†These authors contributed equally.
Academic Editors: Ichiro Wakabayashi and Klaus Groschner
Background: There is controversy over whether non-vitamin K antagonist oral anticoagulants (NOACs) use increase the risk of hepatic impairment in patients with non-valvular atrial fibrillation (NVAF). We conducted a comprehensive assessment using multi-source medical data. Methods: We first performed a systematic search of the PubMed, Embase, and Cochrane Library databases (through 11 August 2021) for randomised controlled trials (RCTs) and real-world studies (RWSs) that reported hepatic impairment events in patients with NVAF administered NOACs or vitamin K antagonists (VKAs) therapy. The primary outcomes were hepatic impairment identified by diagnostic liver injury (DLI) or abnormal liver enzyme (ALE). The secondary outcome was hepatic failure. Relative risks (RRs) for RCTs and adjusted hazard ratios (aHRs) for RWSs were calculated separately using random-effects models. We also conducted a disproportionality analysis by extracting reports of hepatic impairment associated with NOACs from the Food and Drug Administration Adverse Event Reporting System (FAERS) database. Reporting odds ratios (RORs) were calculated to identify the statistical associations between NOACs and hepatic impairment. Scenario analyses were further performed to eliminate event- and drug-related competition bias. Results: A total of 559,873 patients from five RCTs and four RWSs were included in the pooled analysis. For RCTs, NOACs use was not associated with an increased risk of DLI (RR: 0.96, 95% confidence intervals (CI): 0.73–1.28) or ALE (RR: 0.91, 95% CI: 0.69–1.19) compared with VKAs. The merged results of RWSs also showed a similar risk of DLI (aHR: 0.88, 95% CI: 0.72–1.09) or ALE (aHR: 0.91, 95% CI: 0.82–1.00) between NOACs and VKAs. The results of hepatic failure were in accordance with the primacy outcomes. Analyses of individual NOACs did not significantly affect the results. Insights from the FAERS database failed to detect hepatic impairment signals for overall NOACs agents (ROR: 0.34, 95% CI: 0.32–0.37). Scenario analyses confirmed the primary results. Conclusions: Insights from multi-source medical data confirmed that NOACs use was not associated with an increased risk of hepatic impairment in patients with NVAF.
Non-valvular atrial fibrillation (NVAF) is a common arrhythmia worldwide associated with the occurrence of ischaemic stroke [1]. Vitamin K antagonists (VKAs), particularly warfarin, are conventional options to prevent stroke in patients with NVAF [2]. Non-vitamin K antagonist oral anticoagulants (NOACs: dabigatran, rivaroxaban, apixaban, and edoxaban) have demonstrated non-inferior to VKAs with regard to preventing thrombosis and have a lower risk when it comes to intracranial hemorrhage [3, 4, 5]. Persistent monitoring, constricted therapeutic window, and multiple interactions with food or medicine have contributed to the growing use of NOACs [6, 7]. Again, at the moment, NOACs are preferred compared to VKAs for the prevention of ischemic stroke as per ESC (European Society of Cardiology), ACC (American College of Cardiology), NICE (National Institute for Health and Clinical Excellence) guidelines [8, 9].
The first NOAC, ximelagatran, was withdrawn from the market due to an increased rate of hepatotoxicity, causing heightened concerns about liver function adverse effects during NOACs use [10, 11]. To date, quite a few cases of hepatic impairment associated with dabigatran, rivaroxaban, and apixaban have been published [12, 13, 14]. However, no ad hoc randomised controlled trials (RCTs) have addressed this issue. A meta-analysis including 29 RCTs showed that NOACs had an increased risk of drug-induced liver injury compared with the control group [15]. While, the narrow definition of liver impairment, limited inclusion of only RCTs, and the combined controls (including placebo, no-treatment, standard care, non-pharmacological interventions, and any drug) may have resulted in an underestimated or overestimated risk of hepatic impairment.
