Academic Editors: Osama Hamdy and Joanna Mitri
Background: Diabetic neuropathy (DN) is a very frequent microvascular complication of type 2 diabetes mellitus (T2DM). Obesity and physical inactivity are well-known risk factors for T2DM. Fibroblast growth factor 21 (FGF21) is a liver-secreted hormone with several beneficial effects on obesity-related metabolic disorders. We aimed to investigate the effect of short-term physical activity on the levels of FGF21, and its correlation with the severity of peripheral sensory polyneuropathy in T2DM patients. Methods: Thirty patients with DN were enrolled in the study, compared to age- and gender-matched controls. We conducted a six-week aerobic training program, which meant treadmill and cycle ergometers three times a week. Anthropometric and laboratory parameters were measured for each patient before and after intervention. Serum levels of FGF21, TNF-alpha, irisin, leptin and adiponectin were measured by ELISA. The sensory perception threshold (CPT) was quantitatively measured using Neurometer®. Results: We found significant decreases in BMI, waist circumference, HbA1c and TNF-alpha levels. From baseline to six-week follow-up, FGF21 levels were significantly increased in DN patients. Significant negative correlations were shown between the changes in FGF21 levels and BMI, between changes in FGF21 and the improvement of CPT values, and between the changes in FGF21 and TNF-alpha levels. There was no difference in irisin, adiponectin and leptin levels in DN patients after aerobic training program. Conclusions: The physical activity may increase the level of FGF21 in T2DM patients with neuropathy. Our results highlight the importance of regular physical activity in the treatment of diabetic neuropathy.
Diabetic neuropathy (DN) is a quite frequent microvascular complication of type 2 diabetes mellitus (T2DM), which is often diagnosed as distal sensory polyneuropathy. The development of diabetic neuropathy is a multifactorial progression and the precise pathomechanism is not fully clarified. Impaired glucose metabolism is associated with increased reactive oxidative species production, mitochondrial dysfunction and accumulation of glycolytic intermediates that stimulate other metabolic or non-metabolic pathways instead of switching to glycolysis, resulting in activation of polyol, hexosamine, and protein kinase-C pathways. Chronic hyperglycaemia induces the production of reactive oxygen species and increases oxidative stress, which plays an important role in the development of mitochondrial dysfunction in distal sensory polyneuropathy [1].
Previous research indicates that physical activity may improve the neurological function and impaired nerve conduction in DN [2]. Physical activity and regular exercise are effective interventions to reduce the development and progression of diabetic neuropathy. Moreover, it is important that patients with polyneuropathy perform regular physical activity under appropriate health and physiotherapy supervision. Uncontrolled exercise due to peripheral nerve damage may contribute to the development of ulcers and diabetic foot syndrome, as the insensitive foot is unable to mediate the nociceptive stimuli required to elicit protective behavior, which affects peripheral sensation and vasomotor regulation of foot circulation [3]. However, a recent meta-analysis has demonstrated that controlled exercise is a beneficial non-pharmacological intervention for the management of diabetic foot, especially in increasing nerve velocity conduction in the lower extremities. Exercise in diabetic patients may have additional benefits, such as skin sensitivity and intraepidermal nerve density, which may delay the normal course of diabetic peripheral neuropathy, as well as delay skin damage and ulceration [4]. According to the statement of American Diabetes Association, structured lifestyle modification which include at least two and a half hour per week physical activity and dietary changes, are also recommended to delay the progression of microvascular complications in T2DM. Moreover, all diabetic patients with or without peripheral neuropathy should perform both aerobic and resistance training for optimal glycemic control [5].
Regular exercise in diabetes has a favorable effect on insulin resistance, especially in the liver and muscle tissue. Several factors may contribute to insulin resistance in liver, including the formation of reactive oxygen species, genetic factors, aging, and mitochondrial dysfunction. Fibroblast growth factor 21 (FGF21) is a liver-secreted hormone with several beneficial effects on obesity-related metabolic disorders and insulin resistance. FGF21 enhances glucose uptake and oxidation in an insulin-independent manner by inducing the expression of glucose transporter-1 in adipocytes and skeletal myocytes [6]. Previous experimental and human studies have shown that physical activity may increase the serum levels of FGF21 in T2DM [7]. According to a recent meta-analysis, acute exercise significantly increased the serum concentration of FGF21, regardless of body weight and obesity level [8]. However, it is still not clarified how FGF21 may improve the process of mitochondrial oxidation [9].
