†These authors contributed equally.
Academic Editors: Brian Tomlinson and Takatoshi Kasai
Background: Retinol binding protein 4 (RBP4), a biomarker for insulin
resistance in type 2 diabetes (DM), is increased in heart failure. This
case-control study aims to determine the association between serum RBP4 levels
and diabetic cardiomyopathy (DCM). Methods: Demographic and clinical
data were obtained from 245 DM patients and 102 non-diabetic controls. RBP4
levels were measured using ELISA. The association between RBP4 and DCM was
evaluated using multivariate logistic regression and restricted cubic splines
(RCS) in DM patients. Results: We showed that serum RBP4 levels were
higher in DCM patients than in DM patients without DCM or the controls.
Multivariate analysis adjusted by age, gender, body mass index, diabetes
duration, left ventricular ejection fraction, insulin treatment, triglycerides,
low-density lipoprotein cholesterol, estimated glomerular filtration rate,
diabetic retinopathy, diabetic nephropathy, diabetic neuropathy and log
N-terminal proBNP showed a significant association between RBP4 and DCM (highest
vs. lowest tertile OR 16.87, 95% CI: 6.58, 43.23, p
Type 2 diabetes mellitus (DM) was associated with an increased risk of any left ventricular systolic and diastolic dysfunction [1]. Diabetic cardiomyopathy (DCM) was initially described as a pathophysiological condition in which heart failure occurred in diabetic patients without coronary artery disease, hypertension, and valvular heart disease [2]. Epidemiological studies in the U.S. showed a prevalence of DCM is 9.3% in the general population, and 19–26% of diabetic patients suffered from heart failure [3]. Meanwhile, 16.9% of the diabetic patients had diabetic cardiomyopathy and 54.4% had diastolic dysfunction [1]. Mortality from heart failure is among the leading causes of death in patients with DM, constituting a worldwide health and economic burden [4, 5]. However, most of the patients with DCM may not have any overt symptoms or signs of cardiac dysfunction before progressing to symptomatic heart failure. There is an urgent need for reliable and available biomarkers for DCM detection, identify a suitable biomarker will help in the recognition and management of DCM [6]. Therefore, screening of DCM patients may facilitate the early intervention and individualized management and improve the cardiovascular prognosis of diabetic patients [7].
Retinol binding protein 4 (RBP4) is a secreted protein of 21-kDa that transports retinol (vitamin A) in the circulation [8]. The majority of RBP4 is produced in the liver and adipocytes where dietary retinoids are stored and cleared. RBP4 is secreted into the plasma as an RBP4–retinol complex that delivers retinol to extrahepatic tissues [9]. Recent evidence suggests that it may function as an adipokine associated with metabolic homeostasis and elevated RBP4 levels are associated with insulin resistance [10]. Transgenic overexpression of RBP4 or chronic RBP4 administration induces whole-body insulin resistance and RBP4 deletion improves insulin action in mice [11, 12]. Serum RBP4 level is also correlated with visceral adiposity, body mass index (BMI), dyslipidemia, inflammation, and incipient nephropathy in patients with DM [10, 13, 14]. Interestingly, clinical observations showed that increased circulating RBP4 was associated with chronic heart failure (CHF), and elevated serum RBP4 was correlated with a worse outcome in elderly patients with CHF [15, 16]. RBP4 was also associated with the severity of insulin resistance in patients with obesity, impaired glucose tolerance, or DM [17]. These findings suggested that RBP4 plays a pernicious role in the cardiovascular complication of diabetes.
The association of RBP4 with cardiac dysfunction and metabolic disorders suggested its potential as a biomarker in the diabetic population. However, the relationship between RBP4 and DCM remains unclear. Therefore, we performed this case-control study to evaluate the association of serum RBP4 concentrations with the risk of DCM in patients with DM.
A total of 245 patients with DM admitted to the second affiliated hospital of
Soochow University (Suzhou, Jiangsu, China) for diagnostic coronary angiography
due to chest discomforts from January 2017 to December 2019 were consecutively
enrolled in this study. 102 controls without DM were selected from a healthy
population undergoing routine physical examination during the same period. DM was
defined according to the criteria of the American Diabetes Association
(hemoglobin A1c (HbA1c) level
Coronary heart disease was defined as stenosis of at least 50% of the luminal
diameter in at least one major coronary artery branch evaluated by coronary
angiography [19]. Patients with coronary heart disease, idiopathic dilated
cardiomyopathy, hypertension (SBP
 Fig. 1.
