†These authors contributed equally.
Academic Editor: Gianluca Campo
The potential modifiable factors for remote ischemic conditioning (RIC) in
reducing contrast-associated acute kidney injury (CA-AKI) in patients with acute
myocardial infarction (AMI) have not been investigated. The aim of this
meta-regression was to address these issues.We searched Pubmed, Embase and the
Cochrane Library database for published randomized controlled trials (RCTs) with
registration number CRD42020155532. Nine RCTs comprising of 1540 subjects were
included in our meta-analysis. Compared with control group, RIC was associated
with reduced incidence of CA-AKI [(9 studies, 1540 subjects, relative risk (RR)
0.51, 95% confidence intervals (CI) 0.35 to 0.76, p = 0.000, I
Acute myocardial infarction (AMI) is one of the leading causes of mortality and morbidity globally. Emergency percutaneous coronary intervention (PCI) was recommended as the standard therapy to perfuse the ischemic myocardium, especially for the ST-segment elevated myocardial infarction (STEMI) [1, 2]. However, reperfusion seems like as a double-edged sword, acting the role of rescuing but causing injury for the myocardium. The latter is also known as ischemia-reperfusion injury (IRI) [3]. IRI during emergency PCI not only directly causes damage to heart, but also inducing systematic inflammatory response potentiating the impairment of vital organs such as kidney [4, 5].
Contrast-associated acute kidney injury (CA-AKI), as defined by the increment of
serum creatinine
We reported this meta-analysis following the guidance of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis)statement [30]. We searched Pubmed (Medline), Embase and the Cochrane Library database (http://www.cochrane.org) (up to June 2020) with registration number CRD42020155532. We also manually searched reference lists of the retrieved articles. The key words used in search were (ischemic post-conditioning or post-conditioning or ischemic pre-conditioning or pre-conditioning or remote ischemic conditioning) paired with (myocardial infarction or myocardial injury or percutaneous coronary intervention or acute kidney injury or contrast induced nephropathy).
The primary endpoint was CA-AKI, which was diagnosed by increment of serum
creatinine
Inclusion criteria for the retrieved studies were as follows: (1) prospective RCT design; (2) performed in the patients with AMI (including STEMI and non-STEMI (NSTEMI)); (3) inclusion of outcomes of CA-AKI; (4) inclusion of multivariable-adjusted or unadjusted relative risk (RR)/hazard ratio (HR) and their corresponding 95% confidence intervals (CI); or provided the number of events and total population in each group;
Two authors (Yuehua Li and Ying Lou) assessed the quality of the RCTs by the
Cochrane criteria including adequacy of random sequence generation, allocation
concealment, blinding of participants and personnel, blinding of outcome
assessment, incomplete outcome data, intention-to-treat analysis and other bias
[31]. Trials scored one point for each item addressed. If 3 quality criteria were
not met, the study was classified as having high risk of bias; others were
classified as having moderate (3–5 points) or low (
Data were extracted by two independent authors (Yuehua Li and Ying Lou). Discrepancies were resolved by group discussion. The extracted data included source of study (author, publication year, country), population characteristics [protocol, conditioning, patients number, mean age, male proportion, percentage of smoking, diabetes mellitus (DM), hypertension, hyperlipidemia, multi-vessel disease, left anterior descending (LAD)branch involving, use of angiotensin-converting enzyme inhibitor(ACEI)/angiotension receptor blocker (ARB), beta-blocker (BB) and statin, follow-up period], the clinical endpoints, RRs or HRs and their corresponding 95% CI.
