higher CHADS-VASc score was independently associated
with a poor outcome in CAD patients with sinus rhythm [6, 7, 8].
Early risk stratification is also important for ACS patients to help clinicians
to determine prognosis and therefore guide management strategies. A number of
prognostic models have been developed including Global Registry of Acute Coronary
Events (GRACE) [9], thrombolysis in myocardial infarction (TIMI) [10], and
platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using
Integrilin therapy (PRUSUIT) [11]. Among these, the GRACE risk score has been
externally validated and proved to display the best discriminative performance
[12]. The GRACE score at admission [age, systolic blood pressure (SBP), heart
rate, serum creatinine, cardiac arrest at admission, elevated cardiac biomarkers,
ST-segment deviation and Killip class at presentation] is established and widely
accepted for assessing death or myocardial infarction from admission to six
months after discharge [13]. Meanwhile, another prediction model, the GRACE score
at discharge [age, history of CHF, history of myocardial infarction (MI), heart
rate, SBP, ST-segment depression, serum creatinine, elevated cardiac enzymes and
no in-hospital PCI] also derived by the implemented GRACE registry, has been
established a robust tool for predicting 6-month post-discharge mortality in
patients with ACS [14].
For patients with AF and ACS, it still remains unclear whether
CHADS-VASc or GRACE score may be useful to assess the risk of major
adverse cardiovascular and cerebrovascular events (MACCEs) and thus guide the
anticoagulant regimens. Therefore, in the present study we aim to compare the
prognostic values of CHADS-VASc, GRACE score at admission and GRACE
score at discharge in predicting long-term MACCEs in patients with AF and ACS or
undergoing PCI.
2. Materials and Methods
2.1 Study Population and Data Collection
This is an observational, prospective, single-center registry. From January 2017
to December 2018, a total of 1408 patients with AF (new-onset, paroxysmal,
persistent, long-standing or permanent) who were diagnosed with ACS or referred
for PCI were consecutively enrolled in the present study. All participants aged
18 years and patients unable/unwilling to finish the follow-up were excluded
from the analysis. Demographic characteristics, medical history, clinical exams,
laboratory tests and discharge medications were collected from the medical
records. The classification of AF was in accordance with 2020 ESC guideline [5].
Hypertension was defined by self-reported and diabetes mellitus (DM) was defined
by either self-reported or hemoglobin A1c 6.5%. Information of heart
failure status was obtained by viewing medical records retrospectively. The
laboratory results at admission included hemoglobin (Hb), serum potassium,
creatinine, estimated glomerular filtration rate (eGFR), increase in cardiac
troponin I (cTNI), N-terminal pro-B-type natriuretic peptide (NT-proBNP),
low-density lipoprotein cholesterol (LDL-C), international normalized ratio (INR)
and HbA1c. Left ventricular ejection fraction (LVEF) was measured by experienced
physicians using echo-cardiography.
Patients were categorized into three risk groups according to the GRACE score at
admission (low: 108, intermediate: 109–140, high: 140), the GRACE
score at discharge (low: 88, intermediate: 89–118, high: 118) and
CHADS-VASc score (low: 1–2, intermediate: 3–4, high: 4)
respectively.
2.2 Endpoints
Cardiovascular (CV) death was adjudicated as any death with a demonstrable
cardiovascular cause or any death that was not clearly attributable to a
noncardiovascular cause. MI (myocardial infarction)was defined according to the
third universal definition of MI [15]. Ischemic stroke was adjudicated as an
episode of neurological dysfunction caused by focal cerebral, spinal, or retinal
infraction and transient ischemic attack (TIA) was defined as focal cerebral
ischemic event with symptoms lasting 24 hours. Ischemia-driven coronary
revascularization included all coronary revascularization during follow-up that
were performed in the context of MI and those for worsening symptoms in
combination with evidence of myocardial ischemia. Systemic embolism was defined
as new acute limb ischemia or objective evidence of sudden loss of perfusion of a
limb or an organ. Every adverse event was carefully reviewed by an independent
clinical event adjudication committee.
The primary outcome of interest was MACCEs defined as cardiovascular (CV)
mortality, myocardial infarction (MI), ischemic stroke or TIA, systemic embolism
and ischemia-driven revascularization in follow-up and these events were analyzed
individually. All-cause mortality was analyzed as a secondary end-point. The
primary safety objective was a composite of major bleeding according to the
Thrombolysis in Myocardial Infarction (TIMI) criteria [16] or bleeding in
need of medical attention.
Follow-up by telephone interviews or clinic visits were scheduled every 6 months
lasting for 36 months. Every adverse event or bleeding was carefully reviewed by
an independent clinical event adjudication committee. The study was approved by
the ethics committee of Fuwai Hospital and was conducted in accordance with the
Declaration of Helsinki. All subjects provided written consent form before
participation.
2.3 Statistical Analysis
For baseline characteristics, categorical variables were displayed as
frequencies (percentages), and continuous variables were expressed as means
standard deviations (SD) or medians with interquartile range (IQR) if
they were not normally distributed. Normality was evaluated using the
Shapiro-Wilk W-test. Continuous variables were compared using independent
Student’s t-test or Mann-Whitney test as appropriate while categorical
variables using Pearson’s chi-squared test or Fisher’s exact test. Risk
predictive models were analyzed both as a continuous and class variable. In order
to adjust the effects by potential confounding risk factors, Cox proportional
hazards models were used to perform multivariable analysis. Models included
GRACE, CHADS-VASc score and were adjusted for all the variables not
included in the three risk models which showed an independent association with a
p value 0.10 in the univariable analysis. A backward stepwise
selection algorithm was used. The results were displayed as
hazards ratios (HRs) and their 95% confidence
intervals (95% CI). Kaplan-Meier curves were generated and statistical
differences were assessed by log-rank test (after Bonferroni correction
= 0.0167) in clinical endpoints between subgroups.