Fig. 1.Diagram showing patient flow throughout the trial. Flow chart of study enrollment to illustrate the inclusion and exclusion criteria.
Demographic and clinical data including age, gender, BMI, systolic and diastolic blood pressure, diabetes duration, smoking habits, alcohol consumption, complications related to diabetes, insulin therapy and hypoglycemic drug treatment were recorded. Neuropathy was diagnosed after checking pin prick, vibration sense, ankle reflex, and knee reflex. Retinopathy was detected after examining microdots, blot hemorrhage, hard exudates, soft exudates, and new vessel formation. Nephropathy was noted upon finding urinary albumin in detailed urine reports.
Echocardiography was performed by a certified cardiologist on all participants.
LV ejection fraction (LVEF) was obtained from 2D-images by manual tracing using
the biplane Simpson method in 4- and 2-chamber views. Left ventricular diastolic
dysfunction was determined by pulsed-wave Doppler examination of mitral inflow
(before and during Valsalva maneuver) and by Doppler tissue imaging of the mitral
annulus. We collected data on left atrial volume index, the early (E) and late
(A) trans mitral inflow velocities, early diastolic velocity of the medial
(septal) mitral annulus (e’), non-invasive assessment of left ventricular filling
pressures (E/e’). Normal diastolic function was defined as an E/A between 0.75
and 1.5, normal left atrial volume index (
DCM was diagnosed in patients according to the following criteria: (1) diabetes
mellitus (2) moderate to severe diastolic dysfunction or LVEF
A total of 5 mL venous blood samples were collected from the study participants
in the morning after a 12-h fasting period. After immediate centrifugation at 4
N-terminal pro-B-type natriuretic peptide (NT-proBNP) was measured by biotin
coupled anti-NT-proBNP antibody/streptavidin solid-phase chromatographic
immunoassay with the StatusFirst
Continuous variables with normal or skewed distributions are expressed as mean
