†These authors contributed equally.
Academic Editors: Buddhadeb Dawn and Boyoung Joung
Objectives: Postoperative atrial fibrillation (POAF) is a frequent
complication following cardiac surgery. This study examined the safety and
efficacy of the new DefiPace
Postoperative atrial fibrillation (POAF) is a frequent (20–70%) complication following cardiac surgery, often resulting in prolonged hospital stay and an increased risk of morbidity and mortality [1, 2]. POAF tends to occur one to three days after cardiac surgery, with a peak incidence on postoperative day two [3]. Standard-of-care is to treat POAF using high-dose anti-arrhythmic medication (beta-blockers, amiodarone, magnesium) and external high-energy atrial cardioversion, all of which have either side effects or are particularly time-consuming [1, 2]. Bi-atrial pacing, as a measure to prevent the onset of POAF, has been shown to be successful in many clinical studies and meta-analyses [4, 5, 6, 7, 8, 9, 10, 11, 12], and have been included in the European Guidelines for cardiac surgery patients, even in the absence of a commercially available bi-atrial pacemaker device. Similarly, low-energy internal cardioversion of POAF has also been used for some time [13, 14, 15, 16, 17, 18, 19, 20], and good results have already been achieved with this technique. However, the procedures were less practicable because they required complex fixation of the electrodes, and two separate devices were required for postoperative pacing and low-energy cardioversion. This and the lack of practicable product lines on the market are the likely reasons why both methods of bi-atrial pacing and internal cardioversion, both of which are more comfortable for the patient, did not prevail despite good results.
This study aimed to examine the safety and efficacy of low-energy atrial
defibrillation using a new system named DefiPace
This patient cohort analysis is a retrospective observation of 29 patients after
cardiac surgery (age 65.6
The study was conducted in two phases. Phase one was initiated when the atrial electrodes had initial CE approval (since November 2016) for single or bi-atrial pacing only. In this phase one, the safety and efficacy of the electrodes were observed and thus served as an internal safety control. Electrodes were placed either on the right or left atrium or both. In the second phase starting in 2020 (after CE certification for additional atrial defibrillation in 2019), the bi-atrially implanted electrodes were also used for atrial cardioversion in case of POAF. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki and the study complies with the local medical board’s ethical regulations (Bayerische Landesärztekammer No.: 2021-1067).
The DefiPace
 Fig. 1.
Fig. 1.Osypka DefiPace
The DefiPace
 Fig. 2.