Real-world studies (RWSs) that integrate data related to health status, diagnosis and care of the research object in real-world practice have commonly been considered weaker evidence level than RCTs. However, there is raised awareness that RWSs could expand RCT findings to large patient populations in the real-world environment. Meanwhile, regarding drug-related adverse reactions, data from the Food and Drug Administration Adverse Event Reporting System (FAERS) database could help to verify and supplement the findings of RCTs [16, 17, 18, 19]. To clearly illustrate the association between NOACs and hepatic impairment, we conducted a comprehensive assessment by summarising all evidence from RCTs and RWSs, as well as by performing a disproportionality analysis based upon the FAERS database.
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [20], we electronically searched the PubMed, Embase, and Cochrane Library databases from their inception to August 2021. Detailed search strategies are presented in Supplementary Table 1. The reference lists of the identified records were also checked to determine the eligible articles. Studies were included if they met the following criteria: (I) RCTs or RWSs; (Ⅱ) indication for NOACs was NVAF; (III) with available data reporting liver outcome; and (IV) published in the English language. The RWSs should be based on multicenter or health insurance data offering adjusted or matched results. Abstracts, letters, case reports, one-arm studies, or studies that reported unmatched or unadjusted results were excepted. Two independent reviewers (Z.G. and J.W.) conducted literature screening. Any disagreements were discussed with a third author (B.Z. or Z.L.).
The primary outcomes were
hepatic impairment identified by diagnostic liver injury (DLI)
or abnormal liver enzyme (ALE). The DLI included the clinical
diagnosis of liver injury (including liver disorder, liver failure, hepatitis,
chronic hepatitis, acute hepatitis, ischaemic hepatitis, hepatopathy, hepatic
failure, hepatic congestion, and hepatic steatosis, etc.). ALE was defined as an
increase in the serum levels of transaminases, particularly alanine
aminotransferase (ALT, normal reference range 0–40 U/L) or aspartate
aminotransferase (AST, normal reference range 0–40 U/L)
The following data of included studies were extracted based on the previously designed format: (1) study characteristics; (2) patient demographics; (3) clinical characteristics; and (4) data of recorded hepatic outcomes. Specific information not presented in the publications was further obtained from the website of ClinicalTrials.gov (http://clinicaltrials.gov). The methodological quality of the selected RCTs was appraised with the Cochrane criteria [23, 24]. The bias risk of RWSs was evaluated according to the following four dimensions: methods to adjusting selection bias, existence of residual confounding, approaches to handle covariates regarding time-varying and information and selective reporting of study outcomes [25].
Data analyses were conducted using the STATA software (version 13,
Statacorp, College Station, TX, USA). We
applied forest plots to measure the pooled results for RCTs, and the
random-effects model was used to calculate relative risks (RRs) and their 95%
confidence intervals (CIs). For RWSs, we calculated adjusted hazard ratios (aHRs)
and 95% CIs through random-effects models to estimate the pooled results.
Significant heterogeneity among studies was defined by the Higgins inconsistency
index (I
We performed a retrospective pharmacovigilance study using data from the FAERS database from 2004 Quarter 1 (Q1) to 2021 Q1. MICROMEDEX (Index Nominum) was used as a dictionary for NOACs and VKAs names. Both generic and brand names were used as keywords for FAERS database retrieval. Preferred terms (PTs) refer to the recommended medical terminology that describes the event. Using the Medical Dictionary for Regulatory Activities (MedDRA) v24.0, we subtotalled all hepatic impairment PTs in the adverse events (REAC) files (Supplementary Table 2). Exposure assessment was considered when NOACs were recorded as ‘primary suspect’. The time to onset of adverse events was defined as the start date of NOACs administration to the date of outcome onset.