The baseline characteristics of the controls, DM patients without cardiac dysfunction (NDCM), and DCM patients are shown in Table 1. Compared with the NDCM and control participants, the DCM patients were more likely to be older and had higher levels of TC, and LDL-C. The DCM group had lower eGFR and LVEF than the NDCM and control groups. Compared to the NDCM group, DCM patients showed longer diabetes duration, higher NT-proBNP levels, and a smaller proportion receiving insulin treatment. Serum RBP4 of DCM is higher in DCM than in NDCM (Fig. 2). The incidence rate of retinopathy and neuropathy are higher in DCM than NDCM (Table 1).
 Fig. 2.
Fig. 2.Violin plot of serum RBP4 levels measured with enzyme-linked immunosorbent assay. Control, participants without diabetes (n = 102). NDCM, diabetic patients without cardiac dysfunction (n = 159). DCM, patients with diabetic cardiomyopathy (n = 86).
| Control (n = 102) | DM | p | ||
| NDCM (n = 159) | DCM (n = 86) | |||
| Male, n (%) | 60 (60.0) | 88 (55.3) | 41 (47.7) | 0.311 |
| Age (IQR,years) | 65.00 [60.00, 72.25] | 66.00 [57.50, 74.00] | 69.00 [65.00, 74.00] | 0.027 |
| BMI (IQR, kg/m |
25.82 [23.41, 27.57] | 25.44 (22.17, 27.72) | 26.17(23.81, 28.00) | 0.365 |
| SBP (IQR, mmHg) | 133.00 [123.75, 137.25] | 132.00 [120.00, 138.00] | 133.50 [125.00, 137.00] | 0.949 |
| DBP (IQR, mmHg) | 76.00 [68.00, 84.00] | 75.00 [70.00, 85.00] | 79.50 [70.00, 83.50] | 0.785 |
| Smoke, n (%) | 43 (43.0) | 52 (32.7) | 29 (33.7) | 0.985 |
| ALT (IQR, u/mL) | 24.00 [20.00, 30.00] | 31.00 [23.00, 35.00] | 38.50 [32.00, 44.00] | |
| AST (IQR, u/mL) | 32.00 [27.75, 35.00] | 25.00 [20.50, 32.00] | 36.50 [29.25, 43.00] | |
| CRP (IQR, mg/dL) | 7.00 [5.00, 8.00] | 9.00 [6.00, 12.00] | 11.00 [8.00, 14.00] | 0.001 |
| TC (IQR, mmoL/L) | 4.39 [3.68, 5.15] | 4.40 [3.78, 5.15] | 5.56 [4.79, 6.43] | 0.06 |
| TG (IQR, mmoL/L) | 1.52 [1.1, 2.56] | 1.61 (1.23, 2.22) | 1.58 (1.13, 1.86) | 0.11 |
| LDL-c (IQR, mmoL/L) | 2.50 [1.94, 3.16] | 2.67 [1.98, 3.31] | 3.45 [2.48, 4.30] | |
| eGFR (IQR, mL/min/1.73 m |
93.00 [82.75, 103.00] | 92.00 [83.50, 100.00] | 82.00 [68.00, 93.75] | |
| RBP4 (IQR, |
45.00 [30.00, 56.00] | 45.50 [35.00, 56.83] | 65.00 [54.00, 71.00] | |
| NT-proBNP (IQR, pg/mL) | NA | 278.00 [110.00, 450.00] | 455.00 [130.00, 760.00] | |
| HbA1c (IQR, %) | NA | 7.60 [6.70, 8.50] | 7.70 [6.90, 8.70] | 0.053 |
| LVEF (IQR, %) | 65 [61, 67] | 55 [45, 66] | 48 [45, 56] | |
| peak E velocity (cm/s) | 80 [60, 95] | 80 [70, 100] | 70 [64, 90] | |