We considered the HRs as RRs in the prospective studies. We calculated the RR by
the number of events and total population in the RIC and control group. The
random-effect model was also used in the pooled analysis for the potential
clinical heterogeneity [32]. The heterogeneity was assessed by Q statistic,
I
Publication bias was assessed by Begg’s test and Egger’s test [34]. We performed
the trial sequential analyses (TSA) of CA-AKI or MACE following AMI based on the
data from our pooled analysis (RR and incidence of CA-AKI and MACE) to calculate
the required sample size for the statistical power (Two sided: Type-I error =
0.05;
We initially identified 9223studies by database and manual searching. After exclusion of duplicates and non-relevant studies, 29 potential articles were selected for detailed evaluation. We further excluded 20 articles as shown in Fig. 1. Finally, nine studies were included. Among them, all have reported the impact of RIC on CA-AKI and five about the endpoint of long-term MACE [19, 21, 22, 23, 29].
 Fig. 1.
Fig. 1.Flow Chart of the Trial Selection Process. AMI, acute myocardial infarction; CA-AKI, contrast-associated acute kidney injury; RIC, remote ischemic conditioning.
Tables 1,2 (Ref. [19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29]) showed the main characteristics of the data extracted from the included studies. All included studies were randomized and prospective.Three study scored low risk of bias [19, 20, 21], three with moderate [23, 26, 29] and three with high risk of bias [22, 24, 25] (Supplementary Fig. 1).
| Study | Country | MItype | Protocol Algorithm | Conditioning | Patients No. (RIC vs control) | Age | Male (%) | Diabetes (%) | Hypertension (%) | Smoking (%) |
| Deftereos et al. 2013 [19] | Greece | NSTEMI | 4 |
post-conditioning | 113 vs 112 | 68 | 64 | 36 | 65 | 29 |
| Crimi et al. 2014 [20] | Italy | STEMI | 3 |
post-conditioning | 47 vs 48 | 58.5 | 87.5 | 12 | 53.5 | 53.5 |
