†These authors contributed equally.
Academic Editors: Carmela Rita Balistreri, Takatoshi Kasai and Brian Tomlinson
Background: The management of type II endoleaks (T2ELs) remains
controversial in current literature. Hence, this study aimed to explore the
natural history of isolated type II endoleak after endovascular aneurysm repair
(EVAR) and its influence on long-term outcomes based on a 10-year follow-up at a
tertiary medical center. Methods: From January 2011 to April 2021,
consecutive patients who underwent elective EVAR were reviewed. The demographics,
clinical characteristics, treatment details, imaging information, in the event of
T2ELs, and outcomes were extracted. Results: A total of 287 patients
were included for analysis. Isolated T2EL was identified in 79 patients (27.5%),
while no endoleak was found in 208 patients (72.5%). The mean age at EVAR was
68.1
Endovascular aneurysm repair (EVAR) has become the standard procedure used to treat abdominal aortic aneurysm in patients with suitable anatomy, which is mainly attributed to its lower perioperative mortality, morbidity, and shorter length of hospital stay than conventional open surgery [1, 2, 3]. However, postoperative endoleaks, the persistence of blood flow within the aneurysm sac, are the main causes of sac growth and reintervention compromising the long-term durability of EVAR [4, 5].
There is no debate that type I and type III endoleaks, which involve the stent
landing zones or junction points between the stents, should be treated promptly.
However, the management of type II endoleaks (T2ELs), which account for
approximately half of all endoleaks and are caused by retrograde flow from the
aortic side branches, remains controversial [6, 7, 8]. Some reports have shown that
the T2ELs are a benign entity [9], while others have associated T2ELs with
adverse outcomes such as aneurysm sac growth and aneurysm rupture [10, 11].
Current guidelines such as the 2019 European Society for Vascular Surgery (ESVS)
guideline and Society for Vascular Surgery implementation of clinical practice
guidelines have recommended conservative management and intervention was
indicated for significant sac expansion (
To explore the optimal management strategy for T2ELs, understanding the natural course of T2EL and its influence on long-term outcomes is necessary. Thus, this study aimed to report the natural history of T2EL based on a 10-year study at a single medical center and analyze the long-term effect of T2EL.
This was a single-center retrospectively observational study following the STROBE guidelines [15]. The study was approved by the institutional ethics review board and written informed consent was waived due to the retrospective nature.
From January 2011 to April 2021, consecutive patients who underwent elective EVAR were reviewed and recorded in a database. The criterion for inclusion included patients undergoing elective EVAR for infrarenal degenerative atherosclerotic aortic or aortoiliac aneurysm, preoperative computed tomographic angiography (CTA) within 3 months before EVAR, and at least one postoperative CTA imaging during the follow-up. The criterion for exclusion included EVAR for a ruptured aneurysm, dissection, and penetrating aortic ulcer; Patients with incomplete preoperative or postoperative clinical data or CTA imaging; Patients with a type 1, 3, or 4 endoleak. If patients had T2ELs during the follow-up period, but another endoleak (type 1, 3, or 4) before rupture, these patients were also excluded.
Data were collected from the electronic medical records and radiology information system/picture archiving communication system (RIS/PACS). The collected data included the demographics, anatomical characteristics, operative details, complications, in the event of T2ELs, and outcomes. All medical data were reviewed by two independent reviewers (YLW, FY). Data were stored and analyzed anonymously.
The primary endpoint was survival for patients with or without T2EL. The secondary endpoints were aneurysm sac growth, T2EL-related reintervention, and aneurysm rupture.
An isolated T2EL was defined as a persistent retrograde flow from the aortic
side branches into the aneurysm sac, without signs of other types of endoleak
during the follow-up period. T2EL was classified as early if the endoleak was
detected
All patients undergoing EVAR were prescribed to have a follow-up protocol including assessment of the clinical symptoms and images at 1, 3, 6, and 12 months and then annually thereafter. Patients who demonstrated aneurysm sac growth or endoleaks underwent a more frequent follow-up at 3-month or 6-month intervals. Follow-up data was collected up till October 2021 when follow-up was stopped. The main imaging modality was computed tomography angiography (CTA) and magnetic resonance angiography was performed in patients with contraindication for iodinated contrast media use.