We firstly performed a descriptive analysis to summarize the clinical characteristics of the patients with hepatic impairment. The primary disproportionality analyses were next carried out by case non-case approach [29], which is primarily considered a case-control study, in which controls are all reports in FAERS unrelated to the event of interest (i.e., DLI) [30]. We adopted the reporting odds ratio (ROR) to identify statistical associations between NOACs and hepatic impairment under the diagnosis of NVAF [30]. The ROR is the proportion that compared the odds of an object drug with the other drugs reported in the FAERS database, and the odds are the one particular events versus all other adverse events. ROR was considered statistically significant when a lower limit of 95% CI exceeded one [31, 32]. Scenario analyses were also performed as follows: (I) given that the excessive reporting of bleeding events for anticoagulants may mask alerts that arise from other events, namely event competition effect [33], all reports that mentioned bleeding were removed; (Ⅱ) considering the fact that bleeding could be a clinical manifestation of liver injury, the reports that simultaneously recorded hepatic impairment and bleeding were retained; (Ⅲ) concomitant drugs (including hepatotoxic agents, anti-hepatitis agents, and potential drug interactions with NOACs, as listed in Supplementary Table 3) may underestimate or overestimate the signal detection results of NOACs-related hepatic impairment. Therefore, the reports that involved concomitant drugs were removed. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Our original search identified 622 records from the databases, 467 papers were
excluded after screening the titles and abstracts, and 57 articles underwent
full-text review. Ultimately, 9 studies (5 RCTs and 4 RWSs) fulfilled the
inclusion criteria [34, 35, 36, 37, 38, 39, 40, 41, 42] (Fig. 1). In the RCTs, 42,858 patients took NOACs,
and 29,862 patients took VKAs. The follow-up period ranged from 1.8 to 2.8 years
(Supplementary Table 4). In the RWSs, 238,035 patients were prescribed
NOACs, and 249,118 patients were prescribed warfarin. Propensity score matching
and inverse probability of treatment weights of propensity scores were applied to
correct confounding factors (Supplementary Table 5). As shown in
Supplementary Tables 6,7, variables such as sex, heart failure,
hypertension, diabetes mellitus, stroke, and myocardial infarction were