Receiver-operating curves (ROC) and c-statistics were constructed for MACCE and
all-cause mortality to compare the discrimination performance of the three
models. The statistical difference of c-statistics was evaluated through the
Delong method and the net classification improvement (NRI) was further
calculated.
The software package SPSS version 25.0 (IBM Corporation, New York, NY, USA) and
R version 4.1.2 (R Core Team, Vienna, Austria) were utilized for statistical
analysis. All statistical tests were 2-tailed, with a p value 0.05
considered statistically significant.
3. Results
3.1 Baseline Characteristics
A total of 1408 patients were included and their baseline characteristics
categorized by outcomes were displayed in Table 1. The mean age was 67.3
9.4 years. Nearly three quarters of the patients were male (72.9%) and 471
(33.5%) were admitted through emergency department. Previously to the inclusion
of the study, 419 (29.8%) had received PCI therapy. By the time of recruitment,
302 (21.4%) patients were complicated with congestive heart failure, and the
proportions for hypertension, DM and previous stroke/TIA were 77.3%, 42.6% and
25.2% respectively. At the time of inclusion, more than half of participants
(56.5%) suffered paroxysmal AF and 975 (69.3%) suffered ACS. The median value
of NT-proBNP was 766.95 pg/mL (IQR 234.78–2122.95) and the average LVEF was 56.3
10.0%. At the time of discharge, 1147 (81.5%) patients were prescribed
aspirin while 628 (48.4%) patients received anticoagulant therapy including
warfarin, Rivaroxaban or Dabigatran.
Table 1.Baseline characteristics and medical therapies of the study
population according with primary and secondary outcome.
| Variable |
Total (n = 1408) |
MACCE |
p value |
All-cause death |
p value |
| No (n = 1188) |
Yes (n = 220) |
No (n = 1267) |
Yes (n = 141) |
| Age (years) |
67.3 9.4 |
67.0 9.3 |
69.3 9.8 |
0.001 |
66.7 9.2 |
73.1 9.6 |
0.001 |
| Male , n (%) |
1027 (72.9) |
890 (74.9) |
137 (62.3) |
0.001 |
935 (73.8) |
92 (62.5) |
0.036 |
| Emergency presentation, n (%) |
471 (33.5) |
370 (31.1) |
101 (45.9) |
0.001 |
381 (30.1) |
90 (63.8) |
0.001 |
| Vital signs |
|
|
|
|
|
|
|
| BMI (kg/m) |
25.17 5.08 |
25.26 4.96 |
24.70 5.70 |
0.131 |
25.39 3.76 |
23.20 7.10 |
0.001 |
| SBP (mmHg) |
130.4 19.5 |
130.7 19.2 |
128.8 21.5 |
0.209 |
130.9 19.0 |
126.2 23.6 |
0.025 |
| DBP (mmHg) |
77.1 11.4 |
77.1 11.2 |
76.8 12.3 |
0.738 |
77.2 11.2 |
75.7 12.7 |
0.178 |
| Resting heart rate (bpm) |
78 (64–82) |
70 (64–80) |
75 (66–91) |
0.001 |
70 (64–80) |
78 (67–95) |
0.001 |
| Medical history, n (%) |
|
|
|
|
|
|
|
| Myocardial infarction |
397 (28.2) |
310 (26.1) |
87 (39.5) |
0.001 |
324 (25.6) |
73 (51.8) |
0.001 |
| PCI |
419 (29.8) |
351 (29.5) |
68 (30.9) |
0.689 |
370 (29.2) |
49 (34.8) |
0.175 |
| Heart failure |
302 (21.4) |
209 (17.6) |
93 (42.3) |
0.001 |
230 (18.2) |
72 (51.1) |
0.001 |
| Hypertension |
1088 (77.3) |
909 (76.5) |
179 (81.4) |
0.136 |
977 (77.1) |
111(78.7) |
0.751 |
| Hyperlipidemia |
1037 (73.7) |
872 (73.4) |
165 (75.0) |
0.677 |
930 (73.4) |
107 (75.9) |
0.614 |
| Diabetes |
600 (42.6) |
490 (41.2) |
110 (50.0) |
0.018 |
527 (41.6) |
73 (51.8) |
0.025 |
| Stroke/TIA |
355 (25.2) |
278 (23.4) |
77 (35.0) |
0.001 |
305 (24.1) |
50 (35.5) |
0.004 |
| Chronic kidney disease |
187 (13.3) |
133 (11.2) |
54 (24.5) |
0.001 |
131 (10.3) |
56 (39.7) |
0.001 |
| AF pattern, n (%) |
|
|
|
0.001 |
|
|
0.330 |
| First diagnosed |
106 (7.5) |
87 (7.3) |
19 (8.6) |
|
96 (7.6) |
10 (7.1) |
|
| Paroxysmal |
795 (56.5) |
701 (59.0) |
