Academic Editors: Yoshiaki Kaneko and Peter A. McCullough
Background: Catheter ablation is an effective treatment for
atrial fibrillation (AF), primarily performed in patients who fail antiarrhythmic
drugs. Whether early catheter ablation, as first-line therapy, is associated with
improved clinical outcomes remains unclear. Methods: Electronic
databases (PubMed, Scopus, Embase) were searched until March 28th, 2021.
Randomized controlled trials (RCTs) compared catheter ablation vs antiarrhythmic
drug therapy as first-line therapy were included. The primary outcome of interest
was the first documented recurrence of any atrial tachyarrhythmia (symptomatic or
asymptomatic; AF, atrial flutter, and atrial tachycardia). Secondary outcomes
included symptomatic atrial tachyarrhythmia (AF, atrial flutter, and atrial
tachycardia) and serious adverse events. Unadjusted risk ratios (RR) were
calculated from dichotomous data using Mantel Haenszel (M-H) random-effects with
statistical significance considered if the confidence interval (CI) excludes one
and p
• Catheter ablation of paroxysmal AF aiming at electrical pulmonary vein isolation (PVI) as first line therapy resulted in maintenance of sinus rhythm in drug naïve patients.
• Cryoballoon Catheter Ablation was superior to antiarrhythmic drug (AAD) therapy, significantly reducing Atrial tachycardia recurrence in treatment-naive patients with symptomatic paroxysmal atrial fibrillation.
• Catheter ablation was associated with a similar adverse risk profile as compared to antiarrhythmic drug therapy.
Atrial Fibrillation is the most common cardiac arrhythmia encountered in clinical practice. It is associated with increased morbidity, mortality, and impaired quality of life [1]. It is frequently recurrent and follows a progressive course over time [2]. Current guidelines recommend the use of antiarrhythmic drugs (AAD) as initial therapy for AF and before catheter ablation (CA) is considered [3]. However, antiarrhythmic drugs have limited efficacy and are associated with adverse effects [4].
Multiple randomized controlled trials (RCTs) have shown that CA is superior and safe than AAD in maintaining sinus rhythm and preventing recurrent arrhythmias [5, 6, 7, 8, 9, 10, 11, 12]. CA has also demonstrated improvement in left ventricular ejection fraction (LVEF) and quality of life compared to AAD pharmacotherapy in patients with heart failure [13, 14, 15, 16].
In clinical practice, patients with symptomatic refractory AF or those intolerant to AADs undergo CA. This is mainly preceded by the failure of AADs [17]. However, accumulating evidence has suggested that early ablation in AF with shorter diagnosis-to-ablation times allows better rhythm control [18, 19, 20]. Similarly, the recent Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET 4) showed that early rhythm control results in better clinical outcomes than standard care management [21]. To date, only a few studies have investigated the role of CA as a first-line therapeutic modality for AF patients [22, 23, 24, 25, 26, 27]. Whether early CA halts AF progression or is associated with improved clinical outcomes remains unclear. The best appropriate timing for ablation in patients with symptomatic AF, regardless of AF type, has not been determined.
Considering recent evidence regarding this matter [25, 26, 27], a systematic review and meta-analysis was performed to examine the safety and efficacy of CA vs AAD as first-line treatment for the maintenance of sinus rhythm in symptomatic AF patients.
This meta-analysis was carried out adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) was performed (Fig. 1) [28]. A systematic search was performed on online bibliographic databases including PubMed, Embase, and Scopus. ClinicalTrials.gov and Google Scholar were searched to identify grey literature. No restrictions on date, language and publication type existed wherein the search was conducted from inception until the 28th of March 2021. Using Boolean logic, the following combination of MeSH terms and keywords were used on online databases: “atrial fibrillation”, “radiofrequency ablation”, “cryoablation”, “anti-arrhythmic drug”, “isolation”, and “first-line catheter ablation” “pulmonary vein isolation”. A cross-reference check of previously published meta-analysis on this topic was also performed.