significant different between RCTs and RWSs (p
 Fig. 1.
Fig. 1.Flow chart for selection of eligible studies. HR, hazard ratio; NOACs, non-vitamin K antagonist oral anticoagulants; RCTs, randomized controlled trials; RWSs, real-world studies; U.S.A., United States of America.
For RCTs, NOACs use was not associated with an increased risk of DLI (RR: 0.96,
95% CI: 0.73–1.28, I
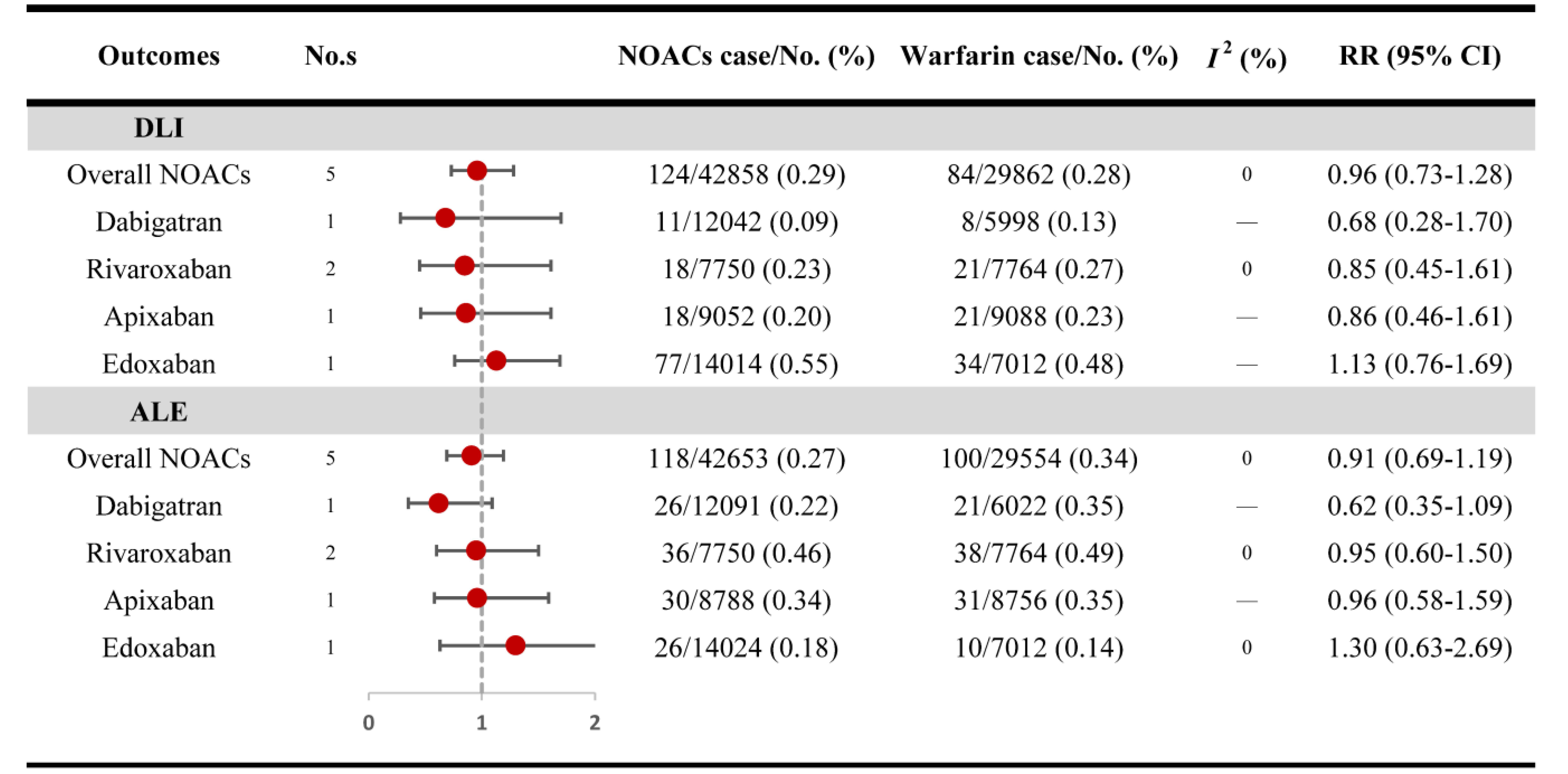 Fig. 2.
Fig. 2.Risk of DLI and ALE in RCTs. DLI, diagnostic liver injury; ALE, abnormal liver enzyme; RCTs, randomized controlled trials; RR, relative risk; CI, confidence interval; NOACs, non–vitamin K antagonist oral anticoagulants.
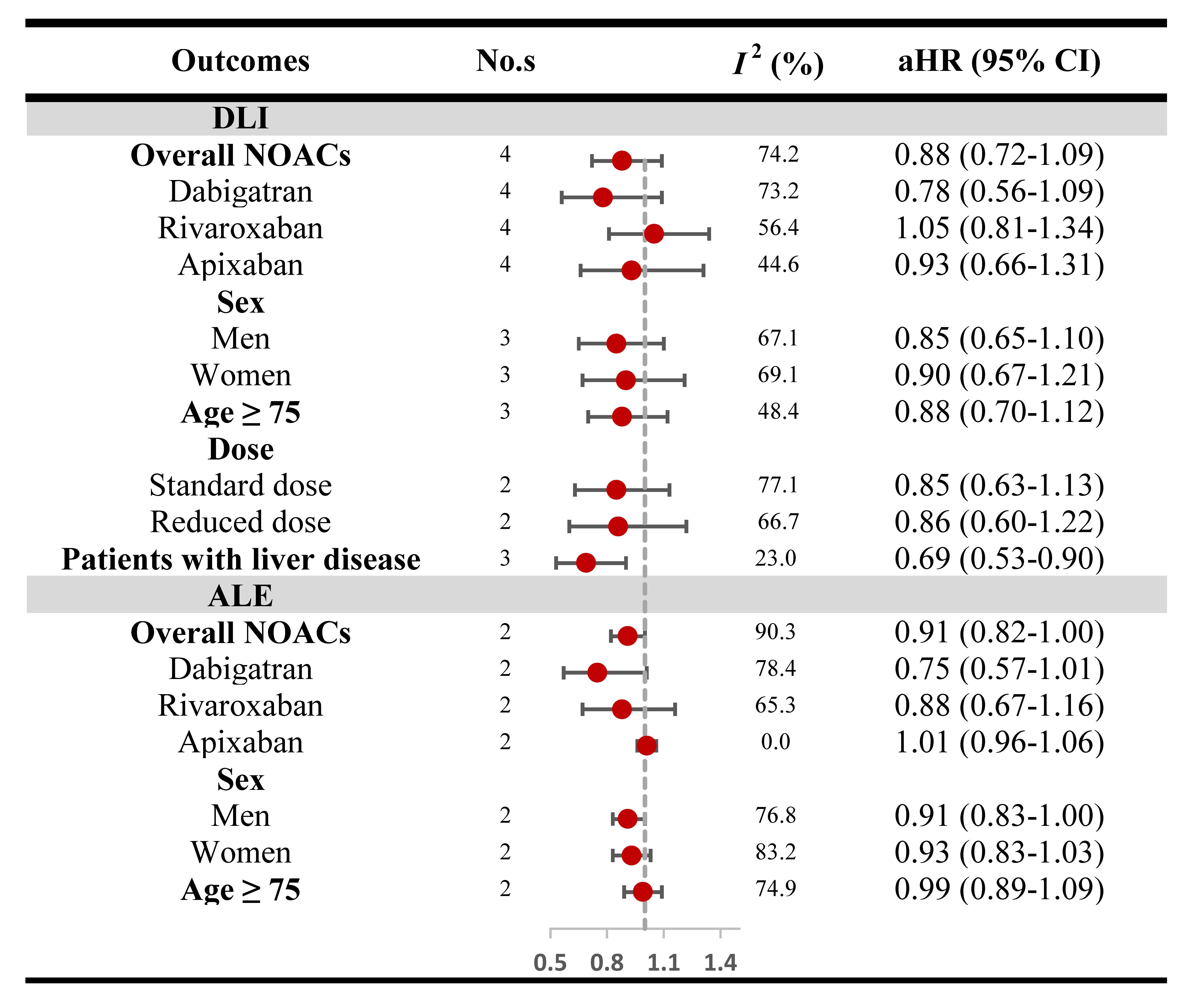 Fig. 3.
Fig. 3.Risk of DLI and ALE in RWSs. DLI, diagnostic liver injury; ALE, abnormal liver enzyme; RWSs, real-world studies; aHR, adjusted hazard risk; CI, confidence interval; NOACs, non–vitamin K antagonist oral anticoagulant.
A total of 1265 reports that used NOACs or VKAs therapy in patients with NVAF
and related to hepatic impairment were documented in the FAERS database from
January 2004 to March 2021. As shown in Supplementary Table 12, 464
(36.68%), 559 (44.19%), 84 (6.64%), 54 (4.27%), and 104 (8.22%) patients