94 (42.7) |
|
725 (57.2) |
70 (49.6) |
|
| Persistent |
462 (32.8) |
364 (30.6) |
98 (44.5) |
|
406 (32.0) |
56 (39.7) |
|
| Long-standing persistent |
41 (2.9) |
33 (2.8) |
8 (3.6) |
|
37 (2.9) |
4 (2.8) |
|
| Permanent |
4 (0.3) |
3 (0.3) |
1 (0.5) |
|
3 (0.2) |
1 (0.7) |
|
| Diagnosis for CAD, n (%) |
|
|
|
0.001 |
|
|
0.001 |
| SCAD |
433 (30.8) |
379 (31.9) |
54 (24.5) |
|
414 (32.7) |
18 (13.5) |
|
| Unstable angina |
471 (33.5) |
402 (33.8) |
69 (31.4) |
|
436 (34.4) |
35 (24.8) |
|
| STEMI |
204 (14.5) |
176 (14.8) |
28 (12.7) |
|
171 (13.5) |
33 (23.4) |
|
| NSTEMI |
300 (21.3) |
231 (19.4) |
69 (31.4) |
|
246 (19.4) |
54 (38.3) |
|
| Laboratory test |
|
|
|
|
|
|
|
| Hemoglobin (g/dL) |
14.25 1.92 |
14.331.83 |
13.822.27 |
0.001 |
14.38 1.83 |
13.14 2.32 |
0.001 |
| Serum potassium (mmol/L) |
4.18 0.45 |
4.17 0.45 |
4.23 0.49 |
0.096 |
4.18 0.45 |
4.21 0.51 |
0.363 |
| Creatinine (mg/dL) |
1.07 0.31 |
1.05 0.29 |
1.16 0.40 |
0.001 |
1.04 0.29 |
1.30 0.44 |
0.001 |
| eGFR (mL/min/1.73 m) |
77.96 22.74 |
79.38 22.47 |
70.31 22.69 |
0.001 |
79.74 22.10 |
61.93 22.13 |
0.001 |
| cTNI elevation |
0.3 (0.0–3.6) |
0.3 (0.0–3.2) |
1.1 (0.0–7.4) |
0.052 |
0.3 (0.0–3.0) |
2.0 (0.3–23.3) |
0.001 |
| NT-proBNP (pg/mL) |
766.95 (234.78–2122.95) |
619.05 (205.03–1691.88) |
1920.5 (704.28–5004.38) |
0.001 |
630.40 (210.90–1692.0) |
3334.4 (1550.20–9368.80) |
0.001 |
| LDL-C (mg/dL) |
89.71 33.64 |
89.71 34.03 |
90.87 32.48 |
0.620 |
89.71 33.64 |
88.94 34.03 |
0.766 |
| INR |
1.15 0.47 |
1.12 0.35 |
1.28 0.84 |
0.008 |
1.12 0.36 |
1.34 0.99 |
0.011 |
| HbA1c (%) |
6.63 1.20 |
6.59 1.18 |
6.86 1.24 |
0.005 |
6.62 1.19 |
6.78 1.22 |
0.159 |
| LVEF (%) |
56.3 10.0 |
57.2 9.3 |
51.5 12.4 |
0.001 |
57.4 9.2 |
46.6 12.1 |
0.001 |
| Medications, n (%) |
|
|
|
|
|
|
|
| Aspirin |
1147 (81.5) |
996 (83.8) |
151 (68.6) |
0.001 |
1053 (83.1) |
94 (66.7) |
0.001 |
| Clopidogrel |
1257 (89.3) |
1054 (88.7) |
203 (92.3) |
0.124 |
1132 (89.3) |
125 (88.7) |
0.801 |
| Ticagrelor |
118 (8.4) |
111 (9.3) |
7 (3.2) |
0.001 |
114 (9.0) |
4 (2.8) |
0.010 |
| Anticoagulant therapy |
682 (48.4) |
528 (44.4) |
154 (70.0) |
0.001 |
612 (48.3) |
70 (49.6) |
0.790 |
| Statin |
1375 (97.7) |
167 (98.2) |
208 (94.5) |
0.003 |
1247 (98.4) |
128 (90.8) |
0.001 |
| ACEi or ARB |
898 (63.8) |
770 (64.8) |
128 (58.2) |
0.067 |
828 (65.4) |
70 (49.6) |
0.001 |
| Diuretics |
550 (39.1) |
425 (35.8) |
125 (56.8) |
0.001 |
447 (35.3) |
103 (73.0) |
0.001 |
| -blocker |
1210 (85.9) |
1017 (85.6) |
193 (87.7) |
0.460 |
1090 (86.0) |
120 (85.1) |
0.798 |
| CHADS-VASc |
3.7 1.8 |
3.6 1.7 |
4.6 1.8 |
0.001 |
3.6 1.7 |
5.1 1.8 |
0.001 |
| GRACE at admission |
126 36 |
123 33 |
142 43 |
0.001 |
122 33 |
161 42 |
0.001 |
| GRACE at discharge |
108 33 |
105 31 |
126 36 |
0.001 |
104 30 |
144 32 |
0.001 |
| MACCE, major adverse cardiovascular and cerebrovascular
events; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood
pressure; PCI, percutaneous coronary intervention; TIA, transient ischemic
attack; AF, atrial fibrillation; CAD, coronary artery disease; SCAD,
stable coronary artery diseases; eGFR, estimated glomerular filtration fraction;
NT-proBNP, N-terminal pro-B-type natriuretic peptide; LDL-C, low-density
lipoprotein cholesterol; INR, international normalized ratio; LVEF, left
ventricular ejection fraction. |
The mean value of CHADS-VASc score was 3.7 1.8 and the
distribution of CHADS-VASc was displayed in Supplementary
Table 1. The average scores of GRACE score at admission and GRACE score at
discharge were 126 36 and 108 33 respectively.