| Yamanaka et al. 2015 [21] | Japan | STEMI | 3 |
pre-conditioning | 47 vs 47 | 67 | 74.5 | 33 | 63.8 | 55.3 |
| Wang et al. 2016 [22] | China | STEMI | 3 |
post-conditioning | 123 vs 128 | 62.6 | 77.7 | 26.7 | 65.3 | 66.9 |
| Cao et al. 2018 [24] | China | STEMI | 4 |
post-conditioning | 36 vs 44 | 59 | 86.2 | 18.8 | 66.3 | NA |
| Caoet al. 2018 [25] | China | STEMI | 4 |
post-conditioning | 29 vs 35 | 59.2 | 87.5 | 18.8 | 65.6 | 71.9 |
| Gaspar et al. 2018 [26] | Portugal | STEMI | 3 |
pre-conditioning | 231 vs 217 | 60 | 80.1 | 27.9 | 50 | 58.9 |
| Moretti et al. 2018 [27] | Italy | NSTEMI | 4 |
pre-conditioning | 107 vs 116 | 72.3 | 67.2 | 38.4 | 89.2 | 16.7 |
| Zhou et al. 2018 [28] | China | ACS | 4 |
pre-conditioning | 50 vs 57 | 69.3 | 60.7 | 47.7 | 56.1 | 20.6 |
| Elbadawi et al. 2018 [23] | USA | STEMI | 3 |
pre-conditioning | 30 vs 30 | 51.5 | 83 | 41 | 32.4 | 70.4 |
| Guo et al. 2019 [29] | China | NSTEMI | 3 |
pre-conditioning | 110 vs 110 | 71.3 | 59.1 | 44 | NA | NA |
| Study | Dyslipidemia (%) | Muti-vessel disease (%) | LAD occlusion (%) | ACEI/ARB (%) | Statins (%) | CA-AKI (RIC vs control) | MACE | |
| Deftereos et al. 2013 [19] | 59 | 55.1 | 54.2 | NA | 17 | 36 | 14/113 vs 33/112 | 14/113 vs 25/112 |
| Crimi et al. 2014 [20] | 31.5 | 35 | 100 | NA | 100 | 100 | 7/47 vs 6/48 | NA |
| Yamanaka et al. 2015 [21] | 55.3 | NA | 42.6 | 95.7 | 5.32 | 16 | 5/47 vs 17/47 | 2/47 vs 7/47 |
| Wang et al. 2016 [22] | NA | 78.9 | NA | NA | NA | NA | 7/123 vs 18 /128 | 9/123 vs 20 /128 |
| Cao et al. 2018 [24] | 12.5 | 18.8 | 46.3 | 33.8 | 68.8 | 100 | 4/36 vs 18 /47 | NA |
| Cao et al. 2018 [25] | 20.3 | 12.5 | 48.4 | 42.2 | 85.9 | 100 | 3/29 vs 11/35 | NA |
| Gaspar et al. 2018 [26] | 50 | 11.6 | 44 | 35.9 | 15.2 | 29.2 | 45/231 vs 45/217 | 19/231 vs 28/217 |
| Moretti et al. 2018 [27] | 67.4 | NA | NA | NA | NA | NA | 10/57 vs 15/54 | NA |
| Zhou et al. 2018 [28] | 25.2 | NA | NA | 67.3 | 72.9 | 95.3 | 5/50 vs 15/57 | 2/50 vs 3/57 |