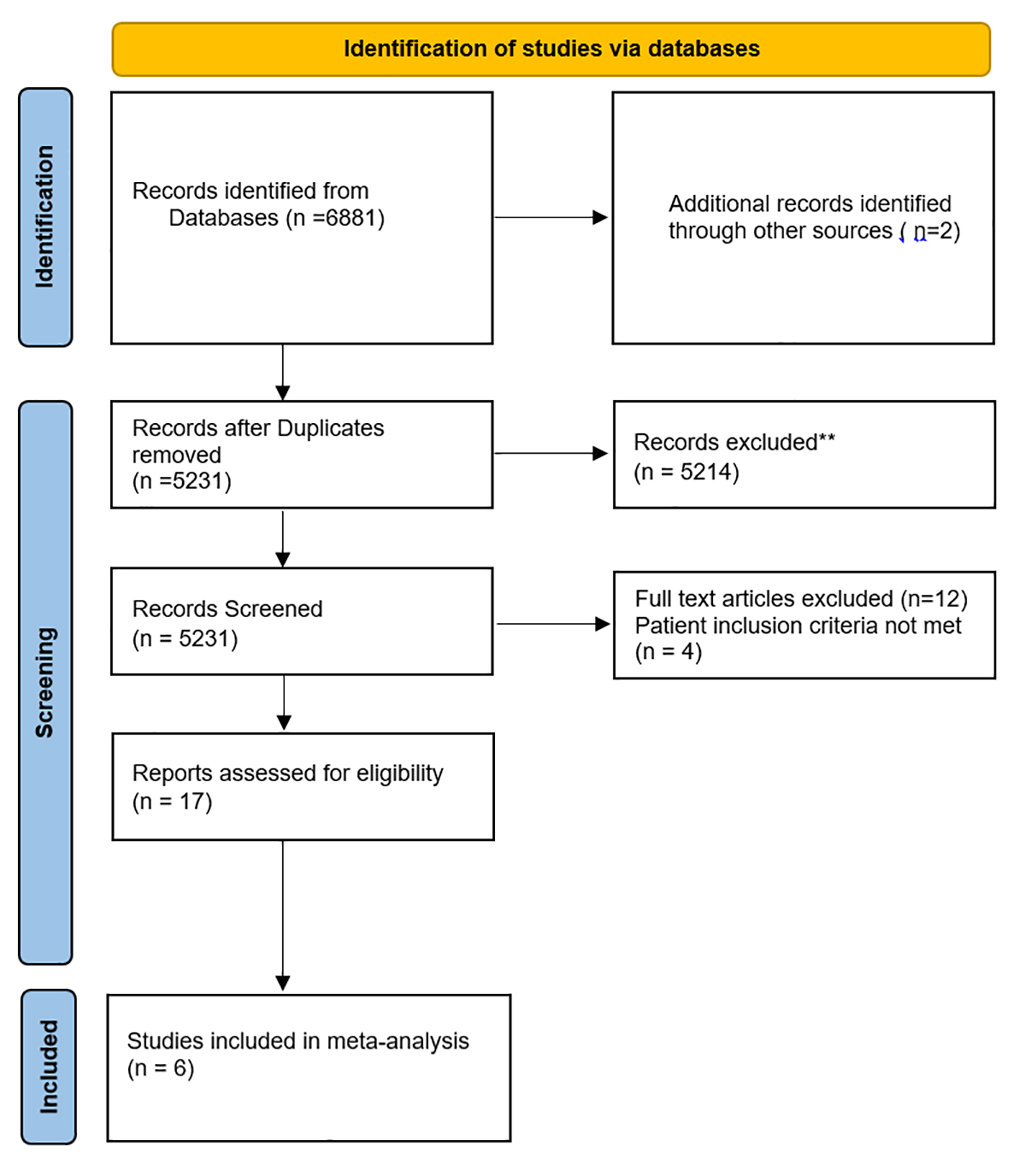 Fig. 1.
Fig. 1.PRISMA Flow of the search strategy for systematic review and meta-analysis on ablation versus antiarrhythmics as first line therapy for atrial fibrillation.
Eligibility criteria for the analysis included studies with an adult patient
population (age
Patients younger than 18 with a history of scheduled use of class IC or class
III antiarrhythmic drugs at therapeutic doses were excluded. Patients were also
excluded if they had a previous ablation for AF, a left atrial diameter
Two independent reviewers (A.A.R. and S.P) screened titles, abstracts and searching reference lists of included studies (backward snowballing). Extracted data was verified by the reviewers and duplicates were removed using Endnote X9 (Clavariaye Analytics, Chandler, AZ, US). The senior author arbitrated any discrepancies concerning the evaluation of the studies. The study design, demographic characteristics, and various outcomes were extracted. No language restrictions were made. For the quality assessment of included RCTs in the systematic review and meta-analysis, evaluation with the Revised Cochrane Risk of Bias tool (ROB 2) was employed to ascertain the quality of studies by two independent reviewers (A.A.R and H.N) [29].