3.2 Clinical Outcomes and Multivariable Analysis
The clinical outcomes classified by GRACE and CHADS-VASc subgroups
were shown in Table 2. During follow-up, 220 (15.6%) primary outcomes occurred,
of which 99 (7.0%) patients suffered CV mortality, 39 (2.8%) suffered MI, 57
(4.0%) experienced stroke or TIA, 56 (4.0%) received coronary revascularization
driven by ischemic symptoms and only 6 (0.4%) suffered systemic embolism. 136
(9.7%) all-cause death occurred and 24 (1.7%) patients experienced major
bleeding. Based on CHADS-VASc score, 380 (27.0%) patients were at
low risk, 584 (41.5%) at intermediate risk and 444 (31.5%) at high risk.
Regarding the GRACE score at admission, 515 (36.6%) patients were categorized as
low risk, 517 (36.7%) at intermediate risk and 376 (26.7%) at high risk. The
rates for GRACE score at discharge were 29.0%, 38.0% and 33.0%.
Table 2.Adverse events at 36 months follow-up according to
CHADS-VASc and GRACE score.
| Endpoints |
|
CHADS-VASc |
GRACE score at admission |
GRACE score at discharge |
|
Total (n = 1408) |
1, 2 (n = 380) |
3, 4 (n = 584) |
4 (n = 444) |
108 (n = 515) |
109–140 (n = 517) |
140 (n = 376) |
88 (n = 408) |
89–118 (n = 536) |
118 (n = 464) |
| MACCE |
220 (15.6) |
29 (7.6) |
79 (13.5) |
112 (25.2) |
57 (11.1) |
69 (13.3) |
94 (25.0) |
36 (8.8) |
69 (12.9) |
115 (24.8) |
| Cardiovascular mortality |
99 (7.0) |
7 (1.8) |
29 (5.0) |
63 (14.2) |
9 (1.7) |
26 (5.0) |
64 (17.0) |
3 (0.7) |
18 (3.4) |
78 (16.8) |
| Myocardial infarction |
39 (2.8) |
2 (0.5) |
11 (1.9) |
26 (5.9) |
7 (1.4) |
11 (2.1) |
21 (5.7) |
2 (0.5) |
14 (2.6) |
23 (5.0) |
| Stroke/TIA |
57 (4.0) |
6 (1.6) |
25 (4.3) |
26 (5.9) |
20 (3.9) |
21 (4.1) |
16 (4.3) |
16 (3.9) |
19 (3.5) |
22 (4.7) |
| Ischemia-driven revascularization |
56 (4.0) |
14 (3.7) |
23 (4.0) |
19 (4.3) |
27 (5.2) |
21 (4.1) |
8 (2.2) |
17 (4.2) |
29 (5.4) |
10 (2.2) |
| Systemic embolism |
6 (0.4) |
0 (0.0) |
5 (0.9) |
1 (0.2) |
2 (0.4) |
3 (0.6) |
1 (0.3) |
2 (0.5) |
1 (0.2) |
3 (0.7) |
| All-cause mortality |
136 (9.7) |
8 (2.1) |
43 (7.4) |
85 (19.1) |
11 (2.1) |
38 (7.4) |
89 (23.7) |
4 (1.0) |
27 (5.0) |
105 (22.6) |
| Major bleeding |
24 (1.7) |
5 (1.3) |
10 (1.7) |
9 (2.1) |
6 (1.2) |
13 (2.5) |
5 (1.4) |
5 (1.2) |
13 (2.4) |
6 (1.3) |
| Minor bleeding |
24 (1.7) |
2 (0.5) |
10 (1.7) |
12 (2.7) |
3 (0.6) |
9 (1.7) |
12 (3.2) |
1 (0.2) |
9 (1.7) |
14 (3.1) |
| Data presented as number of events and 36-month Kaplan-Meier estimates: n (%).
Abbreviations: MACCE, major adverse cardiovascular and cerebrovascular events;
TIA, transient ischemic attack. |
Kaplan-Meier curves for MACCE and all-cause mortality in patients at low,
intermediate and high risk based on different scores were plotted in Fig. 1.
Higher CHADS-VASc was obviously associated with increased risk of
MACCEs and all-cause mortality during follow-up. However, patients presented with
GRACE score at admission 108 compared to those with GRACE at admission
between 109 and 140 gained similar risk of primary outcome (log rank p =
0.221). The GRACE at discharge score was also unable to significantly stratify
low and intermediate risk in MACCEs (p = 0.042 0.0167 after
Bonferroni correction ). After adjusting potential cofounders including
emergency presentation, AF patterns, subtypes of CAD, baseline hemoglobin, INR,
HbA1c, NT-proBNP, LVEF, use of aspirin, Ticagrelor and use of coagulant therapy,
CHADS-VASc (HR 1.184, 95% CI 1.091–1.284, p 0.001) and
GRACE score at discharge (HR 1.009, 95% CI 1.004–1.014, p 0.001)
were independent predictors for subsequent MACCEs as continuous variables.