| Elbadawi et al. 2018 [23] | 76.7 | 10 | NA | NA | NA | NA | 1/30 vs 5/30 | 4/30 vs 2/30 |
| Guo et al. 2019 [29] | NA | 35 | NA | 86.4 | 75.9 | 94.5 | 12/110 vs 18/110 | NA |
| NOTE: ACEI, angiotensin-converting enzyme inhibitor; ACS, acute coronary syndrome; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; CA-AKI, contrast-induced nephropathy; LAD, left anterior descending; MACE, major adverse cardiovascular events; MI, myocardial infarction; NA, not available; RIC, remote ischemic conditioning. | ||||||||
All studies have reported the endpoint of CA-AKI and five reported the risk of
MACE. Compared with the control group, the group with RIC was associated with
reduced risk of CA-AKI (9 studies, 1540 subjects, RR 0.51, 95% CI 0.35 to 0.76,
p = 0.007, I
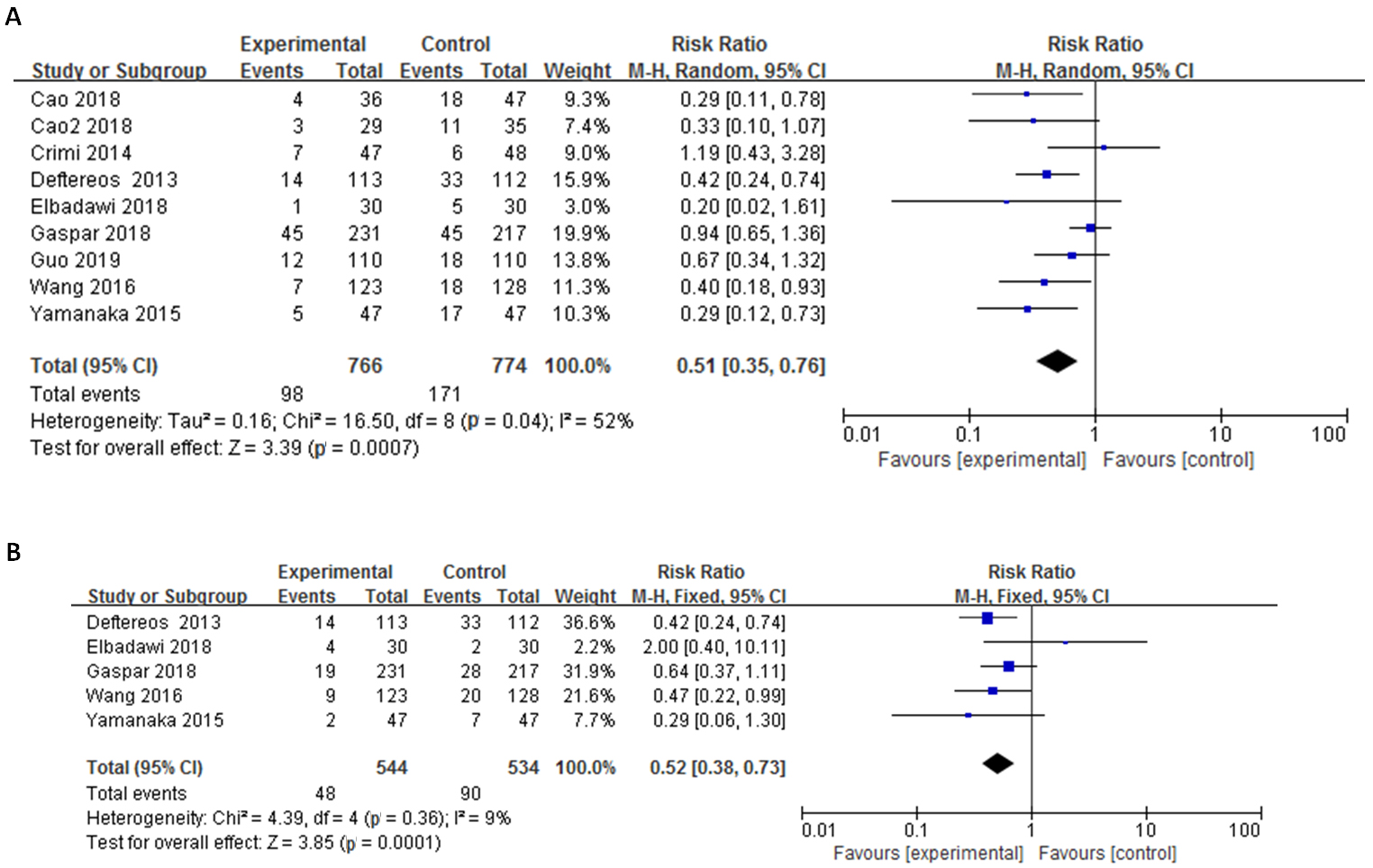 Fig. 2.
Fig. 2.Funnel plot of RIC in Reducing CA-AKI (2A) and MACE (2B) in AMI. Meta-analysis of the effect of RIC in reducing CA-AKI (A) and risk of MACE (B) in the condition of AMI. AMI, acute myocardial infarction; CI, confidence intervals; CA-AKI, contrast-associated acute kidney injury; MACE, major adverse cardiovascular events; RIC, remote ischemic conditioning.
For the main endpoint of CA-AKI, we performed both meta-regression and subgroup analyses by specific study characteristics which was mentioned above. In univariable meta-regression, the percentage of hypertension at baseline was negatively related to risk of CA-AKI (regression coefficient = –0.05, p = 0.021) (Table 2, Fig. 3). This factor was further confirmed by subgroup analysis [for subgroup with hypertensive patients over 58% (mean): RR 0.36, 95% CI 0.25 to 0.52 vsless than 58% (mean): RR 0.72, 95% CI 0.40 to 1.30, p for subgroup difference 0.008] (Table 3). The subgroup analysis also has indicated that the proportion of age, male, DM, conditioning, quality score might be possible modifiable factors, however these factors were not verified by meta-regression analyses (Tables 3,4).
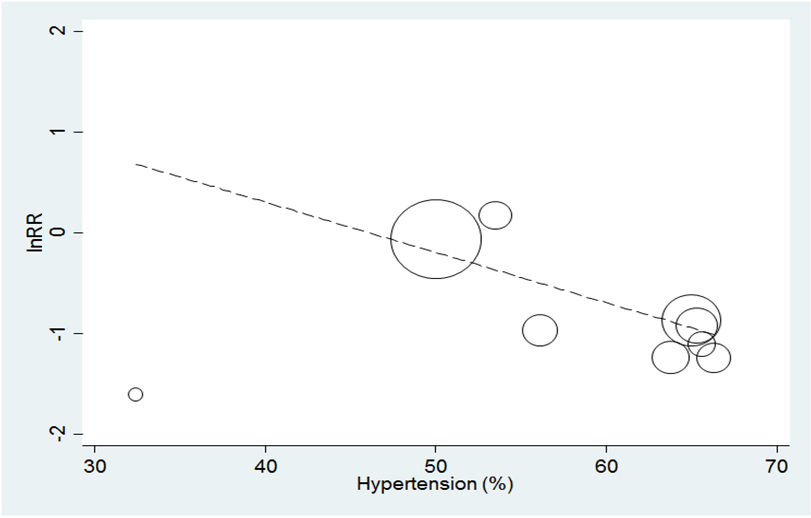 Fig. 3.
