Academic Editor: Ezra Abraham Amsterdam
We present a case series of three patients that underwent myocardial contrast echocardiography (MCE) in the setting of recent chest pain, as paradigmatic examples of the usefulness of contrast-echocardiography with very-low mechanical index imaging in the context of rest wall motion assessment. Moreover, we analysed the pertinent literature about the use of rest MCE in the context of chest pain of unknown origin, showing its diagnostic and prognostic impact. We think that MCE could play a key role in detecting chest pain subtended by previously unknown coronary artery disease (CAD). For example, in pts without significant electrocardiogram (ECG) modifications or in whom high sensitivity troponins show only borderline increase (still below the upper limit) or have no clearly significant delta. In such cases the more sensitive evaluation of wall motion (WM) powered by MCE could add diagnostic information, above all in pts with severe CAD but apparently normal WM at standard echocardiography.
In this case series we present three patients (pts) that underwent myocardial contrast echocardiography (MCE) in the setting of recent chest pain, as paradigmatic examples of the usefulness of contrast-echocardiography with very-low mechanical index imaging in the context of rest wall motion assessment.
The first patient was a 57 years old man with no cardiovascular risk factors who presented to the emergency department (ED) due to chest pain on effort and also at rest. The ECG showed mild abnormalities on the anterior leads, and there was a mild increase of the troponin I levels. At transthoracic echocardiography (TTE) left ventricular wall motion (WM) was apparently normal with no regional abnormalities.
The second patient was a 40-year-old man with hypercholesterolemia who had chest pain on effort and again also at rest. In this case the ECG was unremarkable and the high sensitivity troponin I was between the limit of detection (LOD) and upper reference limit (URL) with no significant delta between two serial measurements. Again, at TTE there was an apparently normal segmental WM of the left ventricle.
Last patient was a 58-year-old woman with obesity and hypertension who was evaluated at the outpatients clinic for exertional chest pain with few episodes at rest. The ECG was unremarkable and the troponin was not measured at that time. TTE was apparently normal also in this case.
Since the symptoms were very typical in all the three cases and the suspect of coronary artery disease (CAD) was high, we integrated the TTE with contrast administration for better WM assessment. In all the cases, the more accurate evaluation of endocardial border could reveal WM abnormalities in the Left Anterior Descending (LAD) territory: in particular, in case 1 they involved the anterior mid-to-distal wall, the apex and the distal septum (see Fig. 1 for details, and Supplementary Videos 1,2), in case 2 the distal anterior wall, the apex and the distal septum (see Fig. 2 and Supplementary Videos 3,4), in case 3 the mid-to-distal septum, the apex and the latero-apical wall (see Fig. 3 and Supplementary Videos 5,6).
 Fig. 1.
Fig. 1.TTE of patient 1 comparing standard views (at the top) with MCE (below). MCE shows WM and MP abnormalities in the anterior mid-to-distal wall, the apex and the distal septum (see arrows). 4CH, 4-chambers view; 2CH, 2-chambers view; 3CH, 3-chambers view; IS, Infero-septal wall; AL, antero-lateral wall; I, Inferior wall; A, Anterior wall; IL, infero-lateral wall; AS, antero-septal wall.
 Fig. 2.
Fig. 2.TTE of patient 2 comparing standard views (at the top) with MCE (below). MCE shows WM and MP abnormalities in distal anterior wall, the apex and the distal septum (see arrows). 4CH, 4-chambers view; 2CH, 2-chambers view; 3CH, 3-chambers view; IS, Infero-septal wall; AL, antero-lateral wall; I, Inferior wall; A, Anterior wall; IL, infero-lateral wall; AS, antero-septal wall.
 Fig. 3.
Fig. 3.TTE of patient 3 comparing standard views (at the top) with MCE (below). MCE shows WM and MP abnormalities in the mid-to-distal septum, the apex and the latero-apical wall (see arrows). 4CH, 4-chambers view; 2CH, 2-chambers view; 3CH, 3-chambers view; IS, Infero-septal wall; AL, antero-lateral wall; I, Inferior wall; A, Anterior wall; IL, infero-lateral wall; AS, antero-septal wall.