Patients with T2ELs were routinely managed conservatively and intervention was
indicated for significant aneurysm sac growth
The categorical variables were expressed as numbers with percentages and the
continuous variables, as mean with standard deviation (SD). Continuous variables
were compared using Student’s t-test or Wilcoxon rank-sum test, and
categorical variables were compared using Fisher exact test. The freedom from
all-cause mortality was analyzed using the Kaplan-Meier method, and the log-rank
test was used for subgroup comparisons. Cox regression analysis was used to
identify the independent factors associated with overall survival. Only variables
with a p-value
During a period of 10 years (from January 2011 to April 2021), 449 patients
underwent EVAR at our institution. After applying the exclusion criteria, a total
of 287 patients were included for analysis. An isolated T2EL was identified in 79
patients (27.5%, 79/287), while no endoleak was found in 208 patients (72.5%,
208/287). Among T2ELs, 53.2% (42/79) originated from the lumbar artery, 26.6%
(21/79) originated from the inferior mesenteric artery, 5.1% (4/79) originated
from the midsacral artery, 13.9% (11/79) originated from the lumbar and inferior
mesenteric artery, and 1.3% (1/79) originated from the lumbar artery and
midsacral artery. 24.1% (19/79) of T2ELs showed a flow between
| Isolated type II endoleak (n = 79) | No endoleak (n = 208) | p-value | ||
| Age, years | 67.9 |
68.3 |
0.765 | |
| Gender | 0.133 | |||
| Male | 60 (75.9) | 174 (83.7) | ||
| Female | 19 (24.1) | 34 (16.3) | ||
| Hypertension | 60 (75.9) | 171 (82.2) | 0.232 | |
| Diabetes | 13 (16.5) | 38 (18.3) | 0.720 | |
| Smoking | 24 (30.4) | 76 (36.5) | 0.328 | |
| Hyperlipidemia | 36 (45.6) | 99 (47.6) | 0.759 | |
| Ischaemic heart disease | 27 (34.2) | 65 (31.3) | 0.635 | |
| Pulmonary disease | 12 (15.2) | 37 (17.8) | 0.601 | |
| Preoperative aneurysm characteristics | ||||
| Proximal neck diameter, mm | 20.0 |
20.0 |
0.919 | |
| Proximal neck length, mm | 40.0 |
40.9 |
0.700 | |
| Proximal neck angle, degrees | 43.3 |
39.5 |
0.219 | |
| Abdominal aortic aneurysm diameter, mm | 60.8 |
60.2 |
0.620 | |
| Maximum iliac diameter, mm | 15.0 |
15.3 |
0.338 | |
| Stent types | 0.705 | |||
| Medtronic | 58 (73.4) | 163 (78.4) | ||
| Microport | 17 (21.5) | 33 (15.9) | ||
| Cordis | 2 (2.5) | 5 (2.4) | ||
| Lifetech | 2 (2.5) | 7 (3.4) | ||
| Oversizing, % | 23.1 |
22.6 |
0.400 | |
| Continuous data are presented as mean | ||||
Among the 79 patients with isolated T2ELs, 33 (41.8%, 33/79) were early and 46 (58.2%, 46/79) were late T2ELs. Spontaneous resolution of T2ELs was identified in 29 patients (36.7%, 29/79). Persistent T2ELs were observed in 50 patients (50/79, 63.3%). No sac growth was seen in 33 patients (66%, 33/50) and these patients were managed conservatively. The remaining 17 patients (34%, 17/50) showed significant aneurysm sac growth, which made the operators consider the secondary intervention. However, 6 of them declined intervention due to various reasons and thus were managed conservatively. The remaining 11 patients underwent interventional embolization for T2ELs. Of which, 8 patients underwent transarterial embolization of the corresponding lumbar artery and 3 patients underwent transarterial embolization of the corresponding inferior mesenteric artery. There was a technical failure in 3 interventions (lumbar artery embolization) which with the small and/or tortuous feeding vessels left because it was unable to cannulate into these vessels. Following the intervention, 2 patients had complete resolution of T2ELs and 9 patients had persistent T2ELs. Among the patients with persistent T2ELs, 2 patients (2/9) still showed progressive aneurysm sac growth, and one of them died from aneurysm rupture (technical failure as stated above); the remaining 7 patients (7/9) showed no aneurysm sac growth (Fig. 1).
 Fig. 1.
Fig. 1.Management and outcome of patients with type II endoleaks (T2ELs). Isolated T2ELs were identified in 79 patients. Spontaneous resolution of T2ELs was identified in 29 patients (36.7%, 29/79). Persistent T2ELs were observed in 50 patients (63.3%, 50/79). No sac growth was seen in 33 patients (66%, 33/50) and these patients were managed conservatively. The remaining 17 patients (34%, 17/50) showed significant sac growth. Six of them declined intervention due to various reasons and the remaining 11 patients underwent interventional embolization for T2ELs. Following the embolization, 2 patients had complete resolution of T2ELs and 9 patients had persistent T2ELs. Among the patients with persistent T2ELs, 2 patients (2/9) still showed progressive sac growth, and one of them died from aneurysm rupture; the remaining 7 patients (7/9) showed no sac growth.