Pulmonary-vein isolation (PVI) was the primary intention of the catheter ablation group. During the cryoballoon procedure, operators performed pulmonary-vein isolation by the fluoroscopic guided device placement at each pulmonary-vein antrum and advanced towards the pulmonary vein to obtain occlusion. The balloon was filled with liquid refrigerant for cooling the tissue.
In the radiofrequency group, operators performed pulmonary-vein isolation using electro anatomical navigation and created a contiguous circular lesion around each pulmonary-vein antrum with point-by-point applications of radiofrequency energy.
The primary outcome of interest was the first documented recurrence of any type of atrial tachyarrhythmia. The follow-up period ranged from 1–2 years. Arrhythmia recurrence was defined as any symptomatic or asymptomatic; AF, atrial flutter, and atrial tachycardia that occurred outside the blanking period of 90 days. The blanking period was defined as the first 90 days after the index ablation procedure or drug initiation. The current Heart Rhythm Society consensus statement on catheter ablation for atrial fibrillation recommends the use of a 3-month blanking period immediately post-ablation to accurately characterize the long-term clinical outcomes of catheter ablation procedures [3].
A separate analysis was performed for studies that have specifically evaluated cryoballoon CA vs AAD therapy as a first-line treatment strategy in patients with symptomatic AF.
Secondary outcomes included symptomatic atrial tachyarrhythmia (AF, atrial flutter, and atrial tachycardia) and serious adverse events. A separate analysis was performed for cardiovascular adverse events, defined as ischemic and embolic events (myocardial infarction, stroke or transient ischemic attack), hospitalizations for heart failure, major bleeding, pulmonary vein stenosis, atrio-esophageal fistula, pericardial complications (effusion, hemorrhage, tamponade, and perforation), syncope, and life-threatening arrhythmias. Quality of life (QoL) was also examined when reported by included studies.
Statistical analyses were performed using Review Manager (RevMan) [Computer
program] Version 5.4 Cochrane Collaboration (The Cochrane Community, London, UK).
The Cochran-Mantel Haenszel method was used with the random-effects model to
calculate unadjusted risk ratios (RR) for the primary and secondary endpoints.
The estimated effect size was reported as a point estimate and 95% confidence
interval (CI). An alpha criterion of p-value
The initial search yielded 6881 results, of which 5231 articles were screened for title and abstract. Consequently, records were removed due to ineligibility (reviews, editorials, non RCTs, ongoing trials, and abstracts). The search strategy is shown in Fig. 1. A full-text screening of 17 articles led to the inclusion of 6 studies, with 1212 participants (609 patients in the CA group and 603 patients in the AAD group) [22, 23, 24, 25, 26, 27]. Quality assessment findings of the included studies are summarized in Supplementary Figs. 1,2.
Baseline demographics, comorbidities and characteristics of studies included in
the meta-analysis are summarized in Table 1 (Ref. [22, 23, 24, 25, 26, 27]). The
follow-up period ranged from 1–2 years. The average age was 56.6 years in both
groups. Approximately 70% of patients were men in both groups. The type of CA
was radiofrequency in three studies [22, 23, 24] and cryoablation in another three
studies [25, 26, 27]. Five studies [23, 24, 25, 26, 27] included patients with paroxysmal AF and
one study involved 35 patients with paroxysmal AF and two patients with
persistent AF [22]. Holter monitor and 12-lead electrocardiography was the most
frequently used method for monitoring [22, 23, 24, 26, 27], while one study stated the
use of an implantable cardiac device [25]. The CA had a 37.2% and a 6.9%
prevalence of hypertension and diabetes, while in the AAD cohort, 39.8% patient
population had hypertension and 10% had diabetes, respectively. There was a
prior history of stroke or transient ischemic attack in 2.4% and 2.9% of
patients in the ablation and AAD groups. Out of 609 patients in the Ablation