Nevertheless, when treated as categorical variables, both CHADS-VASc
(low vs. intermediate: HR 1.449, 95% CI 0.935–2.244, p = 0.097; low
vs. high: HR 2.226, 95% CI 1.436–3.453, p 0.001) and GRACE at
discharge (low vs. intermediate: HR 1.268, 95% CI 0.839–1.917, p =
0.260; low vs. high: HR 1.631, 95% CI 1.066–2.498, p = 0.024) only
retained the ability to identify high-risk subgroups. The multivariable analysis
revealed that GRACE score at admission was not a predictor for MACCEs. The
CHADS-VASc (continuous: HR 1.348, 95% CI 1.216–1.494, p 0.001), GRACE at admission (continuous: HR 1.013, 95% CI 1.008–1.018,
p = 0.002) and GRACE at discharge (continuous: HR 1.026, 95% CI
1.020–1.032, p 0.001) were strong and independent predictors for
all-cause mortality no matter they were treated as continuous or categorical
variables. Regarding the safety endpoints, none of the three risk models provided
sufficient predictive ability. The detailed relations between three models and
outcomes were shown in Table 3.
 Fig. 1.
Fig. 1.
Kaplan-Meier curves for 36-month adverse events in patients at
low, intermediate and high risk based on three risk scores. (a)
CHADS-VASc for MACCEs. (c) GRACE score at admission for MACCEs. (e)
GRACE score at discharge for MACCEs. Event free survival from all-cause mortality
based on (b) CHADS-VASc. (d) GRACE at admission and (f) GRACE at
discharge.
Table 3.Multivariable analysis of the CHADS-VASc and GRACE
scores for the outcomes of MACCE, all-cause mortality and major bleeding.
|
|
MACCE |
|
All-cause mortality |
|
Major bleeding |
|
| HR (95% CI) |
p |
HR (95% CI) |
p |
HR (95% CI) |
p |
| CHADS-VASc (continuous) |
1.184 (1.091–1.284) |
0.001 |
1.348 (1.216–1.494) |
0.001 |
0.995 (0.770–1.286) |
0.972 |
|
1–2 |
Reference |
|
Reference |
|
Reference |
|
|
3–4 |
1.449 (0.935–2.244) |
0.097 |
2.878 (1.285–6.446) |
0.010 |
0.933 (0.304–2.860) |
0.903 |
|
4 |
2.226 (1.436–3.453) |
0.001 |
5.457 (2.469–12.061) |
0.001 |
1.082 (0.335–3.497) |
0.895 |
| GRACE at admission (continuous) |
1.004 (1.000–1.008) |
0.061 |
1.013 (1.008–1.018) |
0.001 |
0.992 (0.978–1.006) |
0.275 |
|
108 |
Reference |
|
Reference |
|
Reference |
|
|
109–140 |
1.089 (0.755–1.571) |
0.648 |
2.863 (1.450–5.653) |
0.002 |
3.148 (1.000–9.518) |
0.050 |
|
140 |
1.32 (0.786–2.229) |
0.292 |
5.309 (2.727–10.336) |
0.001 |
0.966 (0.207–4.510) |
0.966 |
| GRACE at discharge (continuous) |
1.009 (1.004–1.014) |
0.001 |
1.026 (1.020–1.032) |
0.001 |
0.992 (0.977–1.007) |
0.300 |
|
88 |
Reference |
|
Reference |
|
Reference |
|
|
89–118 |
1.268 (0.839–1.917) |
0.260 |
4.219 (1.471–12.101) |
0.007 |
1.573 (0.550–4.498) |
0.399 |
|
118 |
1.631 (1.066–2.498) |
0.024 |
11.666 (4.177–32.585) |
0.001 |
0.638 (0.171–2.383) |
0.504 |
| Adjusted for emergency presentation, atrial fibrillation
patterns, subtypes of coronary artery disease, hemoglobin, NT-proBNP at
admission, LVEF, INR, anticoagulant therapy, use of aspirin, use of ticagrelor. |
3.3 Comparison of Risk Stratification Models
ROC curves of CHADS-VASc and GRACE scores for predicting MACCEs or
all-cause mortality were shown in Fig. 2. The c-statistics and NRI analyses were
shown in Supplementary Table 2. As continuous variables, the
c-statistics for primary outcomes were 0.677 for CHADS-VASc (95% CI
0.647–0.717), 0.629 for GRACE at admission (95% CI 0.585–0.673) and 0.699 for
GRACE score at discharge (95% CI 0.659–0.740). The GRACE at discharge and
CHADS-VASc scores had comparable prognostic value for MACCEs
(p = 0.281) but the GRACE score at admission had worse discrimination
accuracy compared to CHADS-VASc score (p = 0.041, NRI:
–13.21%, 95% CI –21.60% to –7.38%). As for all-cause mortality, the
CHADS-VASc score (c-statistics: 0.750, 95% CI 0.705–0.794) proved
to have similar risk stratification as GRACE score at admission (c-statistics:
0.775, 95% CI 0.732–0.818). Nevertheless, the GRACE at discharge achieved
statistically stronger discrimination ability (c-statistics: 0.846, 95% CI
0.813–0.880) compared to CHADS-VASc (NRI: 45.13%, p
0.001).