Fig. 3.Meta-regression plot on the incidence of CA-AKI against proportion of hypertension. Meta-regression analysis of the percentage of hypertension as a modifiable factor for CA-AKI. CA-AKI, contrast-associated acute kidney injury; RR, risk ratio.
| Baseline characteristics | Number of trials | Risk of CA-AKI | |||
| Coefficient | 95% CI | I |
p | ||
| Age | 10 | –0.012 | (–0.095 to 0.072) | 47.54 | 0.75 |
| Male proportion | 10 | 0.004 | (–0.037 to 0.045) | 50.57 | 0.82 |
| Diabetes proportion | 10 | –0.010 | (–0.051 to 0.031) | 50.94 | 0.59 |
| Hypertension proportion | 9 | –0.050 | (–0.090 to –0.010) | 9.01 | 0.02 |
| Smoking proportion | 8 | 0.003 | (–0.029 to 0.034) | 55.61 | 0.83 |
| Dyslipidemia proportion | 8 | 0.002 | (–0.030 to 0.034) | 62.34 | 0.88 |
| Multi-vessel disease proportion | 8 | –0.006 | (–0.007 to 0.027) | 39.23 | 0.49 |
| LAD branch occlusion | 6 | 0.016 | (–0.023 to 0.056) | 71.29 | 0.31 |
| ACEI/ARB use proportion | 6 | –0.005 | (–0.033 to 0.024) | 56.72 | 0.68 |
| Beta-blocker use proportion | 8 | 0.002 | (–0.014 to 0.017) | 60.73 | 0.82 |
| Statin use proportion | 8 | –0.0004 | (–0.015 to 0.014) | 59.22 | 0.95 |
| Protocol | 11 | 0.003 | (–0.025 to 0.032) | 45.91 | 0.79 |
| Area | 11 | –0.366 | (–1.082 to 0.349) | 36.36 | 0.28 |
| Conditioning time | 11 | 0.358 | (–0.322 to 1.038) | 30.92 | 0.26 |
| Qulity score | 11 | 0.102 | (–0.109 to 0.313) | 42.71 | 0.30 |
| ACEI, angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; CI, confidence interval; CA-AKI, contrast-associated acute kidney injury; LAD, left anterior descending; RIC, remote ischemic conditioning. | |||||
| Characteristic | Risk of CA-AKI | ||||
| Data points, No | Pooled RR (95% CI) | p |
p | ||
| All studies | 11 | 0.52 (0.38 to 0.72) | 0.07 | ||
| age | |||||
| 6 | 0.55 (0.31 to 0.95) | 0.491 | 0.049 | ||
| 4 | 0.45 (0.31to 0.64) | 0.048 | |||
| Male proportion | |||||
| 4 | 0.45 (0.31to 0.64) | 0. 048 | 0.049 | ||
| 6 | 0.55 (0.31 to 0.95) | 0.491 | |||
| Percentage of hypertension | |||||
| 4 | 0.72 (0.40 to 1.30) | 0.156 | 0.000 | ||
| 5 | 0.36 (0.25 to 0.52) | 0.942 | |||
| Percentage of diabetes | |||||
| 4 | 0.58 (0.33 to 1.02) | 0.042 | 0.031 | ||
| 5 | 0.44 (0.311 to 0.62) | 0.573 | |||
| Percentage of multi-vessel diseases | |||||
| 4 | 0.46 (0.20 to 1.05) | 0.041 | 0.215 | ||
| 4 | 0.55 (0.36 to 0.85) | 0.262 | |||
| Percentage of LAD occlusion | 0.205 | ||||
| 5 | 0.45 (0.25 to 0.89) | 0.012 | |||
| 1 | 1.19 (0.43to 3.29) | 0 | |||
| Percentage of dyslipidemia | |||||
| 4 | 0.46 (0.24 to 0.87) | 0.197 | 0.230 | ||
| 4 | 0.49 (0.25 to 0.96) | 0.016 | |||