Moreover, even if the use of small boluses of contrast is not ideal for the
study of myocardial perfusion (MP), the assessment of WM using real-time very low
mechanical index (
All the three-pts had severe left anterior descending coronary artery (LAD) stenosis which turned out to be sub-occlusive in all of the three pts. The first two pts underwent coronary angiography (Figs. 4,5), whereas the last patient underwent computed tomography (Fig. 6 and Supplementary Video 7) and will soon undergo coronary angiography as well.
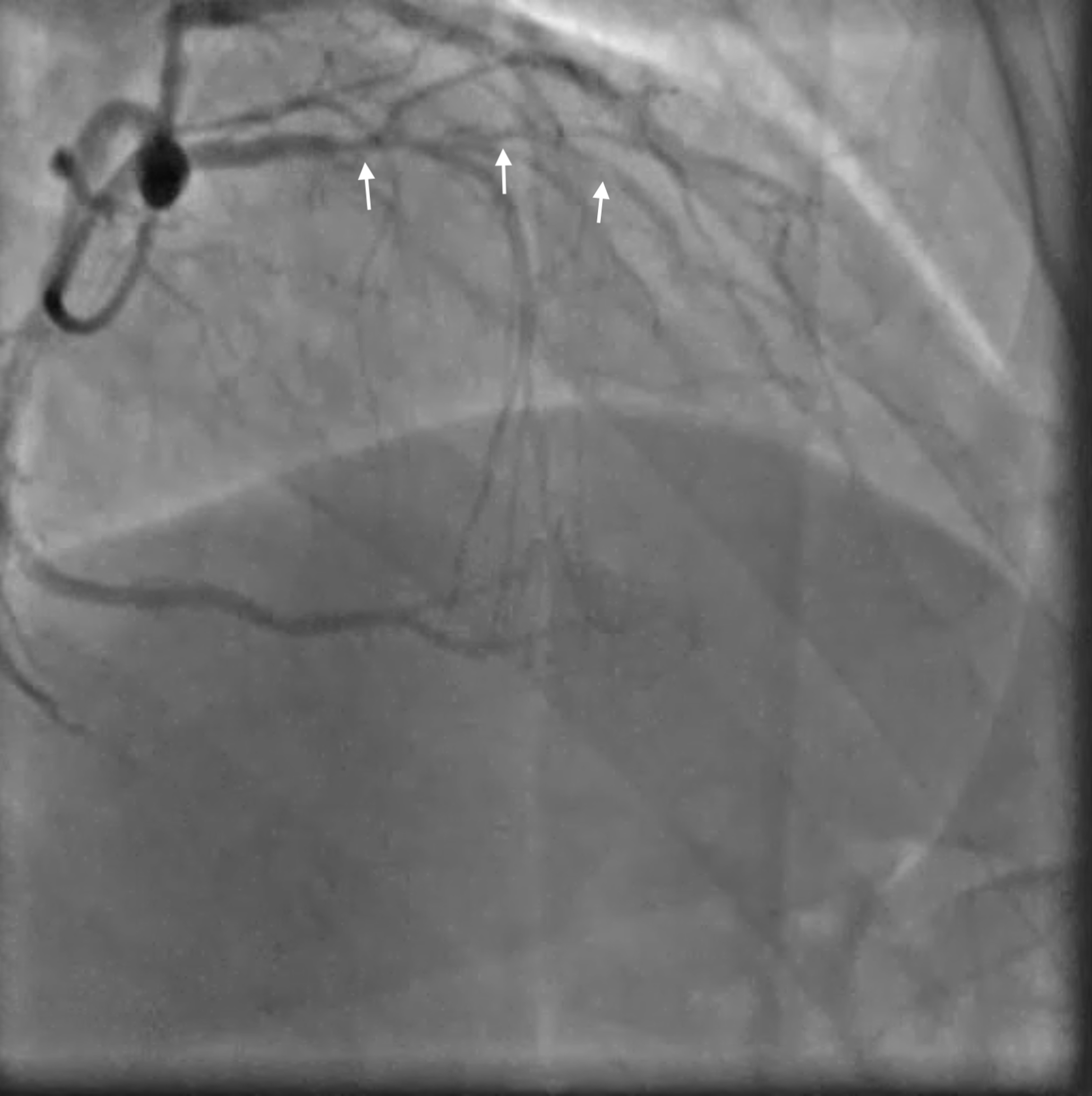 Fig. 4.