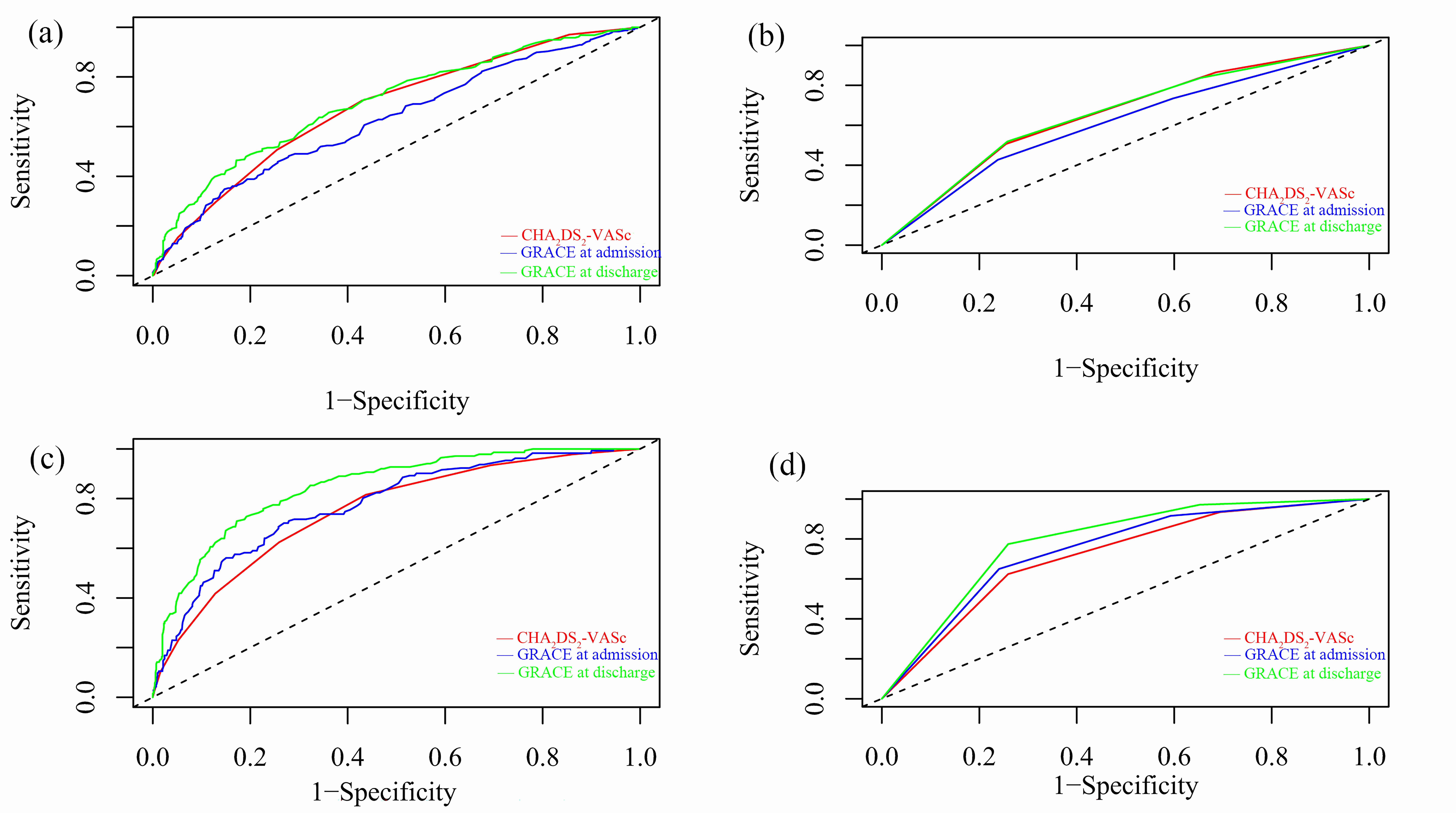 Fig. 2.
Fig. 2.
ROC curves for predicting MACCEs or all-cause mortality during
follow-up. Three scores were treated (a) as continuous variables for MACCEs. (b)
as categorical variables for MACCEs. (c) as continuous variables for all-cause
mortality. (d) as categorical variables for all-cause mortality.
3.4 Subgroup Analyses
To assess whether predictive performance differed depending on sex, primary
outcome was also analyzed comparing CHADS-VASc, GRACE at admission
and GRACE at discharge in subgroups defined by sex. The cumulative incidence of
MACCEs during follow-up was shown in Fig. 3. CHADS-VASc and GRACE at
discharge significantly stratified high-risk patients across male and female in a
consistent manner (p = 0.216 and
p = 0.088, respectively). In addition, three risk models
remained associated with the risk of all-cause death independent of sex category
(p = 0.982 for CHADS-VASc;
p = 0.857 for GRACE at admission;
p = 0.977 for GRACE at discharge). We did not detect any
relevant interaction with the predictive values in any of subgroups. The results
of subgroup analyses were displayed in Supplementary Table 3.
 Fig. 3.
Fig. 3.
Cumulative incidence of MACCEs according to sex during 36 months
follow-up. The predictive performance of CHADS-VASc, GRACE at
admission and GRACE at discharge scores on MACCEs was consistent between male and
female patients (all p for interaction 0.05).
4. Discussion
In the present study, we assessed prognostic values of the
CHADS-VASc, GRACE at admission and GRACE at discharge scores in
adverse outcomes among AF patients with ACS or undergoing PCI during 3-year
follow-up. We demonstrated that higher CHADS-VASc and GRACE at
discharge scores were independently associated with increased risk of MACCEs, but
the GRACE score at admission was not. The fact that CHADS-VASc and
GRACE at discharge demonstrated comparable discriminative capacity meanwhile
GRACE at admission provided relatively lower discrimination further supported
this viewpoint. For prediction of all-cause mortality, three models displayed
good discriminative capacity. The GRACE at discharge showed better predictive
ability. A significant discrimination improvement of GRACE at discharge compared
to the CHADS-VASc was detected. In addition, none of the three scores
had an acceptable value in predicting major or minor bleeding during the
follow-up.