were on dabigatran, rivaroxaban, apixaban, edoxaban, and
warfarin, respectively. Hepatic impairment adverse events were most commonly
reported in patients aged
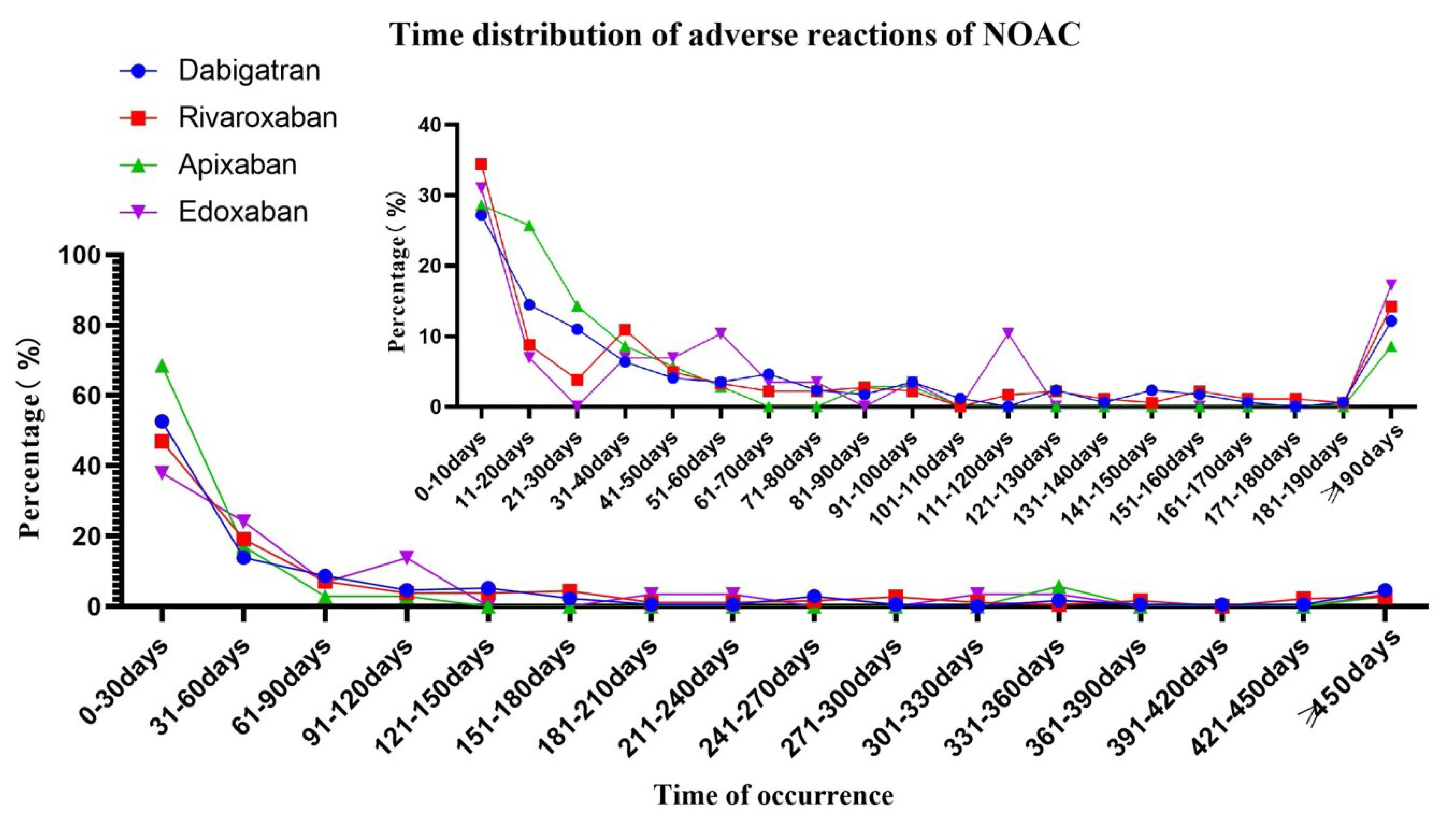 Fig. 4.
Fig. 4.Time distribution of hepatic impairment with NOACs. NOACs, non-vitamin K antagonist oral anticoagulants.
Overall, the primary disproportionality analysis failed to detect signals of hepatic impairment, both for NOACs (ROR: 0.34, 95% CI: 0.32–0.37) and warfarin (ROR: 0.37, 95% CI: 0.31–0.46). Also, no signals were detected in the subgroup analyses, including individual NOACs, sex, older people, and different definitions of hepatic impairment (Table 1 for NOACs and Supplementary Table 13 for warfarin). Scenario analyses are presented in Supplementary Table 14. In scenario one, event competition bias was unconfirmed after the cases with bleeding were removed. No valuable signal was observed in scenario two after retaining reports that simultaneously recorded hepatic impairment and bleeding. In scenario three, the results were also consistent with the primacy disproportionality analysis after removing the reports containing concomitant hepatotoxic agents, anti-hepatitis agents, and potential drug interactions with NOACs.