group, 248 (42.9%), 204 (44.6%), 44 (10.2%) and 64 (23.6%) were on oral
anticoagulation,
| Variable | RAAFT-1 | MANTRA-PAF | RAAFT-2 | EARLY-AF | STOP-AF First | Cryo-FIRST | |
| 2005 [22] | 2012 [23] | 2014 [24] | 2020 [25] | 2020 [26] | 2021 [27] | ||
| Sample (n) | 32/35 | 146/148 | 66/61 | 154/149 | 104/99 | 107/111 | |
| Ablation/AAD | |||||||
| Age | 53/54 | 56/54 | 56.3/54.3 | 57.7/59.5 | 60.4/61.6 | 50.5/54.1 | |
| Male (n) | NA | 100/106 | 51/45 | 112/102 | 63/57 | 76/72 | |
| CAD risk factors | |||||||
| Hypertension | 8/10 | 43/53 | 28/25 | 57/55 | 58/57 | 33/40 | |
| Diabetes Mellitus | NA | 6/10 | 1/4 | NA | 15/17 | 1/4 | |
| Stroke/TIA | NA | 6/5 | 3/4 | 4/5 | 2/3 | 0/1 | |
| Left atrial size mean (SD), (mm) | 41 |
40 |
40 |
39.5 |
38.7 |
37.0 | |
| LV ejection fraction mean (SD), % | 53 |
NA | 61.4 |
59.6 |
60.9 |
62.8 | |
| CHA2DS2-VASc score | N/A | N/A | |||||
| 0 | 92/80 | 49/38 | |||||
| 1 | 37/49 | 33/40 | |||||
| 2 | 13/14 | 13/15 | |||||
| 3 | 3/4 | 4/10 | |||||
| 4 | 1/1 | 3/2 | |||||
| Type of AF | 35 patients had paroxysmal AF and 2 had persistent AF | Paroxysmal | Paroxysmal | Paroxysmal | Paroxysmal | Paroxysmal | |
| Type of CA | Radiofrequency–Pulmonary Vein Isolation | Radiofrequency–Pulmonary Vein Isolation | Radiofrequency–Pulmonary Vein Isolation | Cryoballoon–Pulmonary Vein Isolation | Cryoballoon–Pulmonary Vein Isolation | Cryoballoon–Pulmonary Vein Isolation | |
| Monitoring | 24-hour Holter monitoring | 7-day Holter-monitor recording | Holter, transtelephonic monitor, or rhythm strip | Implantable cardiac monitoring device | 12-lead ECG | 12-lead ECG and 7-day Holter. | |
| Medications | |||||||
| Oral anticoagulation | NA | NA | 35/19 | 103/96 | 72/68 | 38/49 | |
| 19/23 | NA | 40/36 | 85/92 | 6/9 | 54/56 | ||
| Calcium channel blockers | NA | NA | 14/13 | 11/10 | 10/4 | 9/15 | |
| Aspirin | NA | NA | 38/29 | NA | 21/13 | 5/7 | |
| AAD tolerable dose | oral flecainide 100–150 mg twice daily, propafenone 225–300 mg 3 times daily, sotalol 120–160 mg twice daily | Flecainide (200 mg/day) or propafenone (600 mg/day) | Flecainide 176 mg; Propafenone 487 mg; Dronedarone 60 mg | NA | NA | NA | |
| Study design | |||||||
| Study | Prospective RCT | Prospective RCT | Prospective RCT | Prospective RCT | Prospective RCT | Prospective RCT | |
| Year | 2005 | 2012 | 2014 | 2020 | 2020 | 2021 | |
| Center | Multi-center | Multi-center | Multi-center | Multi-center | Multi-center | Multi-center | |
| Study Period | 2001–2002 | 2005–2009 | 2006–2010 | 2017–2018 | 2017–2019 | ||
| Sample size | 67 | 294 | 127 | 303 | 203 | 218 | |
| Follow-up Duration | 12 months | 24 months | 24 months | 12 months | 12 months | 12 months | |
| Main outcomes | Recurrence of AF, Hospitalization and QoL assessment. | Burden of AF, freedom from any AF, freedom from symptomatic AF and QoL assessment. | First documented atrial tachyarrhythmia (symptomatic or asymptomatic AF, atrial flutter, or atrial tachycardia), symptomatic recurrences of atrial tachyarrhythmia and QoL assessment. | First documented recurrence of any atrial tachyarrhythmia (AF, atrial flutter, or atrial tachycardia), freedom from symptomatic arrhythmia, the AF burden, and QoL assessment. | Freedom from initial failure of the procedure for atrial arrhythmia recurrence post 90-day blanking period, serious adverse events. | Freedom from any AA recurrence (at least one episode of AF, atrial flutter, or atrial tachycardia) lasting | |
| Results | Ablation was superior to AAD. | No significant difference between the treatment groups. | Ablation compared with AAD resulted in a lower rate of recurrent atrial tachyarrhythmia. | Ablation compared with AAD resulted in a significantly lower rate of recurrent atrial tachyarrhythmia. | Ablation as initial therapy was superior to AAD for the prevention of atrial arrhythmia recurrence. | Ablation as initial therapy was superior to AAD therapy significantly reducing AA recurrence in treatment naive patients with paroxysmal AF. | |
Five out of six studies reported the recurrence of any atrial tachyarrhythmia
(Symptomatic/Asymptomatic; AF, Atrial flutter, atrial tachycardia) [22, 23, 24, 25, 26].