| peak A velocity (cm/s) | 70 [50, 80] | 65 [52, 79] | 45 [40, 87] | |
| E/A velocity ratio | 1.2 [0.7, 1.4] | 1.4 [0.9, 1.6] | 1.7 [1.55, 2.1] | |
| e’ (medial mitral annulus, cm/s) | 15 [12, 18] | 12 [7, 14] | 10 [7, 12] | |
| E/e’ | 7 [5, 10] | 10 [6, 11] | 13 [12, 18] | |
| Left atrial volume index (mL/m |
23 [20, 35] | 27 [22, 38] | 35 [28, 45] | |
| Diabetes duration (IQR, years) | NA | 7.00 [5.00, 11.50] | 12.00 [9.25, 15.00] | |
| Diabetic retinopathy n (%) | NA | 36 (22.6) | 31 (36) | 0.03 |
| Diabetic nephropathy n (%) | NA | 27 (17) | 18 (20.9) | 0.49 |
| Diabetic neuropathy n (%) | NA | 35 (22) | 32(37.2) | 0.02 |
| Oral medication, n (%) | NA | 145 (91.2) | 81 (94.2) | 0.558 |
| Insulin therapy, n (%) | NA | 88 (55.3) | 24 (27.9) | |
| Data were presented as median (interquartile range) or n (%). BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; ALT, alanine aminotransaminase; AST, aspartate aminotransaminase; CRP, C-reactive protein; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; RBP4, retinol binding protein 4; NT-proBNP, N terminal-pro hormone BNP. | ||||
The prevalence of DCM among the tertile of RBP4 were 9.8%, 32.0%, and 61.4%,
respectively. The OR of DCM were increased in patients with ascending tertile of
RBP4 (P
| RBP4 (ug/mL) | NT-proBNP (pg/mL) | P | |||||||
| 45–59.8 | P |
170–440 | P |
||||||
| No. of DCM cases (%) | 82 (33.47) | 75 (30.61) | 88 (35.92) | 81 (33.06) | 74 (30.2) | 90 (36.73) | |||
| Unadjusted OR | 1.00 | 4.97 (1.99, 12.41) | 16.7 (6.92, 40.72) | 1.00 | 0.80 (0.39, 1.63) | 2.37 (1.25, 4.48) | 0.005 | ||
| Adjusted OR | |||||||||
| Model 1 |
1.00 | 4.49 (1.76, 11.41) | 15.78 (6.38, 39.01) | 1.00 | 0.78 (0.38, 1.62) | 2.26 (1.18, 4.32) | 0.009 | ||
| Model 2 |
1.00 | 4.27 (1.64, 11.08) | 16.87 (6.58, 43.23) | 1.00 | 0.64 (0.30, 1.37) | 2.13 (1.09, 4.16) | 0.018 | ||
| AUROC | 0.63 (0.57, 0.69) | 0.80 (0.75, 0.85) | |||||||
AUROC, area under the receiver operator characteristic curve; PROC, p value for the comparison of area under; ROC, curves for RBP4 and NT-proBNP to predict DCM. | |||||||||
There is significant difference of AUROC between RBP4 and NT-proBNP for diagnose
DCM of diabetes (p
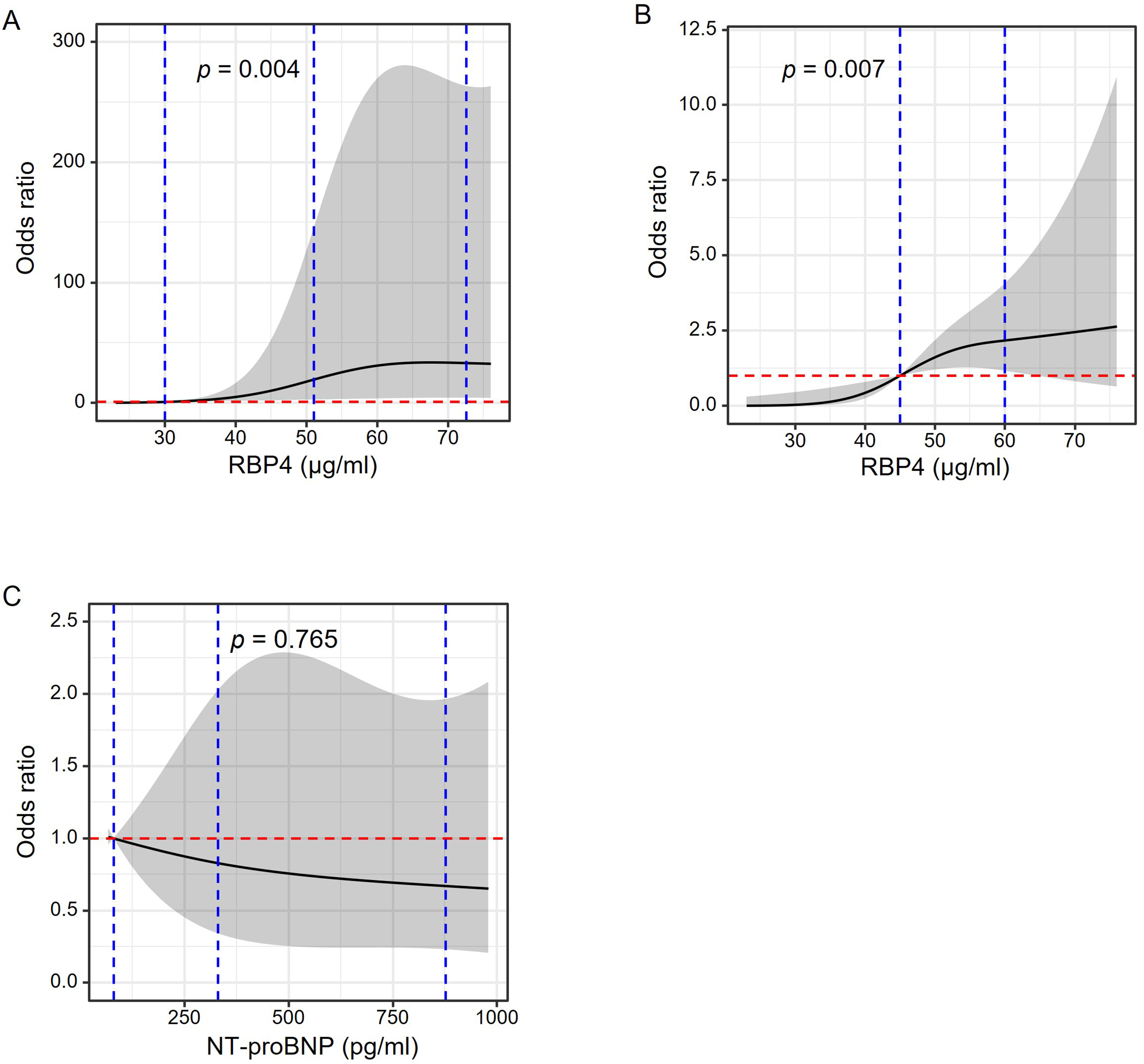 Fig. 3.