There were no differences in baseline characteristics between patients with
isolated T2ELs and those without any endoleaks (Table 1). During the follow-up
period, patients with isolated T2ELs had a higher incidence of aneurysm sac
growth than patients without endoleaks (21.5% vs 4.3%, p
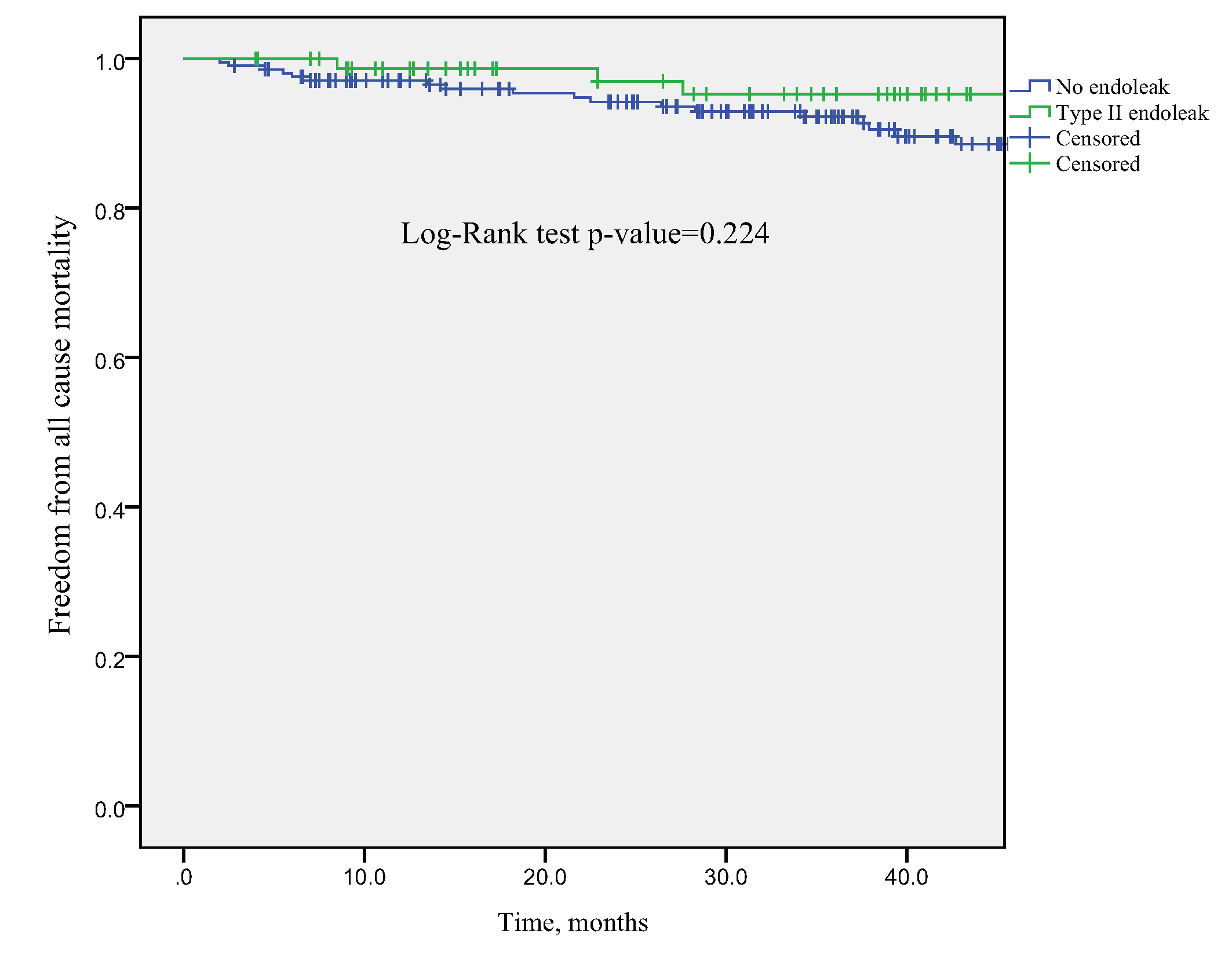 Fig. 2.