Recurrence occurred in 160 of 577 (27.7%) patients who underwent CA and 223 of
457 (45%) patients on AAD. Our meta-analysis revealed a 37% reduction in the
risk of recurrence of any type of atrial tachyarrhythmia (RR 0.63; 95% CI
0.55–0.73; p
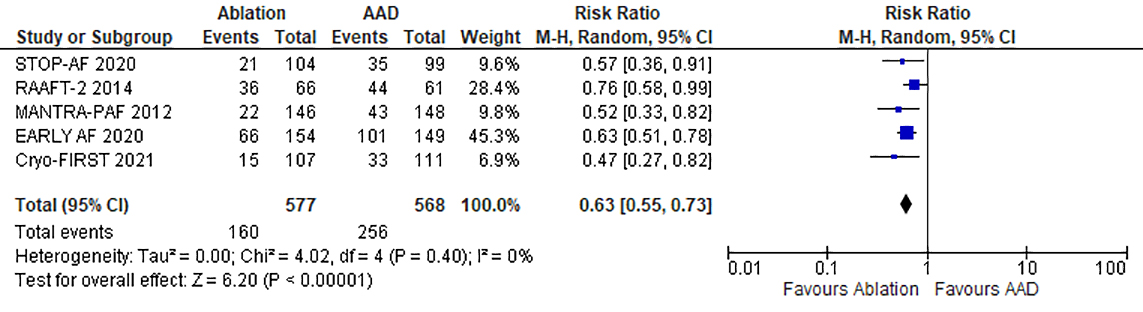 Fig. 2.
Fig. 2.Forrest plot comparing the primary efficacy endpoint of catheter ablation to antiarrhythmic drug therapy as first line treatment for atrial fibrillation.
Cryoablation as an ablation procedure was evaluated in three recent studies.
There was a 40% reduction in the risk of recurrence of any types of atrial
tachyarrhythmia (RR 0.60 95% CI 0.50–0.72; p
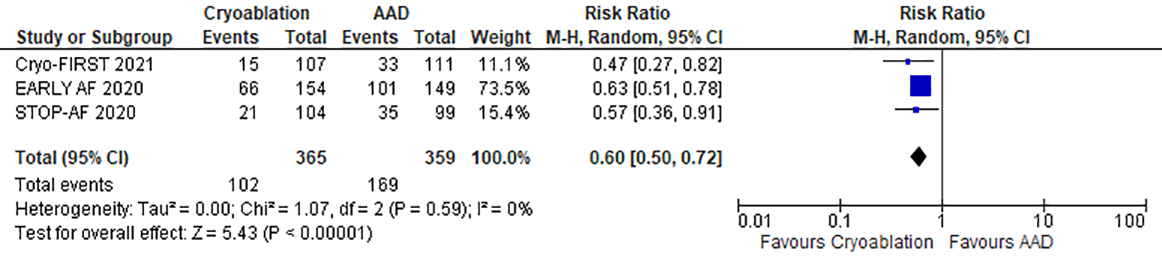 Fig. 3.
Fig. 3.Forrest plot comparing the primary efficacy endpoint of cryoablation to antiarrhythmic drug therapy as first line treatment for atrial fibrillation.
Four out of five studies reported recurrence of symptomatic Atrial
Tachyarrhythmia [22, 23, 24, 25]. A secondary outcome of symptomatic recurrence of AF,
atrial flutter or atrial tachyarrhythmia was reported in 83 patients of 398
patients (20.8%) in the CA group compared with 141 (35.8%) in the ablation
group. A better response was observed in the CA group with a 47% reduction in
the risk of recurrence of symptomatic atrial tachyarrhythmia (RR 0.53; 95% CI
0.32–0.87; p = 0.01). There was moderately high heterogeneity among the
studies included for the analysis (I
 Fig. 4.
Fig. 4.Forrest plot comparing catheter ablation to antiarrhythmic drug therapy (as first line treatment for atrial fibrillation) for the recurrence of symptomatic tachyarrhythmia.