| Percentage of smoking | |||||
| 2 | 0.41 (0.25 to 0.66) | 0.857 | 0.062 | ||
| 6 | 0.54 (0.31 to 0.94) | 0.039 | |||
| Percentage of ACEI/ARB use | |||||
| 3 | 0.50 (0.21 to 1.22) | 0.032 | 0.094 | ||
| 3 | 0.46 (0.27 to 0.76) | 0.310 | |||
| Percentage of beta-blocker use | |||||
| 3 | 0.52 (0.26 to 1.07) | 0.01 | 0.343 | ||
| 5 | 0.52 (0.32 to 0.84) | 0.244 | |||
| Percentage of statin use | |||||
| 3 | 0.52 (0.26 to 1.07) | 0.010 | 0.343 | ||
| 5 | 0.52 (0.32 to 0.84) | 0.244 | |||
| Study quality | |||||
| High risk | 6 | 0.42 (0.29 to 0.63) | 0.697 | 0.044 | |
| Moderate/low risk | 5 | 0.62 (0.39 to 1.00) | 0.034 | ||
| By protocol time | |||||
| 2 | 0.41 (0.26 to 0.66) | 0.923 | 0.086 | ||
| 9 | 0.55 (0.38 to 0.80) | 0.069 | |||
| By conditioning | |||||
| Pre-conditioning | 5 | 0.61 (0.40 to 0.92) | 0.395 | 0.035 | |
| Post-conditioning | 6 | 0.43 (0.30 to 0.63) | 0.097 | ||
| By type of myocardial infarction | 0.348 | ||||
| STEMI | 7 | 0.49 (0.29 to 0.83) | |||
| NSTEMI | 2 | 0.51 (0.33 to 0.80) | |||
| By area | 0.067 | ||||
| China | 5 | 0.44 (0.30 to 0.65) | |||
| others | 6 | 0.59 (0.37 to 0.95) | |||
| ACEI, angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction;
ARB, angiotension receptor blocker; CI, confidence interval; CA-AKI,
contrast-associated acute kidney injury; LAD, left anterior descending; NSTEMI,
non-STEMI; RIC, remote ischemic conditioning; STEMI, ST-segment elevated
myocardial infarction. | |||||
To confirmed the pooled effect sizes of CA-AKI or MACE as true estimated effect, the required sample sizes for the CA-AKI is 959 (RR reduction = 50.0%, incidence of Control arm = 22.0%), and MACE (RR reduction = 50.0%, incidence of Control arm = 17.0%) is 561. However, the same size of CA-AKI (1540 vs 959) or MACE (1078 vs 561) is enough for the estimated effect (Supplementary Figs. 4,5).
The publication bias of CA-AKI was not observed by Begg’s adjusted rank correlation test (p = 0.35) and Egger’s test (p = 0.06) (Supplementary Fig. 6).
In this meta-analysis of nine RCTs including 1540 subjects, we found that RIC was an effective strategy in reducing the incidence of CA-AKI in patients with AMI, with a profound protection associated with a 0.51-fold lower risk. What’s more, our meta-regression showed that the effect of RIC seemed to be more beneficial for the hypertensive patients. In addition, RIC was also showed long-term protective effect with a reduce risk of MACE for long term (RR 0.52). To our knowledge, this is the first meta-regression analysis focusing on the modifiable factors for renoprotection of RIC in AMI patient.