Fig. 2.Kaplan-Meier analysis, freedom from all-cause mortality comparing patients with isolated type II endoleaks vs no endoleaks. No significant difference in overall survival was found between patients with or without isolated type II endoleaks.
| Isolated type II endoleak (n = 79) | No endoleak (n = 208) | p-value | |
| Follow-up time, months | 47.9 |
40.8 |
0.077 |
| Sac growth | 17 (21.5) | 9 (4.3) | |
| All cause death | 5 (6.3) | 21 (10.1) | 0.321 |
| Continuous data are presented as mean | |||
| Univariable analysis Hazard ratio (95% CI) | Multivariable analysis Hazard ratio (95% CI) | ||
| Age, years* | 1.085 (1.036–1.135) | 1.081 (1.033–1.132) | |
| Gender | 1.490 (0.625–3.555) | 1.248 (0.516–3.017) | |
| Proximal neck diameter | 1.018 (0.854–1.212) | ||
| Proximal neck length | 0.995 (0.972–1.018) | ||
| Proximal neck angle | 1.004 (0.988–1.021) | ||
| Abdominal aortic aneurysm diameter | 1.006 (0.971–1.043) | ||
| Maximum iliac diameter | 1.008 (0.810–1.254) | ||
| Stent types | |||
| Medtronic | 0.244 (0.071–0.840) | 0.294 (0.085–1.021) | |
| Microport | 0.407 (0.101–1.634) | ||
| Cordis | 0.000 | ||
| Lifetech | Reference | ||
| Sac growth | 0.712 (0.244–2.074) | ||
| Oversizing, % | 1.007 (0.920–1.103) | ||
| * Variables significantly related to survival in univariable and multivariable analysis; CI, confidence interval. | |||
There were no differences except for stent types in baseline characteristics between patients with early isolated T2ELs and patients with late isolated T2ELs (Table 4). During the follow-up period, 1 patient died of aneurysm rupture in the early T2ELs group and 4 patients died from non-aneurysm-related causes. Spontaneous resolution rate, aneurysm sac growth rate, and all-cause mortality showed no differences between patients in the early T2ELs group and late T2ELs group (Table 5).
| Early type II endoleak (n = 33) | Late type II endoleak (n = 46) | p-value | ||
| Age, years | 69.4 |
66.8 |
0.268 | |
| Gender | 0.301 | |||
| Male | 27 (81.8) | 33 (71.7) | ||
| Female | 6 (18.2) | 13 (28.3) | ||
| Hypertension | 25 (75.8) | 35 (76.1) | 0.973 | |
| Diabetes | 4 (12.1) | 9 (19.6) | 0.379 | |
| Smoking | 13 (39.4) | 11 (23.9) | 0.140 | |
| Hyperlipidemia | 13 (39.4) | 23 (50.0) | 0.351 | |
| Ischaemic heart disease | 9 (27.3) | 18 (39.1) | 0.273 | |
| Pulmonary disease | 5 (15.2) | 7 (15.2) | 1.000 | |
| Preoperative aneurysm characteristics | ||||
| Proximal neck diameter, mm | 20.4 |
19.7 |
0.094 | |
| Proximal neck length, mm | 41.3 |
39.1 |
0.565 | |
| Proximal neck angle, degrees | 44.0 |
42.7 |
0.818 | |
| Abdominal aortic aneurysm diameter, mm | 60.3 |
61.1 |
0.703 | |
| Maximum iliac diameter, mm | 14.8 |
15.2 |
0.347 | |
| Stent types | ||||
| Medtronic | 23 (69.7) | 35 (76.1) | ||
| Microport | 8 (24.2) | 9 (19.6) | ||
| Cordis | 1 (3.0) | 1 (2.2) | ||
| Lifetech | 1 (3.0) | 1 (2.2) | ||
| Oversizing, % | 22.9 |
23.2 |
0.783 | |
| Continuous data are presented as mean | ||||
| Early type II endoleak (n = 33) | Late type II endoleak (n = 46) | p-value | |
| Follow-up time, months | 49.4 |
46.8 |
0.722 |
| Spontaneous resolution | 13 (39.4) | 16 (34.8) | 0.675 |
| Sac growth | 7 (21.2) | 10 (21.7) | 0.955 |
| All cause death | 3 (9.1) | 2 (6.3) | 0.700 |
| Continuous data are presented as mean | |||
Given the conflicting reports regarding whether T2ELs are a benign entity or related to adverse outcomes, debates remain regarding the optimal management strategy for T2ELs. The present study was conducted to report the natural history of T2EL and its influence on long-term outcomes based on a 10-year study at a tertiary medical center. The presented study found that the incidence of an isolated T2EL was 27.5% (79/287), and 36.7% (29/79) of T2ELs showed spontaneous resolution; patients with an isolated T2EL showed a higher aneurysm sac growth rate than patients without endoleak; no significant difference in overall survival was found between patients with or without an isolated T2EL; no significant differences in overall survival and sac growth were identified in patients with an early T2EL and patients with a late T2EL.