QoL was reported only in three of the six included studies. It was measured by
using the 36-item Short Form General Health Survey (SF-36) in 1 study [22] and
European Quality of Life 5 Dimension (EQ-5D) in 2 studies [24, 25]. The study
that used SF-36 reported an improvement in QoL both in the physical and mental
domains in patients who underwent CA. A significant improvement (WMD 11 95% CI
8–12; p
All six studies reported the overall adverse effects after treatment and were
included in the meta-analyses [22, 23, 24, 25, 26, 27]. The details of adverse events are
described in Supplementary Table 1. The number of adverse events were
111 of 609 (18.2%) patients who underwent CA and 124 of 603 (20.5%) patients
who were on AAD. However, there was no significant difference in the incidence of
major adverse events between ablation and AAD (RR 0.93; 95% CI 0.68–1.27;
p = 0.64) with homogenous findings (I
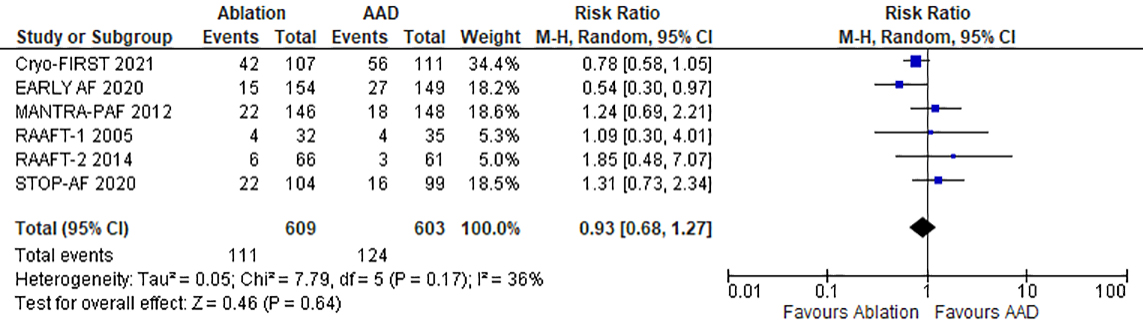 Fig. 5.
Fig. 5.Forrest plot comparing catheter ablation to antiarrhythmic drug therapy (as first line treatment for atrial fibrillation) for the incidence of adverse events.
The cardiovascular events have been described in Supplementary Table 2.
Out of 609 patients in the ablation arm and 603 patients in the AAD arm, 61
(10%) and 75 (7.9%) experienced cardiovascular events, respectively. Using a
random-effects model, we determined that the catheter ablation group had similar
odds as compared to the AAD group in experience cardiovascular adverse events (RR
0.90; 95% CI 0.56–1.44; p = 0.65), with no heterogeneity among studies
(I
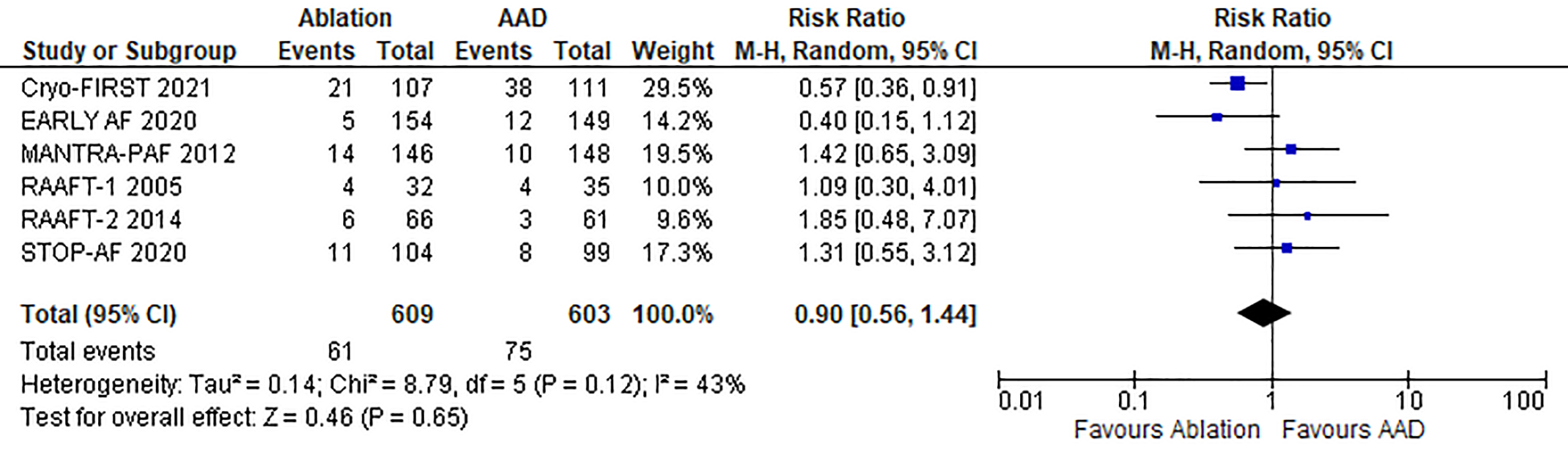 Fig. 6.