The effect of RIC from previous studies in reducing CA-AKI in patients with AMI was inconsistent. We combined all available RCTs and found that RIC was an effective strategy in preventing RIC. Our study was in line with previous two meta-analyses which have showed that RIC was beneficial for prevention of acute kidney injury in patients with PCI, coronary artery bypass grafting and other cardiac surgeries [35, 36]. However, these two meta-analyses did not discriminate the effect of RIC in different conditions separately. Our study has found that RIC was not only effective in reducing the incidence of CA-AKI in AMI, but also associated with an improved long-term prognosis. The current meta-analysis has extended previous finding that that RIC was effective in reducing CA-AKI in elective PCI [37, 38]. In addition, our meta-analysis has indicated that RIC also showed a long-term cardiac protection in reducing MACE. This result was consistent with recent studies which have reported that RIC was an benefit for AMI patents in reducing MACE, heart failure as well as myocardial edema levels, myocardial salvage index [26]. Moreover, the TSA results showed that, assuming future trials record the same event rates as the published trials, they did not need additional participants to provide future meta-analysis with the power to confirm the benefit for CA-AKI and MACE. This meaningful and intriguing finding indicates that RIC is probably an effective renoprotection strategy in the condition of AMI.
The effect of RIC in reducing CA-AKI for AMI may be influenced by some modifiable factors. Our meta-regression has showed that hypertension status was negatively associated with the reduced risk of CA-AKI. What’s more, our subgroup analyses have showed that the group with more percentage of hypertensive patients was benefit more (RR 0.36 vs 0.72) from RIC treatment under the condition of AMI. Our results were in lined with previous studies which have reported that RIC was effective in reducing systolic blood pressure about 5 mmHg [39, 40]. In addition, hypertension is a well-known risk factor for AMI and our results have also suggested that RIC might be more effective for some high-risk patients. Our study was consistent with previous trials which have also suggested that RIC was more effective in kidney protection for high-risk patients undergoing PCI or cardiac surgery [41, 42]. Nevertheless, the influential effect of hypertension in RIC-induced renoprotection needs more large sample-size and high-quality clinical trials to verify in future.
Results from our meta-analysis indicated that RIC was an effective strategy in reducing CA-AKI and MACE for AMI. This meaningful and intriguing finding indicates that RIC is probably an effective renoprotection strategy in AMI. Hence, routine performance of RIC would be helpful for renal protection under the condition of acute ischemic events. Future experimental studies are needed to explore the mechanism about the RIC for kidney protection. In addition, large randomized controlled trials are necessary to extend the investigation of the effect of RIC for renal protection in both cardiac and non-cardiac conditions.
Our meta-analysis has some limitations. First, we did not use the multivariable adjusted RR for the effect size, resulting inpotential residual confounders. Second, the excluded studies which performed in patients with acute coronary syndrome might influence on the effect size. Third, five included studies were from China. Although our subgroup analyses have indicated that area was not a modifiable factor, it needs further investigation for RIC in reducing CA-AKI for AMI. Finally, our meta-analysis used pooled data, rather than individual data, which restricted detailed analysis for the potential confounding factors.
Our meta-analysis of nine RCTs comprising 1540 patients has demonstrated that RIC remains an effective strategy in reducing CA-AKI for AMI, especially for the hypertensive group. Routine RIC in AMI should be recommended for renal protection.
Conceptualization, CZ and HP; methodology, YLi; software, YLi; validation, YLi, YLo, HP, and CZ; formal analysis, YLi; investigation, YLo; resources, YLo; data curation, YLo; writing—original draft preparation, YLi; writing—review and editing, YLo, HP, and CZ; visualization, CZ; supervision, CZ; project administration, HP; funding acquisition, HP, and CZ. All authors have read and agreed to the published version of the manuscript.
Not applicable.
Not applicable.
This work was supported by the Clinical Research Foundation of Fuwai Hospital (No. 2016-ZX033), and the National Natural Science Foundation of China (No. 81970290 and 81760096).
The authors declare no conflicts of interest.