The co-existence of AF and the need for PCI is a much more complicated situation
compared to suffering from AF or CAD alone. Existing evidence reports the
incidence of ACS with concomitant AF between 6% to 22%, with an increased
incidence in elderly and female patients [17, 18]. AF is a well-established marker
of poor short- and long-term prognosis in patients with ACS and is associated
with an increased risk of overall mortality. An analysis derived from 1558205 ACS
patients observed that patients with AF had significantly longer and more
complicated hospital stays with nearly double adjusted in-hospital mortality
[19]. Pilgrim et al. [20] showed that among patients with CAD undergoing
revascularization with drug-eluting stents (DES), AF conferred a rising risk of
both all-cause mortality and ischemic stroke during four-year follow-up. Similar results were obtained from sub analysis of the Global Registry of Acute
Coronary Events (GRACE) study where ACS patients with concomitant AF were more
likely to have a complicated in-hospital course than those without AF [21].
Meanwhile, in a large-scale, prospective registry including 29,679 consecutive
patients presenting with AF, a prior ACS conferred higher adjusted risks of
stroke, systemic embolism, all-cause mortality and CV mortality [22]. In the
present study, we reported that the 3-year incidences of composite MACCEs,
all-cause mortality, CV mortality reached 15.6%, 9.7% and 7.0% respectively.
In the EPICOR (long-tErm follow-uP of antithrombotic management patterns In acute
CORonary syndrome patients) Asia study, 6.2% patients experienced the composite
endpoint of death, MI and ischemic stroke and 3.6% suffered all-cause death
(including 1.3% cardiovascular-related) within 2 years. Although our analysis
was from a different group of patients in EPICOR Asia, it could be predicted that
ACS combined with AF had a numerically higher relative risk in long-term adverse
events compared to ACS alone. Whether AF contributes to the onset of ACS or if
ACS leads to AF is beyond the scope of this paper as we lack the precise
information about the time of appearance of these diseases. However, previous
studies observed that AF could promote inflammation that could cause a
prothrombotic state and eventually coronary artery occlusion [23]. In addition,
AF with high heart ventricular rates might yield symptoms of myocardial ischemia
characterized by an imbalance between demand and blood supply [24]. Conversely,
CAD affecting the atrial branches could result atrial scarring and remodeling to
form a substrate conducive for consequent persistent AF [25]. In the past three
decades, catheter ablation has evolved to a well-established treatment option for
AF patients to obtain rhythm control. The safety and effectiveness of ablation in
increasing freedom from recurrences and lowering AF burden during one year
follow-up has been documented in multiple clinical trials [26, 27]. While the
study lacked information on catheter ablation, there is no randomized controlled
trial sufficiently large to properly evaluate a reduction in thromboembolic
events compared with antiarrhythmic dugs [5].
The CHADS-VASc score has been widely used for the assessment of
thrombo-embolic risk and guiding antithrombotic therapy in AF or atrial flutter.
A growing number of studies have assessed the predictive accuracy of
CHADS-VASc score in patients with CAD. Podolecki et al. [28]
included 2647 consecutive acute myocardial infarction (AMI) patients without AF
and found that the risk of ischemic stroke and all-cause death in
CHADS-VASc 4 increased 4-fold compared to
CHADS-VASc = 1. Besides, every point in CHADS-VASc score
was independently associated with 41% increase in stroke risk and 23% increase
in mortality. Both CHADS and CHADS-VASc scores were evaluated
in a study of 929 AF patients referred for PCI. A high CHADS-VASc
score was predictive of all-cause mortality and MACCE at 12-months while the
CHADS score could only predict MACCEs. CHADS and
CHADS-VASc were not associated with major bleeding [29]. These
findings were further supported by another survey enrolling 13,422 ACS patients
which demonstrated that a higher CHADS-VASc score was associated with
an increased risk of 1-year mortality after adjusting for in-hospital treatments
[30]. However, the available studies mainly targeted ACS or ACS undergoing PCI as
research population and there is little evidence evaluating the association
between risk models and stable coronary artery disease (SCAD) patients undergoing
elective PCI. Also, previous study often calculated c-statistics or utilized
Kaplan-Meier curves to illustrate the predictive performance of
CHADS-VASc score. There are no sufficient data regarding the impact
of post-discharge antithrombotic regimens as it plays an important role in
long-term outcomes. In the current study, we included 433 (30.8%) SCAD patients
who were eligible for elective PCI, among whom 54 suffered MACCE and 18 died
within 3 years. We found that CHADS-VASc score had a good predictive
performance in MACCE (c-statistic: 0.677 as continuous) and all-cause death
(c-statistics: 0.750 as continuous) throughout the entire range of the score. Of
note, the thromboembolic risk was approximately 1.184 times greater for each
point increase in the CHADS-VASc score, while low and intermediate
category reclassified by CHADS-VASc had comparable risk of MACCEs
after adjusting for covariates. These findings could not be attributed to poor
predictive performance for the cutoffs were chosen on the basis of previous
literature subjectively. It is the first to consider not only clinical
presentations and laboratory results but also post-discharge medications as
potential risk factors. Patients who experienced MACCE were less likely to be
prescribed Aspirin and Ticagrelor but more frequently to receive anticoagulant
therapy at discharge. Multivariable Cox regression analysis including
antithrombotic regimens as covariates showed that CHADS-VASc score
was an independent risk factor for MACCEs and all-cause mortality. Besides,
CHADS-VASc 4 could increase the risk of MACCEs by double and
all-cause death by 5 times compared to CHADS-VASc equal to 1 or 2.