Fig. 6.Forrest plot comparing catheter ablation to antiarrhythmic drug therapy (as first line treatment for atrial fibrillation) for the incidence of cardiovascular adverse events.
On visual assessment, the funnel plot was symmetrical with an equal number of
studies on each side of the vertical axis. There was no publication bias
demonstrated. Egger’s test for the assessment of publication bias was
non-significant (2-tailed p
This meta-analysis of six RCTs, including 1212 patients, compares the CA’s efficacy and safety outcomes as first-line therapy versus AAD in symptomatic AF patients. The main findings of this meta-analysis are as follows: (i) CA (Cryoballoon or Radiofrequency- Pulmonary vein Isolation) resulted in a significantly lower rate of any recurrent atrial tachyarrhythmias when used in antiarrhythmic naïve patients. (ii) This effect was observed irrespective of follow-up duration (short versus long term), and type of catheter ablation (radiofrequency versus cryoablation). (iii) CA was not associated with an increase in the rates of overall severe adverse events and cardiovascular adverse events; while procedural adverse events included tamponade, pulmonary stenosis and pericardial effusion.
To our knowledge, this is the first comprehensive meta-analysis to compare clinical outcomes of CA versus AAD as first-line therapy for symptomatic AF and included the results of the recent EARLY-AF, STOP-AF First and Cryo-FIRST trials [25, 26, 27]. A prior meta-analysis from 2015 explored the impact of CA and AAD as initial therapy but was underpowered due to paucity of data and a limited number of included studies [32]. In addition to exploring more endpoints, the current study is the first to include both radiofrequency and cryoballoon studies to compare CA and AAD as first-line treatments for AF.
Radiofrequency ablation (RFA) and cryoballoon ablation (CBA) are both safe and effective options for treating atrial fibrillation. In a recent meta-analysis comparing RFA and CBA, there were no statistically significant differences between the two energy sources regarding AF/atrial tachycardia-free survival and overall adverse events [33]. CBA has emerged as a promising alternative with comparable efficacy to RF ablation for the maintenance of sinus rhythm, primarily in patients with paroxysmal AF. Triggers originating from the pulmonary and thoracic veins appear to be the primary mechanism of subjects with paroxysmal AF [34]. Extending the role of CBA aiming at the electrical pulmonary vein as a novel catheter ablation strategy, the current study supports the use of CBA as initial first-line therapy, like that observed with radiofrequency ablation. These findings from the current meta-analysis are in concordance with the recent EARLY-AF, STOP-AF First and Cryo-FIRST trials [25, 26, 27].
Although more successful than AAD, most ablations in clinical practice are performed in patients who fail AAD due to either inefficacy or side effects [17]. However, this conventional “standard of care” consisting of the first attempt of rhythm control with AAD may represent a delay in an optimal treatment. Drug therapy often fails due to adverse events and arrhythmia breakthroughs, and the time that passes between AAD introduction and failure can cause a delay in time-to-ablation, during which progressive electro-anatomical remodeling may render AF more refractory. A shorter diagnosis-to-ablation time has been associated with better ablation outcomes [18, 19, 20]. Similarly, a prior study from our center showed that markers of atrial remodeling such as hemodynamic strain and inflammation progress with longer diagnosis-to-ablation times in persistent AF.
Furthermore, the longer diagnosis-to-ablation time has been associated with worse AF-related outcomes such as heart failure and death [35]. In the progressive course of AF, atrial scarring and fibrosis can occur, reducing the ability to maintain sinus rhythm as observed in the DECAAF trial [36]. Finally, the ARISTOTLE trial showed a higher risk of stroke at advanced stages in AF progression [37], which further supports earlier ablative intervention; but this requires further investigation. This data and the current findings suggest implications for the timing of early aggressive management of AF and its favorable impact on disease progression. Moreover, progression of AF is less common with CA intervention than AAD therapy [35, 36, 37]. Therefore, CA provides the most significant benefit earlier in the disease.