It is well known that skeletal muscle produces and releases cytokines during
exercise, which are termed myokines. Irisin is a myokine expressed by physical
activity with insulin-sensitizing properties and derived from the C-terminal
cleavage of the fibronectin type III domain containing 5 (FNDC5) transmembrane
proteins [10]. This proteolytic process is mediated by the peroxisome
proliferator-activated receptor-gamma coactivator-1-alpha (PGC-1
In addition to energy storage, adipose tissue produces a variety of
adipocytokines, including leptin, adiponectin and others, thus having potential
endocrine function. Leptin may directly improve insulin resistance in diabetic
mice by increasing the oxidation of free fatty acid (FFA) [12]. Previous research
has shown that decreased circulating adiponectin is positively associated with
insulin resistance and the markers of cardiovascular disease in T2DM subjects
[13]. Furthermore, an increased proinflammatory response is observed in leptin
resistance during obesity and physical activity may reduce inflammation by
improving leptin resistance in T2DM [14]. Tumor necrosis factor alpha (TNF-alpha)
is a pro-inflammatory adipokine associated with insulin resistance and
In the current study, we aimed to investigate the changes of FGF21 level and their relationships with other inflammatory markers and adipokines in T2DM patients with distal sensory polyneuropathy after six-week of aerobic exercise training program. We hypothesized significant correlations between the changes of FGF21 level and the severity of peripheral sensory neuropathy in T2DM patient after physical activity.
Our study group included 30 adult individuals with T2DM and distal sensory
polyneuropathy (9 men and 21 women; the mean age: 61.97
Study design flowchart of diabetic patients with neuropathy are depicted on Fig. 1. After final enrolment, DN patients were instructed to march with trekking
poles and the aerobic exercise training program was supervised by a
physiotherapist and corrected if needed. Glucose levels were determined
immediately after the training and one hour later. If significant drop of serum
glucose levels were measured, antidiabetic therapy has been modified according to
the needs of exercise. The subjects had to perform the exercises for 6 weeks, 3
days a week, occasionally for 70 minutes. The exercise program whose duration has
progressed gradually (from 50% to 80% of maximum heart rate), included 10
minutes of stretching movements until warm-up, then 50 minutes of aerobic
training (treadmill and bicycle ergometers), followed by 10 minutes of relaxation
activity to cool down. Before and after the intervention, we measured the cardio
fitness levels by VO
 Fig. 1.
Fig. 1.Study design flowchart of type 2 diabetic patients with and without neuropathy.
Peripherial blood samples were withdrawn after 12-hour overnight fast into Vacutainer® tubes. After centrifugation, routine clinical parameters (i.e., creatinine, uric acid, glucose, hemoglobin A1c—HbA1c, triglyceride, total cholesterol, low-density lipoprotein-cholesterol—LDL-C, high-density lipoprotein-cholesterol—HDL-C) were measured with Cobas 6000 autoanalyzer (Roche Ltd., Mannheim, Germany) in the Department of Laboratory Medicine, University of Debrecen, Faculty of Medicine, Debrecen, Hungary. High-sensitivity C-reactive protein (hsCRP), ApoA1, ApoB and Lp (a) levels were determined by immune-turbidimetric assays. Total cholesterol (TC), triglyceride, HDL-C and LDL-C levels were measured by enzymatic colorimetric tests. All measurements were performed according to the manufacturers’ recommendation. Samples for further ELISA determinations were kept at –80 °C.
Serum FGF21 concentration was detected by commercially available sandwich enzyme immunoassay (Human FGF21 ELISA, Biovendor, Brno, Czech Republic) with intra-assay coefficient variations ranging from 1.6 to 2.4% and inter-assay coefficient variations ranging from 3.1 to 3.5%, respectively. Determination of FGF21 level in sera were performed according to the manufacturer’s recommendation. The values were presented as pg/mL with the assay range of 30–1920 pg/mL and 7 pg/mL limit of detection.
We measured serum levels of TNF-alpha by using commercially available sandwich enzyme immunoassay according to the recommendations of the manufacturer (R&D Systems Europe Ltd., Abington, England). The intra-assay coefficient variations were 1.9–2.2% and inter-assay coefficient variations were 6.2–6.7%, respectively. The values were presented as pg/mL with 0.156–10 pg/mL assay range and 0.049 pg/mL sensitivity.