| Outcomes | N | ROR (95% CI) | |
| Overall NOACs | 1161 | 0.34 (0.32–0.37) | |
| Individual NOACs | |||
| Dabigatran | 464 | 0.46 (0.42–0.51) | |
| Rivaroxaban | 559 | 0.41 (0.37–0.44) | |
| Apixaban | 84 | 0.94 (0.75–1.17) | |
| Edoxaban | 54 | 1.00 (0.76–1.31) | |
| Sex | |||
| Men | 577 | 0.36 (0.33–0.40) | |
| Women | 493 | 0.31 (0.28–0.35) | |
| Age |
484 | 0.38 (0.34–0.42) | |
| Narrow outcome definitions | |||
| DLI | 610 | 0.39 (0.36–0.43) | |
| ALE | 650 | 0.31 (0.28–0.34) | |
| Hepatic failure | 229 | 0.50 (0.42–0.59) | |
| NOACs, Non-vitamin K antagonist oral anticoagulants; ROR, reporting odds ratio; CI, confidence interval; DLI, diagnostic liver injury; ALE, abnormal liver enzyme. | |||
Oral anticoagulant treatment is the principal priority for patients with NVAF [43]. It is well known that all NOACs undergo some degree of hepatic metabolism [44, 45], and previous studies are controversial as to whether NOACs use increases the risk of hepatic impairment in patients with NVAF [15, 34, 35, 36, 37]. Therefore, it is crucial to understand the influence of NOACs on liver function. In the present study, we performed a comprehensive assessment to examine this issue based on the pooled analysis of RCTs and RWSs, as well as a disproportionality analysis of the pharmacovigilance database. Our main findings were: (I) in RCTs, NOACs use was not related to an increased risk of ALE and DLI versus VKAs; (II) the pooled results based on RWSs were in line with RCTs; (III) no hepatic impairment signal was detected for NOACs in the pharmacovigilance study; (IV) the above results were also tenable in individual NOACs.
Although seemingly rare, case reports and case series have described hepatic
impairment linked to the use of NOACs [12, 13, 14, 46]. Unfortunately, issues
containing widespread underreporting of adverse events and selectively submitting
recently approved drugs may lower the quality of these
findings. One systematic review has assessed the liver injury risk of NOACs [15].
However, the data of this review only drew from phase III RCTs and not from
individual patients, which is a potential source of bias. Several RWSs concerning
the risk of hepatic impairment in patients with NVAF receiving NOACs have been
presented recently. Alonso et al. [34] found that compared with VKAs,
dabigatran, rivaroxaban, and apixaban reduced the liver injury risk by 43%,
12%, and 30%, respectively. However, the included participants were without
distinction as to whether they had prior liver disease. Subsequent studies
improved their study design by considering patients with liver disease history
[34, 35]. Notably, neither of these studies used liver enzyme elevations to
identify hepatic impairment, which might reduce the sensitivity of the outcome.
Until recently, Zhao et al. [37] adopted a laboratory test outcome as an
objective measure for the definition of liver injury. Based on the above
limitations, we carried out a reliable pooled analysis, which featured a large
number of patients, comprehensive outcome definitions, and considerate subgroups
to obtain a robust conclusion. The baseline characteristics of RCTs and RWSs
differed, while NOACs showed no increased risk of DLI and ALE compared to
warfarin in both RCTs and RWSs, thereby supporting and extending the RCT findings
to large patient populations in real-world clinical practice. Certainly, it is
worth noting that I
The FAERS database represents a primary source of information for detecting rare adverse event signals [47]. However, post-marketing data based on FAERS concerning the hepatic impairment of NOACs are limited. Recently, Raschi et al. [48] found a disproportionality signal of drug-induced liver injury for rivaroxaban, which was not emerged for dabigatran, based on the FAERS database. However, compared with the United States prescribing information and previous pre-marketing data on phase III studies [49], the ROR of rivaroxaban (ROR: 2.08, 95% CI: 1.34–3.08) was higher than expected, approximating drugs with warning for liver injury. Furthermore, the conclusions of apixaban and edoxaban are still unclear due to insufficient reports. Hence, we updated a disproportionality analysis based on the reports extracted from the FAERS database. Meanwhile, we analyzed the reporting patterns of NOACs in actual clinical application scenarios. Our data indicate that nearly a quarter of reports mention bleeding, and a considerable amount of hepatic impairment reports combined hepatotoxic or interacting drugs, which is consistent with a current study published by McDonald et al. [50] found a large part of adverse events associated with dabigatran were due to concomitant prescription. Thus, we finally indicated no positive signals for NOACs, even when the serial scenarios were tested. Another finding from the pharmacovigilance study was that the occurrence time of hepatic impairment related to NOACs was primarily within three months, indicating that we should closely monitor liver function early after NOACs administration. In contrast, adverse liver events due to NOACs are increasingly rare over time, which may be favorable for patients with NVAF receiving long-term medication.