Fig. 2.Schematic drawing of a human heart [front (a) and back view (b)] showing the atrial temporary wire electrodes in place. The distal 10 cm of the electrode (anode) formed as a zigzag were placed at the right (a) and left atria (b), respectively. On the right side (b), back view of the heart, the electrodes were either placed via transvers sinus (1) or fixed on the left atrium (2).
There are different types of TMA® electrodes available. They vary only in the fixation mechanisms: the ends of the cathode or anode are available with or without a needle. The type of electrode is chosen by physician preference. The operating function is identical.
The temporary left atrial electrodes were either transferred through the
transverse sinus for placement at the left atrium without fixation (Figs. 2b, 3)
or fixated on the epicardial or pericardial surface based on the preference of
the individual surgeon. In case the electrodes were fixed, the anode
(defibrillation zigzag) was either placed and fixed between the free wall of the
left atrial appendage or fixed to the pericardium and the left upper pulmonary
vein (Fig. 2b). The cathode was placed one to two cm distal from the anode. The
anode of the TMA® wire to the right atrium was placed and fixated
to the free right atrial wall between the superior and inferior vena cava (Fig. 4); and the cathode was placed at the sinus node one to two cm distal to the
anode. The electrodes were placed and stitched with meticulous care at the atria,
and guided carefully in the pericardium to ensure smooth extraction. Special
attention was given to bypass grafts to prevent damage during extraction. The
proximal ends of the electrodes were lead through the skin of the patient’s chest
and secured with a suture. The three connection cables (two from
TMA® wires and one from the ventricular wire) are then plugged
into the external device, Osypka DefiPace
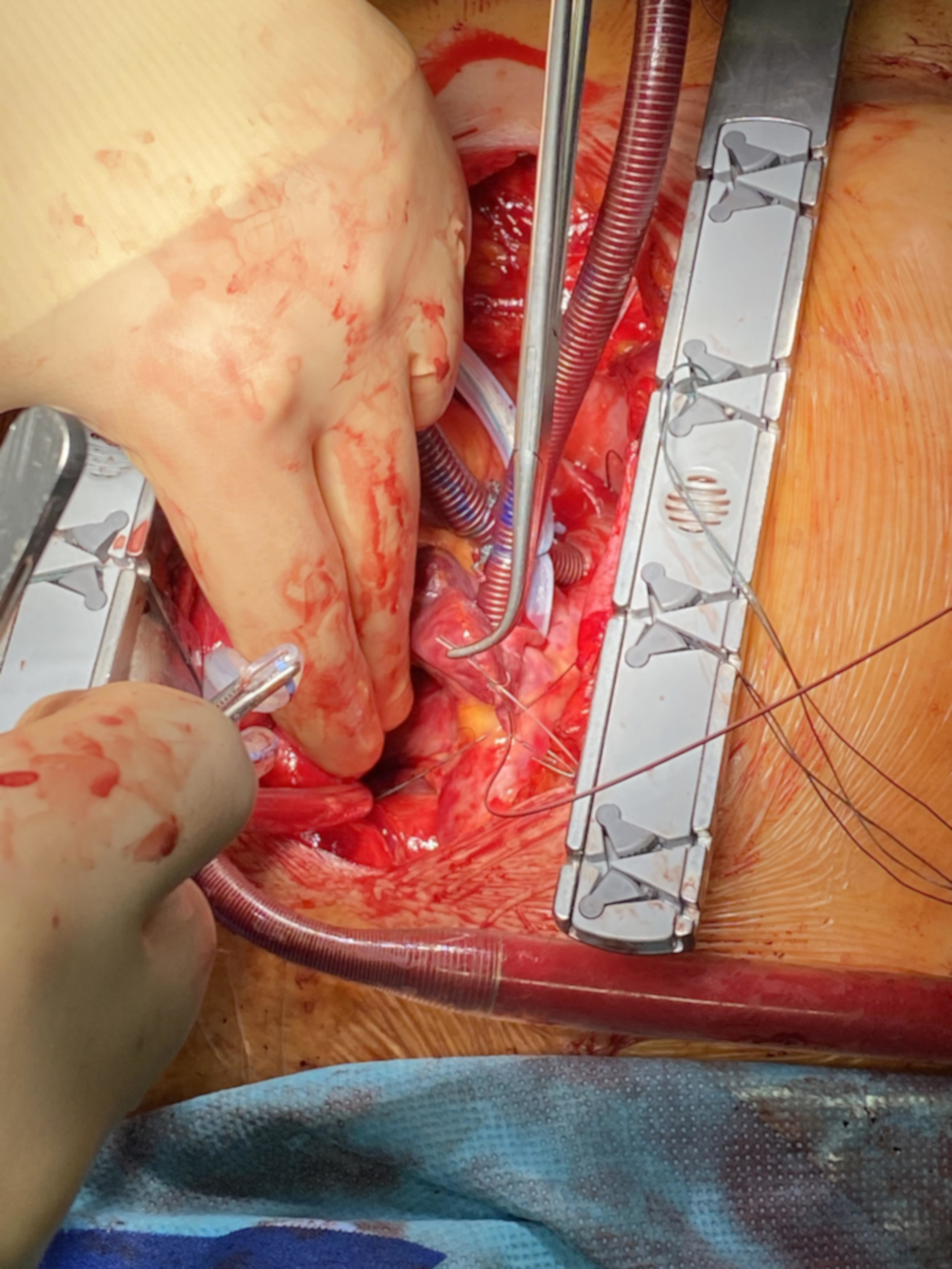 Fig. 3.
Fig. 3.Implantation of the atrial TMA® electrodes without needle: placement of the TMA® wire transferred through the transvers sinus for placement at the left atrium without fixation.
 Fig. 4.
Fig. 4.Implantation of the atrial TMA® electrodes with needles: placement of the right wire with anode stitched to the pericardium and the cathode on the superior vena cava.
Postoperatively, the pacing threshold and sensing (P- and R-wave amplitudes)
were measured in a standard manner up to the 6th postoperative day with the
DefiPace
Twenty-nine patients (mean age: 64.6
After training, the implantation of the TMA® electrodes took 4.2
The sensing and the threshold of the electrodes are shown in Table 1. In all patients, the atrial pacing and sensing values for left and right atrial threshold and sensing were acceptable, and pacing was possible except when POAF occurred.