Circulating irisin were measured by competitive ELISA (Human Irisin ELISA,
Biovendor, Brno, Czech Republic). The calibration range was 0.001–5
Serum adiponectin and leptin levels were measured with ELISA (Human Total Adiponectin/Acrp30 Quantikine and Human Leptin Quantikine Immunoassays, R&D Systems Europe Ltd., Abington, England). The coefficient variations of the intra- and inter-assay for measurement of total adiponectin were 2.5–4.7% and 5.8–6.9%, respectively. Intra-assay coefficient variationss were ranging from 3.0% to 3.3%, inter-assay CV-s from 3.5% to 5.4% in case of leptin ELISA. Both adiponectin and leptin are 4.5 hours ELISA with 3.9–250 ng/mL assay range and 0.891 ng/mL sensitivity (adiponectin); and 15.6–1000 pg/mL assay range and 7.8 pg/mL sensitivity (leptin), respectively.
All participants were evaluated in detail for peripheral neuropathy (DN4
questionnaire in screening for neuropathic pain, vibration perception threshold,
quantitative sensory testing) and in-vivo confocal microscopy of the
cornea by ophthalmologist for the diagnosis of distal sensory polyneuropathy.
Peripheral sensory nerve functions were assessed with current perception
threshold testing (CPT) using the Neurometer®. It has been
previously reported that Neurometer® is capable of detecting
distal sensory neuropathy in various diseases, including diabetes mellitus [21].
The CPT testing using Neurometer® delivers sinusoidal alternating
current stimuli at three different frequencies: 5 Hz, 250 Hz and 2000 Hz,
assessing small unmyelinated C-fibre, small myelinated Aß-fibre and large
myelinated Aß-fibre function, respectively. This intensity setting is
performed to approach the CPT value within a
We performed Statistica® TIBCO Software Inc. (2018). Statistica
(data analysis software system), version 13. http://tibco.com during statistical
analyses. Normality of data distribution was checked with the Kolmogorov–Smirnov
and Shapiro-Wilk tests. Relationship between two categorical variables is
calculated by Chi-squared test. In case of normal distribution, the differences
between anthropometric and clinical laboratory values in diabetic controls and
patients before exercise program were analyzed with unpaired Student’s t
test. Data were expressed as means
Clinical and laboratory parameters of DN patients before and after physical activity and data of controls are summarized in Table 1.
| (1) Diabetic patients with neuropathy before aerobic exercise | (2) Diabetic patients with neuropathy after aerobic exercise | (3) Control patients with type 2 diabetes | |
| Number of patients (male/female) | 30 (9/21) | 32 (10/22) | |
| Age of patients (years) | 61.97 |
64.37 | |
| Duration of diabetes (years) | 10.3 |
10.9 | |
| BMI (kg/m |
31.6 |
31 |
29.3 |
| Waist circumference (cm) | 92.2 |
90.07 |
88.82 |
| hsCRP (mg/L) | 3.95 (1.5–9.1) | 3.49 (1.8–6.5) |
1.4 (0.6–2.9) |
| HbA1C (%) | 7.09 |
6.78 |
6.98 |
| Creatinine (µmol/L) | 72.73 |
74.27 |
77.44 |
| Uric acid (µmol/L) | 305.57 |
308.33 |
300.22 |
| Alanine Aminotransferase (U/L) | 34.5 |
32.1 |
36.7 |
| Aspartate Aminotransferase (U/L) | 26.4 |
28.9 |
25.7 |
| Gamma-Glutamyl Transferase (U/L) | 38.6 |
36.3 |
33.8 |
| Triglyceride (mmol/L) | 2.5 |
2.23 |
2.3 |
| Total cholesterol (mmol/L) | 4.79 |
4.77 |
4.8 |
| HDL-cholesterol (mmol/L) | 1.27 |
1.31 |
1.34 |
| nonHDL-cholesterol (mmol/L) | 3.65 |
3.49 |
3.49 |
| LDL-cholesterol (mmol/L) | 3.0 |
2.95 |
2.76 |
| FGF21 (pg/mL) | 140.62 (73.19–373.07) | 168.89 (111.4–513.69) |
133.54 (81.52–281.76) |
| TNF-alpha (pg/mL) | 0.7 |
0.57 |
0.59 |
| Irisin (µg/mL) | 4.32 |
4.3 |
4.73 |
| Adiponectin (µg/mL) | 6.91 |
7.09 |
6.89 |
| Leptin (ng/mL) | 30.72 |
30.59 |
20.93 |
| Current perception threshold (by Neurometer®, mA) | 0.545 |
0.498 |
0.458 |
| Data are presented as mean #: p ##: p $: p $$: p †: p ††: p | |||
Significant decreases in BMI, waist circumference, hsCRP, HbA1c and TNF-alpha levels were observed after 6-week physical activity in DN patients. Circulating FGF21 levels were significantly increased; while CPT values as measured by Neurometer® test were significantly improved after 6-week physical activity in DN patients.