Fig. 3.RCS to evaluate RBP4 and NT-proBNP levels with the risk of DCM
in diabetes. Odds ratio (OR) and 95% confidence interval (CI) were derived from
restricted cubic spline regression adjusted for age, gender, diabetes duration,
body mass index, insulin treatment, left ventricular ejection fraction,
triglyceride, low-density lipoprotein cholesterol (LDL-C), estimated glomerular
filtration rate (eGFR), diabetic retinopathy, diabetic nephropathy, diabetic
neuropathy and Log NT-proBNP (for RBP4 only), with knots placed at the 10th,
50th, and 90th percentiles (A) or tertiles (B) of RBP4 and tertiles of NT-proBNP
(C). Blue vertical dashed lines in panel A indicate RBP4 knot cut-offs placed at
10th (30
We evaluated whether RBP4 improved the discriminative ability for DCM beyond
other risk factors, including clinically relevant factors and significant
covariates based on the univariate analyses. Adding log RBP4 to a basic risk
model including age, BMI, diabetes duration, LVEF, insulin treatment, TG, LDL-C,
eGFR, CRP, log NT-proBNP, retinopathy, nephropathy and neuropathy improved the
c-index from 0.91 to 0.94 (p = 0.024) (Table 3). Improvement in
reclassification by adding log RBP4 to the basic model was evaluated by NRI and
IDI. With risk thresholds at 0.3 and 0.7, low, medium, and high-risk categories
were defined as having
| Model | C-index | Continuous NRI |
Categorical NRI |
IDI | ||||
| Estimate (95% CI) | p value | Estimate (95% CI) | p value | Estimate (95% CI), % | p value | Estimate (95% CI), % | p value | |
| Basic |
0.91 (0.88–0.95) | Ref | Ref | Ref | ||||
| Basic |
0.94 (0.91–0.97) | 0.024 | 87.86 (64.4–111.32) | 15.07 (4.48–25.66) | 0.005 | 7 (3–10) | ||
IDI stands for the difference between mean value of the predicted probability of
DCM for each individual in the new model and the old model. An IDI (95% CI) of
7% (3%–10%) showed that the new model (basic + RBP4) improves predictive
power by 7% over old model (basic model) (p
In this study, we found that levels of RBP4 were elevated in patients with DCM. Higher serum RBP4 was independently associated with the risk of DCM. The addition of RBP4 improved the reclassification and discrimination of a DCM risk model.
DCM often accompanies other comorbidities such as obesity, dyslipidemia, and
vascular disease. In the early stages, only sub-structural changes in
cardiomyocytes are present. Furthermore, identifying DCM before cardiac
dysfunction exacerbates may provide a critical window of time for early
intervention. Computed tomography (CT), magnetic resonance imaging (MRI), and
echocardiography are commonly used to detect DCM. CT is helpful because it
collects end-systolic and end-diastolic volumetric data that can be reconstructed
by automated software, which collects small segments of data along several
cardiac cycles to produce the final image of the computed tomography.
Consequently, this approach yields parameters of the ventricular function that
are instrumental in the diagnosis of DCM. However, radiation exposure and the
side effects associated with the use of contrast media may limit this
methodology. MRI operates with a greater spatial and temporal resolution to
evaluate chamber size, left ventricular EF, and myocardial mass distribution. MRI
also provides extra information about information like myocardial fibrosis and
subclinical ischemia [7]. However, MRI also has some limitations. MRI
may underestimate diastolic dysfunction, is not compatible with some pacemakers
or implantable defibrillators, and may produce claustrophobia in some patients.
Thus, compared to the two methods noted above, ultrasound has obvious advantages.
It uses no radiation, no contrast medium, and is widely used in clinical
evaluations of cardiac function. However, but the early period of cardiac
dysfunction is very difficult to detect without the TDI model at exercise stress
[23]. Therefore, in our study, we chose patients with LVEF
Pathological diagnosis of the myocardium is a reliable assessment of DCM. Pathophysiological features of DCM include accumulation of advanced glycation end products (AGE), cardiomyocyte apoptosis, autophagy, myocardial fibrosis, endothelial dysfunction, left ventricular hypertrophy, and endoplasmic reticulum stress [24, 25, 26]. However, the clinical practice requires sensitive but reliable markers that can be obtained non-invasively and that accurately predict underlying disease and its severity. Several efforts have been made to improve DCM detection by quantification of biomarkers [27, 28, 29]. Our findings show that RBP4, a new adipocytokine, is a useful diagnostic marker of DCM, and circulating RBP4 was valuable in predicting the presence of DCM in diabetics. These findings provide indirect evidence of RBP4 involvement in cardiac remodeling and bring new insights into the pathophysiological role of RBP4 which might be a promising therapeutic target for DCM.
Several possible explanations could explain the association between RBP4 and
DCM. Firstly, RBP4 is a novel polypeptide ligand that has been shown to play a
pivotal role in the regulation of glucose homeostasis and lipid metabolism [30].