The incidence of isolated T2EL in the present study was in line with the
incidence in previously published articles (8%–44%) [16, 17]. 36.7% (29/79)
of T2ELs showed spontaneous resolution in the present study, which was also
consistent with the published reports (30% to 50%) [12]. There was only one
(1.3%, 1/79) aortic rupture that occurred in patients with T2ELs, which was
similar to the rupture rate in current literature (close to 1%) [6]. Patients in
the isolated T2ELs group showed a higher incidence of aneurysm sac growth than
patients in the no endoleak group (21.5% vs 4.3%, p
Among current reports, the treatment modalities for persistent T2ELs management included transarterial embolization, transcaval or translumbar embolization, laparoscopic ligation, sacotomy, or graft explant [20]. However, the treatment of T2ELs is technically challenging and time-consuming, with unsatisfactory clinical success. The recurrence of T2ELs after intervention can be as high as 60% [14, 20]. In the present study, a total of 11 patients with T2ELs received the intervention, but only 2 patients (2/11, 18.2%) showed complete resolution of the endoleak during the follow-up. In Loy’s study, there were 14 patients with T2ELs underwent secondary interventions and only 5 patients (5/14, 35.7%) had complete and sustained resolution of T2EL [21]. Moreover, Sana Mulay et al. [18] recently reported that neither survival nor aneurysm-related outcomes will be improved for patients treated for T2ELs. Based on the above-mentioned results, a more conservative protocol for selecting high-risk T2ELs patients for intervention is rational.
Some studies have reported prophylactic embolization of aortic side branches or aneurysm sac before or during EVAR to reduce the incidence of T2ELs, and results identified this method might be associated with a lower rate of sac growth and incidence of T2ELs [13, 14, 22]. However, most of them are single-center retrospective studies with a small sample size. The exact clinical effect other than the reduction of endoleaks is still needed to be explored. The benefit of selective prophylactic embolization and the economic cost, technical fitness also should be considered. Thus, prospective randomized trials with a large sample size are needed to evaluate the advantages of prophylactic embolization.
In the present study, no differences were found in spontaneous resolution rate, aneurysm sac growth rate, and all-cause mortality between patients in the early T2ELs group and late T2ELs group. While other studies reported that late T2ELs were less likely to have spontaneous resolution than early T2ELs and patients with early T2ELs were less likely to survive than patients with late T2ELs [18]. The different results might be attributed to the following reasons: First, the sample size in our study was small which may cause selection bias. Second, there is no exact definition for the endoleak stage in current literature. For example, the early T2EL was defined as an endoleak visualized on first-year follow-up in Sidloff DA’s study [23]; the early T2EL was defined as an endoleak visualized on 3-month follow-up in Mulay S’s study [18], and the early T2EL was defined as an endoleak visualized on 1-month follow-up by other researchers [14, 17]. Third, the present study may cause information bias. It is possible that some endoleaks were incorrectly categorized or not detected because the CTA imaging mainly detects relatively large endoleaks, more sensitive imaging such as blood pool MRA may be needed to detect small T2ELs [18]. Thus, more studies are needed to explore the exact clinical significance of the stage of T2ELs.
There are some limitations in the present study. First, 36.1% of patients undergoing EVAR were excluded in the present study because most of our patients undergoing EVAR were lost to follow-up. And this could not be improved due to its retrospective nature and low patients’ compliance. Thus, the results of this study should be viewed with an inherent bias due to the retrospective nature and small sample size. Second, this is a single-center study and the follow-up time was variable (range, 2–119.7 months), which may lead to selection bias. Finally, the cause of death in some cases was unknown, it was not possible to evaluate the impact of T2ELs on aneurysm-related mortality.
Type II Endoleak was significantly associated with aneurysm sac growth and no association with survival was observed.
Study conceptualization and design—YLW, FY, YWB; Study supervision—BX; Data collection—WY, CZ, JCL, SGJ, CYW, CTY; Data analysis and interpretation—SJH, CTY, YC, YLW, TQL, JCL; Draft writing—YLW; Draft revising—FY, YWB, CZ, CYW, SGJ, BX; All authors contributed edits, read and approved the final manuscript version.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Wuhan union hospital and written informed consent was waived because of the retrospective nature (Approval number: 20211101).
We would like to express my gratitude to Huimin Zhou (Wuhan Tongji Hospital) who helped us edit the language. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