There were no differences in serum creatinine, uric acid, irisin, adiponectin, leptin, triglyceride, total cholesterol, HDL-C, nonHDL-C, LDL-C levels and enzyme liver parameters in DN patients before and after physical activity.
Circulating TNF-alpha and hsCRP was significantly higher in DN patients compared to controls (Table 1). Although the concentrations of adipocytokines did not change across groups after physical activity, the level of leptin was significantly decreased after physical activity compared to control subject.
Significant negative correlations were observed between the changes in FGF21
levels and BMI (r = –0.4, p = 0.03) (Fig. 2a), between changes in FGF21
and the improvement of CPT values (r = –0.58, p
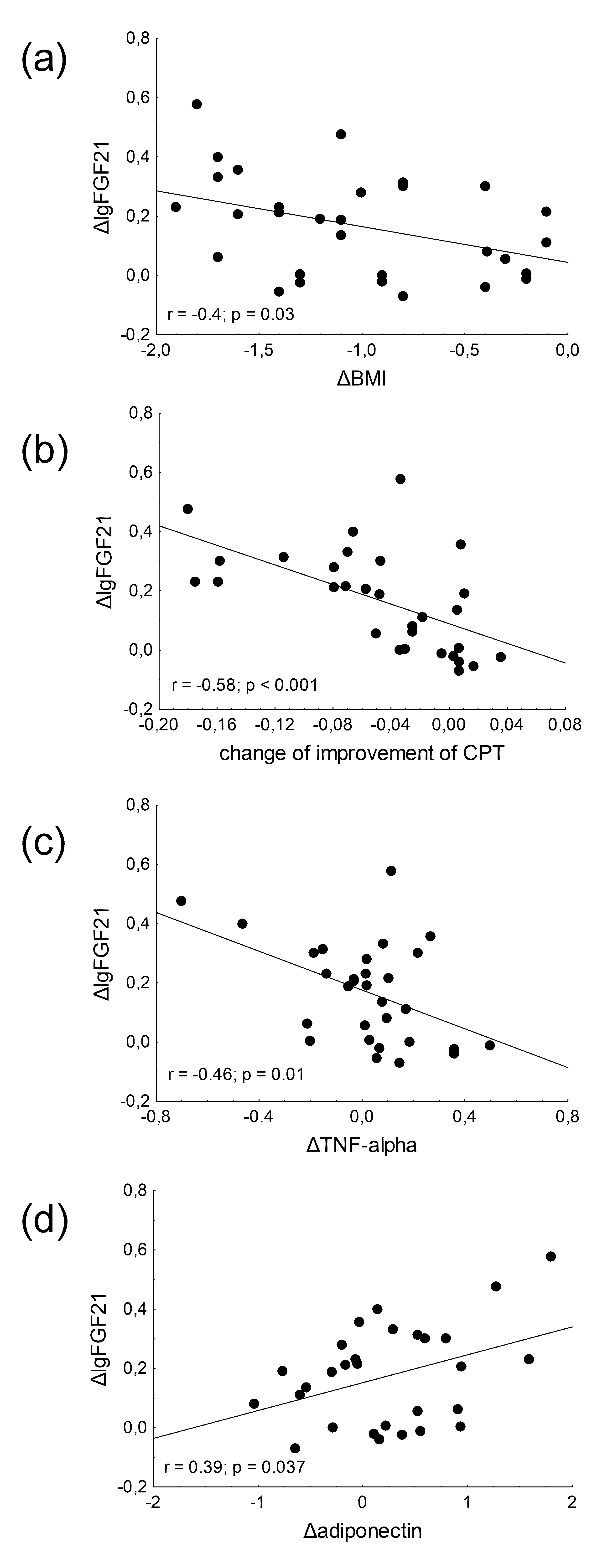 Fig. 2.