| sensing threshold | pacing threshold | |||||
| TMA right atrium (mV) | TMA left atrium (mV) | Ventricular electrode (mV) | TMA right atrium (V/0.5 ms) | TMA left atrium (V/0.5 ms) | Ventricular electrode (V/0.5 ms) | |
| OD | 1.0 |
1.5 |
4.7 |
1.9 |
3.9 |
1.9 |
| 1. pod | 0.9 |
1.3 |
4.4 |
2.1 |
3.9 |
2.1 |
| 2. pod | 0.9 |
1.0 |
3.3 |
2.5 |
4.4 |
2.6 |
| 3. pod | 1.0 |
1.1 |
2.7 |
2.7 |
4.3 |
3.1 |
| 4. pod | 1.0 |
1.2 |
2.7 |
2.5 |
5.0 |
3.2 |
| 5. pod | 1.2 |
1.1 |
3.0 |
2.8 |
4.8 |
3.4 |
| 6. pod | 1.3 |
1.1 |
3.3 |
2.9 |
5.0 |
3.4 |
| Legend: data are expressed as mean | ||||||
In 4 patients, POAF was observed between 2–4 postoperative days. In two
patients, POAF was converted to sinus rhythm using the DefiPace
The wires were removed without complication after 6.2
This study proved the safety and pacing efficacy of left and right atrial
TMA® electrodes during cardiac surgery. Low-energy atrial
cardioversion for postoperative atrial fibrillation using epicardial pacing wires
was first described in 1998 by Liebold et al. [14] in a study with 238
patients. Additional studies by the same team were performed within the next year
[16]. In these studies the wires (so-called TADpole wires) were similar to
bipolar Osypka TME pacing wires, but featured a 10 cm long portion of uninsulated
wire distally to the heart needle. The uninsulated wire portions for pacing and
cardioversion had to be sutured onto the left and right atria with several
stitches [13, 14, 16]. In contrast to the DefiPace
Another multicenter European trial conducted by Kleine et al. [15] with
a total of 296 patients, also using TADpole wires, also showed the suitability of
using epicardial pacing wires for conversion of atrial fibrillation. Sixty-five
patients had a total of 83 episodes of AF treated by TADpole wires with a
conversion rate of 88.5%, using an energy of 6.0
In our retrospective study, bi-atrial pacing was not the primary goal but was performed when atrial pacing was necessary. An overall review of the history of atrial pacing proved that bi-atrial pacing is the most successful treatment of all pacing methods to prevent postoperative atrial fibrillation [4, 5, 6, 7, 8, 9]. A review by Mitchell [10] further details the results of an evaluation of 12 trials of prophylactic atrial pacing involving 1708 patients. Overall, when combining all results regardless of atrial pacing site (right, left, or bi-atrial), or the pacing algorithm used, prophylactic atrial pacing significantly reduced the incidence of postoperative pacing. The meta-analysis from Crystal [11] demonstrated that when all pacing algorithms were combined, there was a statistically significant difference achieved with bi-atrial pacing, reducing the postoperative hospital length of stay by 1.54 days. In another meta-analysis from Burgess [12] it was shown that, while all pacing sites and algorithms combined are beneficial, the only significant result was seen in the bi-atrial pacing group, which reduced atrial fibrillation from an average of 35.3% in the control group to 17.7% in the paced group (OR 0.44, 95% CI 0.31–0.64). In view of these positive outcomes, the European Society of Cardiology (ESC) together with the European Association of Cardio-Thoracic Surgery (EACTS) has added bi-atrial pacing as a recommendation in their guidelines for postoperative treatment of patients to prevent atrial fibrillation. However, studies involving temporary bi-atrial pacing can also be difficult to conduct with the pacing wires that are currently on the market: bi-atrial pacing requires the placement not just of the standard ventricular and right-atrial pacing wire, but also requires a left-atrial pacing wire or wires. These left atrial pacing wires are difficult to attach on the left atrium.
In our study we investigated the DefiPace
Since we have only a few patients in the database who have required atrial
conversion, we cannot give conclusive guidance on the optimal placement of the
electrodes. However, a study is currently underway to answer this and other
questions. The follow-up PMCF Registry Study (ClinicalTrials.gov Identifier:
NCT04804748) will be conducted as an international multicenter study with
10–12 participating centers within Europe. In brief, in the control arm
of the study standard of care will be documented in at least 150 consecutive
patients (based on statistical analysis) that are eligible for cardiac surgery,
of which at least 50 patients develop postoperative atrial fibrillation. Those
patients serving as a control group will be implanted with standard Osypka TME
pacing wires only, which are not intended for bi-atrial pacing or atrial
defibrillation. In the second arm (treatment group), about 300 patients will be
recruited. The TMA® electrodes will be implanted and connected to
the DefiPace
The DefiPace
HM, FV, PL—conceptualization, methodology, project administration, resources, writing – original draft; HM, FV, JG, PK, PL—data curation; HM, GS, DR—formal analysis; HM, FV, JG, EG, DR, PK, PL—investigation; HM—software; HM, PL—supervision; HM, FV, DR, PL—validation; HM, FV, EG—visualization; HM, FV, GS, DR—writing – review & editing. All authors have read and agreed to the published version of the manuscript.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Bayerische Landesärztekammer Munich Germany (approval number: 2021-1067). Patient consent was waived due to the retrospective character of the study and de-identified data set.
Not applicable.
Osypka AG received research funding for the project “Innovative heart stimulation for the treatment of atrial fibrillation” by the Federal Ministry of Education and Research (BMBF, Bundesministerium für Bildung und Forschung) of Germany, grant number 13GW0233.
The authors declare no conflict of interest. Peter Lamm and Helmut Mair are medical consultants for Osypka AG. This did not influence the representation or interpretation of reported research results. Guiseppe Santarpino is serving as one of the Guest editors of this journal. We declare that Guiseppe Santarpino had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Buddhadeb Dawn and Boyoung Joung.