The GRACE ACS score was derived from an international registry of ACS patients
and has been a well-recognized risk system to stratify patients according to
their estimated risk of future death or ischemic events. Several studies have
also tried to evaluate and compare the predictive performance of
CHADS-VASc and GRACE scores to determine which system is more
suitable for AF patients combined with ACS or coronary stenting. Fauchier
et al. [31] aimed to find out the most appropriate score among
CHADS-VASc, GRACE at admission, REACH [32], SYNTAX [33] and
Anatomical and Clinical Syntax II Score (ACSS) [34] to use in the setting of 845
AF with coronary stenting. The results indicated that CHADS-VASc was
the best predictor of stroke and thromboembolic events with a c-statistics of
0.604 and SYNTAX was better to predict MACE with a c-statistics of 0.612. GRACE
at admission was the best to predict all-cause mortality with a c-statistics of
0.682 [31]. Another retrospective study consisting of 1452 consecutive patients
undergoing PCI with a diagnosis of AF demonstrated that the GRACE at admission
but not the CHADS-VASc score was associated with the incidence of
MACEs within 1 year. However, the two scores showed similar predictive
performance in the prediction of all-cause mortality [35]. Although the above
researches came to controversial conclusions, they adopted GRACE score designed
for predicting cumulative six month risk of death or MI other than the GRACE
score developed for predicting post-discharge outcomes. The variables used by
GRACE at discharge involved history of CHF, history of MI and in-hospital PCI
suggesting that more weighting is given to chronic conditions in the
post-discharge system. To the best of our knowledge, this is the first report
comparing CHADS-VASc, GRACE at admission and GRACE at discharge
scores in the same cohort. We found that the CHADS-VASc and GRACE at
discharge scores showed significant prognostic values in long-term MACCEs
according to multivariable analysis as well as c-statistics, while the GRACE at
admission had no impact on predicting MACCEs. Notably, the prognostic ability of
GRACE at discharge score could be weakened after grouping in accordance with
recommendations of the GRACE system. On the other hand, GRACE at discharge
demonstrated significant superiority in predicting all-cause mortality with a
c-statistics of 0.846 (as continuous) to CHADS-VASc score with a
c-statistics of 0.750 (p 0.001). The results were consistent with
the differences of purpose when the two risk scoring systems were established.
The CHADS-VASc score was developed for AF in stratifying high-risk
patients who would sustain thromboembolic events. Nevertheless, the main outcome
measured in designing GRACE at discharge score was all-cause mortality during 6
months follow-up. As discussed above, any risk score has to balance simplicity
and practicality against precision. In the present study, the GRACE at discharge
score should undoubtedly be advocated for evaluating the long-term survival if
conditions permit. The CHADS-VASc only performed modestly in
predicting all-cause mortality, but it could be utilized rapidly if biomarkers or
electrocardiogram information necessary for calculating the GRACE were difficult
to obtain. Furthermore, it was suitable to combine CHADS-VASc with
GRACE at discharge to improve the accuracy of risk stratification, leading to
more effective clinical decision-making and prolonged survival of AF patients
with ACS or undergoing PCI.
The following were several limitations in the present study. First, this is an
observational, prospective, single-center registry and has its inherent residual
confounding bias. Our findings should be carefully interpreted when applied to
external validation cohorts. However, we analyzed a total of 1408 AF patients
with ACS or undergoing PCI and the sample size was comparable to those of similar
researches. Second, we did not assess the prognostic values of the three models
according to the subtypes of AF for we were unable to determine the accurate
order of the presence of AF and ACS. Previous evidence suggested that only
permanent AF was an independent predictor for death in AMI patients treated
invasively [36]. Third, the post-discharge antithrombotic regimens were collected
and treated as a covariate in our study. During 3-year follow-up, the medication
adherence of participants and the possible transitions in dual-antiplatelet
therapy after coronary stenting were unable to be obtained. The changes in
anticoagulant or antiplatelet therapies might have significantly affected the
incidence of ischemic or bleeding events. Further well-designed clinical trials
are needed to compare and validate the prediction performance of several risk
stratification systems for AF patients with ACS or undergoing stent implantation.
5. Conclusions
In the setting of coexistence of AF and ACS or coronary stenting, higher
CHADS-VASc and GRACE at discharge score were independently associated
with increased risk of MACCEs and they had comparable discriminative capacities.
Both CHADS-VASc and GRACE at discharge scores demonstrated good
prognostic values in all-cause mortality. A significant discrimination
improvement of GRACE at discharge was detected compared to
CHADS-VASc. The GRACE at admission score could not identify patients
at high risk of MACCEs. Further studies are needed to validate the clinical
significance of these scores externally or help build a more accurate and
practical risk score.
Author Contributions
RM acquired the data, performed statistical analysis and drafted the manuscript.
YMY designed the research and revised the manuscript. HZ and NS collected data
and provided help in interpreting. JYW and SQL collected the data and helped in
endpoints adjudication. All authors contributed to editorial changes in the
manuscript. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
The study was approved by the ethics committee of Fuwai Hospital and was
conducted in accordance with the Declaration of Helsinki (No. 2017-922).
Acknowledgment
We wish to thank all individuals who agreed to participate in this study. The
authors also thank the staff in Emergency Department in Fuwai Hospital for
patients’ recruitment.
Funding
This research was funded by Capital’s Funds for Research and Application of
Clinical Diagnosis and Treatment Technology, grant number Z171100001017193.
Conflict of Interest
The authors declare no conflict of interest.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3.