Fig. 2.Correlations between changes of fibroblast growth factor 21 (FGF21) levels and (a) change of body mass index (BMI); (b) change of improvement of CPT; (c) changes of tumor necrosis factor alpha (TNF-alpha) levels and (d) changes of adiponectin levels in type 2 diabetic patients with neuropathy. Pearson’s correlation test was performed to investigate the relationship between continuous variables.
We found a significant positive correlation between changes in BMI and TNF-alpha
concentrations (r = 0.39, p
Significant negative correlation was observed between the changes in BMI and
adiponectin levels (r = –0.38, p
We found a significant positive correlation between changes in CPT values and
TNF-alpha concentrations (r = 0.62, p
To our knowledge, this is the first study, to demonstrate significant correlations between the change of FGF21 concentration and change of body mass index, as well as change of TNF-alpha and adiponectin levels in T2DM patients with peripheral neuropathy after six weeks of aerobic exercise. In addition, we firstly analyzed the change in FGF21 levels in diabetic neuropathy and found a significant increase after training program.
In our study, we found a significant increase in FGF21 levels in type 2 diabetic
patients after 6 weeks of physical activity. Previous research has also shown
that physical activity may increase the serum level of FGF21 in T2DM but
neuropathy as a microvascular complication has not been reported in these studies
[7, 24]. A recent retrospecive study also revealed that the levels of serum FGF21
were elevated in patients with higher BMI compared to individuals with normal or
low BMI. Moreover, the FGF21 concentrations were found to be higher in patients
who exercised regularly compared to those who exercised only intermittently or
not at all [25]. our result may be explained by the activating effect of FGF21 on
FGFR1 receptor and
Growing evidence suggests that FGF21 may reduce atherosclerosis in cardiovascular disease [30, 31]. Recent research has demonstrated that FGF21 may inhibit arterial calcification in experimental models of vascular injury via various mechanisms including suppression of endoplasmic reticulum stress-mediated apoptosis and inhibiting the osteogenic transition of vascular smooth muscle cells [32]. Paradoxically, there is a positive association between FGF21 levels and a number of cardiovascular or metabolic diseases, such as coronary heart disease, obesity and T2DM [33, 34]. On the other hand, based on another clinical trial, acute myocardial infarction was also associated with a decrease in circulating FGF21 levels [35]. Thus, a number of studies on this topic have been published with contradictory results in recent years, which have often shown inconsistencies and contradictions between studies in terms of metabolic parameters and medication of patients. The results of our study in patients with DN are in line with previous studies showing that exercise may increase the serum levels of FGF21 not only in patients with T2DM, but also in distal sensory polyneuropathy [7, 24].
It must be noted that there are no data on the effects of aerobic exercise on FGF21 levels in subjects with distal sensory polyneuropathy. This is the first report on beneficial effect of physical activity on FGF21 levels in distal sensory polyneuropathy that strengthens the beneficial effects of physical exercise on sensory symptoms and neuropathic deficits in T2DM patients. The change of FGF21 level correlated with the severity of peripheral sensory neuropathy—defined by Neurometer®—after physical activity. Therefore, increased serum FGF21 levels may predict the clinical response to aerobic exercise. Previous studies have demonstrated a significant improvement in neurological function, affecting both sensorimotor and autonomic components of the peripheral nervous system, in patients with DN during physical exercise programmes [2, 36]. The mechanism of improvement of neuropathic symptoms and nerve function is thought to be related to improvement in endothelial dysfunction and reduction of inflammation in DN. Our data highlight the initial hypothesis that increased FGF21 may be a biomarker of chronic inflammation in DN. However, there are no previous data in the literature on whether physical activity directly or indirectly elevates FGF21 in patients with diabetic neuropathy. Therefore, further studies are necessary to validate our results.