In terms of individual NOACs, dabigatran, rivaroxaban, apixaban, and edoxaban were all found to have no significant difference in hepatic impairment compared with VKAs. According to the research, aging is associated with the severity and poor prognosis of various liver diseases due to the lost ability to maintain homeostasis [51]. Meanwhile, pharmacokinetic research found that dabigatran has a higher maximum serum concentration and the area under the curve in women than in men [44]. Women are identified to suffer more drug-induced liver injury than men [52, 53]. In addition, different dosages of NOACs directly affect the blood concentration of drugs. Therefore, we also focused on sex, older people, and different dosages of NOACs. Again, we obtained the same conclusion. Of note, our current study indicated that NOACs had a greater decline in the risk of DLI than warfarin in patients with a history of liver disease. The different pharmacokinetic profiles of oral anticoagulants may help explain this finding [44]. Compared with warfarin, which is nearly 100% hepatically eliminated, approximately 20%, 65%, 75%, and 50% of dabigatran, rivaroxaban, apixaban, and edoxaban, respectively, were found to be eliminated by the liver [54]. Thus, the active concentration of NOACs was lower in patients with impaired liver function than warfarin, partly explaining the decreased occurrence of hepatic impairment in these fragile patients. Clinical trials of NOACs in patients with liver disease are scarce because patients are often excluded if their baseline liver function is abnormal [37, 38, 39, 40, 41]. Thus, studies are restricted to small cohort studies, and no meta-analysis has been published [34, 35].
Our study has several limitations. First, all of the RCTs included were not specially designed to assess the adverse reaction of NOACs on liver function. Second, the definitions of ALE and DLI were not uniform, likely making the bias of the reported incidence. Third, the methods used to adjust various confounding factors in RWSs were different, posing a challenge for comparability among studies. Fourth, baseline liver function, especially Child-Pugh class, cannot be extracted due to the data deficiency. Finally, the follow-up of four RWSs was relatively insufficient, possibly underestimating the effects of NOACs on liver function. In addition, there are several inherent limitations in pharmacovigilance studies. Primarily, the shortcomings of the FAERS database, such as underreporting, incomplete reporting, false reporting, arbitrariness, and inaccuracy, cannot be avoided by data mining technology. Meanwhile, disproportionality analysis cannot quantify adverse reactions and prove a causal relationship. Nonetheless, our study has several strengths. We comprehensively evaluated the relationship between NOACs and hepatic impairment using a dual approach of pooled analysis and pharmacovigilance studies. In addition, DLI outcome definition alone may result in low positive predictive values due to the misclassification of outcomes [55], we also interpreted hepatic impairment in terms of ALE. Meanwhile, in the disproportionality analysis, we controlled for confounding factors such as events and drug competition that might impact the rate of adverse liver events.
Insights from multi-source medical data confirmed that NOACs use was not associated with an increased risk of hepatic impairment in patients with NVAF. Further well-designed RCTs are needed to confirm these findings.
The data that support the findings of this study are available on reasonable request from the corresponding authors.
ZLL and BZ are guarantors of the entire manuscript. ZCG and JW contributed to the study conception and design, critical revision of the manuscript for important intellectual content. CZ contributed to the data acquisition, analysis, and interpretation. All the authors have read and approved the final version of this manuscript.
Not applicable.
Not applicable.
This work was supported by the Clinical Research Innovation and Cultivation Fund of Ren Ji hospital (RJPY-LX-008), the Development Fund for Shanghai Talents (2020110), Research Project of Drug Clinical Comprehensive Evaluation and Drug Treatment Pathway (SHYXH-ZP-2021-001), Ren Ji Boost Project of National Natural Science Foundation of China (RJTJ-JX-001), and Shanghai “Rising Stars of Medical Talent” Youth Development Program – Youth Medical Talents – Clinical Pharmacist Program (SHWJRS (2019)_072).
The authors declare no conflict of interest.