Many patients are given AAD first in clinical practice, owing to concerns with the invasive nature of catheter ablation. In the current study, the incidence of both overall adverse events and cardiovascular adverse events including ischemic and embolic events (myocardial infarction, stroke, or transient ischemic attack), hospitalizations for heart failure, major bleeding, pulmonary vein stenosis, pericardial complications (effusion, hemorrhage, tamponade, and perforation), syncope, and life-threatening arrhythmias were similar in both CA and AAD groups. The ability of CA procedures to obviate the need for AAD long term may inherently avoid the risks associated with long term use of antiarrhythmics.
Although AADs are not benign, invasive procedures and long-term radiation exposure are similar risks to medical therapy. Complications were reported in 9.35% patients and included pericardial tamponade/effusion (1.8%), symptomatic pulmonary vein stenosis (0.67%), bleeding complications (0.5%), and phrenic nerve injuries (0.5%). Cardiac tamponade was the most common fatal complication of AF ablation, occurring in seven of 609 patients. Supplementary Table 2 summarizes the common complications occurring during CA of AF. The operator’s experience is critical regarding safety issues of catheter ablation of AF. Moreover, most reported adverse events occurred after 30 days. However, the safety profile for procedural risks is excellent, especially at tertiary care centers with experienced operators. CA should be done carefully after weighing the benefits and risks of the procedure.
Several limitations of our study should be acknowledged. The definition of outcomes varied substantially among the included studies. This analysis included studies that differed in the use of Class I or III AADs, types of AF and method of surveillance used to monitor recurrence, with the follow-up period varying from 1–2 years. Five studies included patients with paroxysmal AF, and hence the data is only relevant to patients with paroxysmal AF and should not be extrapolated to patients with persistent AF. This meta-analysis combines the different ablation techniques (RFA and Cryoballoon) to evaluate the primary efficacy endpoints, thereby adding a new dimension to the study while presenting as a limitation.
Furthermore, ablation targets beyond the pulmonary veins are variable and mostly up to operators’ preferences. However, the primary efficacy endpoint was consistent in the ablation arm across all studies. The study has the strength of compiling RCT and confirming the superiority of ablation vs AAD to prevent arrhythmia recurrences without significant risks.
In this study, comparing the efficacy and safety of catheter ablation (RFA and CBA) and antiarrhythmic drugs as first-line therapy for symptomatic AF, catheter ablation aiming at electrical pulmonary vein resulted in a significantly lower recurrence rate of atrial tachyarrhythmia and maintenance of sinus rhythm. Catheter ablation for AF rhythm control is superior to AAD in drug naïve patients.
AAR—Conception of the study, drafting, editing, reviewing, and final approval of the study to be submitted. HML—Help in the design of the study, drafting, editing, reviewing, and final approval of the study to be submitted. SP—Drafting, editing, and final approval of the study to be submitted. SR—Drafting, editing, and final approval of the study to be submitted. SAH—Drafting, editing, and final approval of the study to be submitted. NH—Drafting, editing, and final approval of the study to be submitted. HN—Drafting, editing, and final approval of the study to be submitted. KTR—Drafting, editing, and final approval of the study to be submitted. HS—Drafting, editing, and final approval of the study to be submitted. FY—Drafting, editing, and final approval of the study to be submitted. AM—Drafting, editing, and final approval of the study to be submitted. SC—Drafting, editing, and final approval of the study to be submitted. MBM—Critical revision of the manuscript, editing, reviewing, and final approval of the study to be submitted. AFB—Critical revision of the manuscript, editing, reviewing, and final approval of the study to be submitted.WS—Critical revision of the manuscript, editing, reviewing, and final approval of the study to be submitted. OW—Critical revision of the manuscript, editing, reviewing, and final approval of the study to be submitted. AAH—Critical revision of the manuscript, editing, reviewing, and final approval of the study to be submitted.
Not applicable.
We acknowledge the patients, their referring clinicians, and the brave front-liners that continue to risk their lives to save others.
This research received no external funding.
Oussama Wazni serves a consultant speaker for Boston Scientific and Biosense Webster. The remaining authors have no conflict of interest.