Although the levels of adipokines did not differ in patients with diabetic neuropathy after physical activity, the increasing tendency in adiponectin level was significantly associated with the magnitude of body weight loss and we found a positive correlation between the increase in the adiponectin level and the FGF21 concentration in diabetic neuropathy. FGF21 shows functional similarity to adiponectin, which acts as a downstream effector of FGF21, controling glucose and lipid metabolism in adipocytes and skeletal muscle [37]. Meanwhile, adiponectin may enhance the effect of FGF21 on energy balance and insulin sensitivity in these tissue; thus, the FGF21— adiponectin axis may be implicated in the regulation of glucose and lipid homeostasis [38]. We have also found a significant correlation between the level of serum adiponectin and improvement of neurological function, affecting sensorimotor component of the peripheral nervous system in patients with diabetic neuropathy. Previous research has been reported that decreased adiponectin levels were associated with a significantly increased risk of DN in T2DM patients. Moreover, there was a strong relationship between decreased nerve conduction velocity and adiponectin concentration in chronic inflammation and progression of diabetic sensorimotor neuropathy [39].
Our study revealed that six weeks of aerobic physical activity in patients with DN lead to a significant reduction in the levels of TNF-alpha and hsCRP. Recent studies have demonstrated the efficacy of physical exercise on inflammatory markers in DN [36, 40]. TNF-alpha plays a crucial role in initiating inflammatory processes leading to severe impairment of glucose tolerance and insulin sensitivity which may eventually increase the risk of cardiovascular diseases in T2DM [41]. TNF-alpha stimulates lipolysis in adipose tissue, thus increased plasma concentration of FFA may contribute to atherogenesis in T2DM patients. Moreover, TNF-alpha enhances leptin production, which is known to regulate energy homeostasis by reducing pancreatic insulin secretion and promoting insulin resistance. Therefore, TNF-alpha may indirectly contribute to the development of insulin resistance by inhibiting adiponectin and stimulating leptin via glucose metabolic pathways [41]. Our findings regarding the linear association between the changes of body mass index and TNF-alpha levels were generally consistent with prior research in patients with DN after exercise program [36, 40]. However, aerobic training may have a protective effect against diabetic nerve damage by restoring endothelial function and decreasing the production of the inflammatory cytokine TNF-alpha in T2DM.
While some prior studies have demonstrated positive association or contradictory results, we have not found association between the changes in irisin levels after physical activity. Previous research has shown that FNDC5 expression in skeletal muscle are reduced in obese subjects and circulating irisin levels are related with insulin sensitivity in T2DM [11]. Studies examining the relationship between the circulating irisin levels and training-induced changes have yielded mixed results, with some studies suggesting a strong association and others finding no association [42, 43, 44]. It was hypothesized that the level of serum irisin increased immediately after physical activity and seems to correlate with the intensity of exercise training, as well as prior empirical research suggest the contribution of irisin in the neuroprotective process of physical exercise in T2DM [45, 46]. Therefore, further follow-up studies should be performed to determine the effects of various factors directly or indirectly for changes in levels of irisin in T2DM with peripheral neuropathy.
There are some limitations of our study. Data on other inflammatory biomarkers
and parameters of endothelial dysfunction, arterial stiffness and flow mediated
dilatation would enhance our knowledge about the impactt of physical activity on
chronic inflammation and endothelial dysfunction and its contribution to the
favorable effects on peripheral neuropathy. Evaluation of sural nerve automated
nerve conduction in the diagnosis of peripheral neuropathy could be useful
additional information. Direct measurement on VO
Our results highlight the potential role of regular physical activity in the treatment of diabetic neuropathy. According to our results, physical activity increased the levels of FGF21 in T2DM patients with distal sensory polyneuropathy. Monitoring of FGF21 levels may predict the efficacy of aerobic exercise in diabetic neuropathy. Data on other biomarkers of inflammation and oxidative stress may enhance our knowledge about the impact of physical activity in peripheral sensorimotor neuropathy.
These should be presented as follows: FS and GP designed the research study. ÁM and AS performed the research. IS and ZB provided help and advice on methodology. HL, MH ans IS analyzed the data. FS, HL and MH wrote the manuscript. GP and PK critically revised manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Regional Ethics Committee of the University of Debrecen and the Medical Research Council (protocol code: 5287-2/2019/EKU, date: 07/03/2019).
The authors say thank to Ferencné Lénárt and Zsanett Molnár Hámoriné for the excellent technical assistance.
The work is supported by the Bridging Fund—University of Debrecen to MH, Faculty of Medicine; by the Hungarian Diabetes Association.
The authors declare no conflict of interest.