A Clinical study showed that serum RBP4 levels
One important finding of our study is that the duration of diabetes is an independent factor for the risk of DCM. Diabetes duration was a recognized risk factor for diabetic complications in diabetic subjects [38] and the presence of AGE deposition in the hearts of patients was related to the duration of diabetes [39]. We did not find a significant correlation between RBP4 and FBG in diabetes patients and Fedders R et al. [40] reported that increasing circulating RBP4 did not affect glucose homeostasis in mice with liver-specific overexpression of RBP4. This result suggests that RBP4 is not always associated with glucose levels. In our study, insulin therapy may be the reason for the result between glucose and RBP4. Also, we find that insulin use in the DCM group is lower than in the NDCM group, which indicate that partially reason of DCM in diabetes was related with insulin use. Animal studies in the low-dose streptozotocin-induced diabetic rat show that markers of diabetic cardiomyopathy were markedly ameliorated following insulin replacement indicating that insulin replacement can reduce complications of diabetes including cardiomyopathy [41]. Therefore, our research provides a little support for clinical prevention of diabetic cardiomyopathy. It is necessary to investigate effect of the insulin use on DCM patients in the future.
According several other studies [42, 43, 44], they show a positive correlation between RBP4 and LDL-C. Our result shows that BMI was not significantly different among the groups, because correlation analysis showed that RBP4 was positively correlated with BMI [42]. To minimize the effect of BMI on RBP4 concentrations, we calculated the reclassification and discrimination for DCM by serum of RBP4 in patients with DM, which show RBP4 is a valuable marker for the risk of DCM. RBP4 is cleared from the circulation by the kidneys [45]. Decreasing eGFR was associated with higher levels of RBP4 in hypertension [42]. RBP4 increased in DCM was associated with reducing renal clearance, rather than increasing secretion of adipocytes, which might also account for our finding. When controlled with eGFR, diabetic nephropathy and other parameters, we still found RBP4 is the risk factor for DCM in patients with diabetes. Thus, renal dysfunction is not enough to explain the higher RBP4 concentrations in DCM. Although age and gender were shown in other studies to influence the levels of RBP4 [46, 47, 48], in our study the age of DCM group has a higher level than NDCM group, in order to eliminate this interference factor, we adjusted HR by multivariate logistic regression analyses, the RBP4 level was independently predictive of DCM in diabetes.
Our study has several limitations. First, this is a case-control study that could not establish the causative role of RBP4 in DCM prediction. Secondly, the sample size was relatively small. Our study enrolled diabetic patients with moderate and severe diastolic dysfunction, the mild diastolic dysfunction of diabetes which accounts for more diabetic samples did not include. This design can better ensure more reliable conclusions. Thirdly, we could not follow up with incident DCM with only a single echocardiographic evaluation. We will address these points with a larger prospective cohort in our future studies.
This study investigated the RBP4 levels in DM patients. We found that serum RBP4 levels were higher in DCM patients than in DM patients without DCM. Moreover, the elevated serum levels of RBP4 are associated with the risk of DCM in patients with DM. The results suggested the role of RBP4 was a potential biomarker for the diagnosis of DCM in DM.
XG and HS conceived the study and designed the study protocol. TY, YJ, and YF collected the study sample. JZ performed the echocardiographic analyses. TY and HP conducted the literature review and statistical analysis. TY and YJ drafted the manuscript. TY, YF, HG, HL reviewed the manuscript for intellectual content, made revisions as needed. All authors contributed to editorial changes in the manuscript and read and approved the final manuscript.
The protocol of the present study was performed according to the principles of the Declaration of Helsinki and approved by the local research and ethics committee of the second affiliated hospital of Soochow University (IRB No. JD-LK-2020-031-01). Written informed consent was obtained from all subjects before participation.
We would like to thank Pamela Harding from Henry Ford hospital (Detroit, MI, USA) for her assistance in the preparation of this manuscript.
This work was supported by the National Natural Science Foundation of China (Grant numbers 81900140, 82170831, 82070838), Science and Technology Development Plan of Suzhou City, China (Grant numbers SYS2019061, SYSD2018097), MSD cholesterol Fund of China Heart House - China Cardiovascular Association (Grant number 2017-CCA-Msdlipid-014), and Young Investigator Pre-Research Foundation of the Second Affiliated Hospital of Soochow University (Grant number SDFEYQN1717).
The authors declare no conflict of interest.