Fig. 4.Coronary Angiogram in patient 1. The angiogram shows severe sub-occlusive multiple stenosis (arrows) in the LAD course.
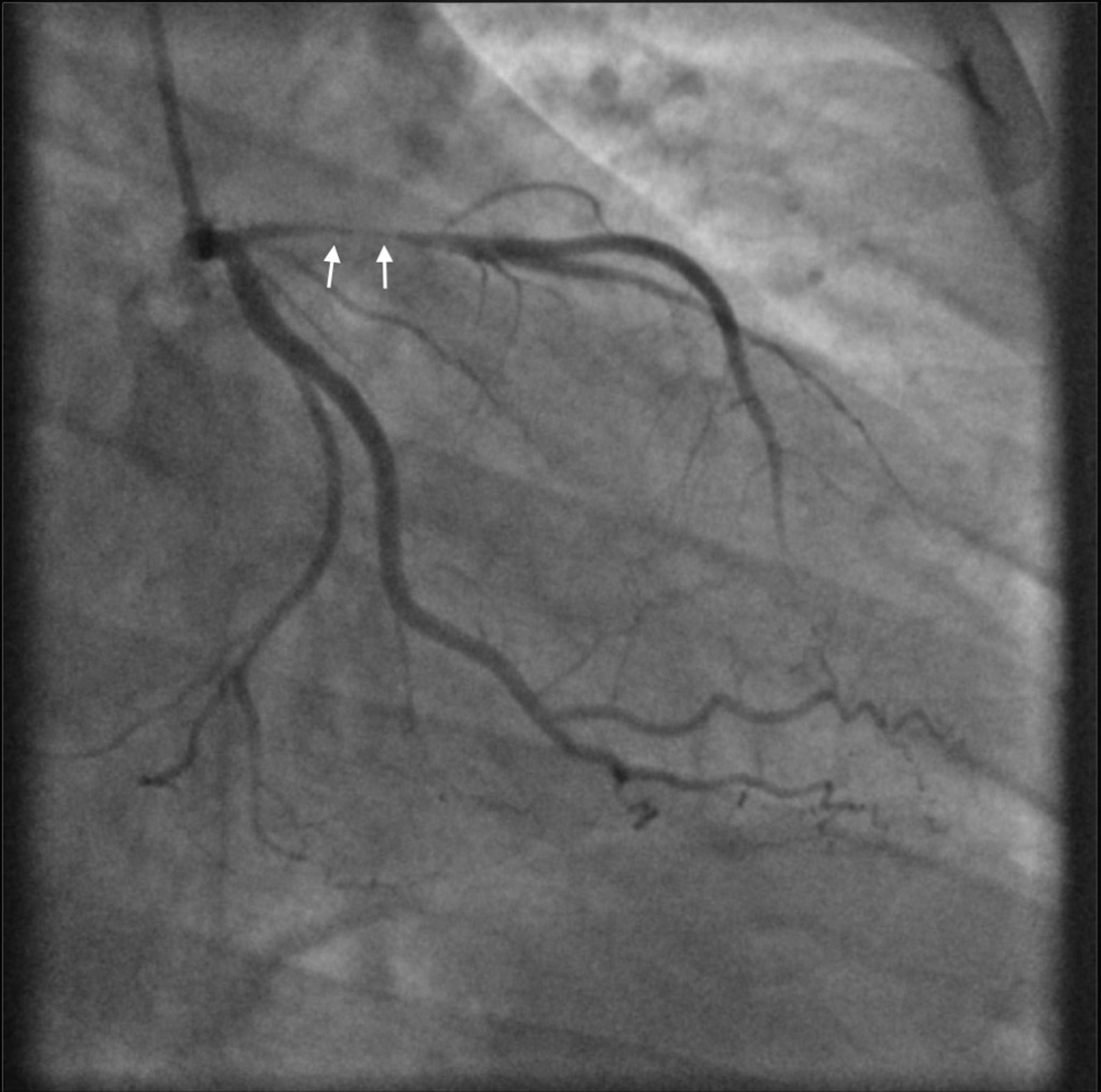 Fig. 5.
Fig. 5.Coronary Angiogram of patient 2. The angiogram shows a sub-occlusive focal proximal stenosis of the LAD (arrows).
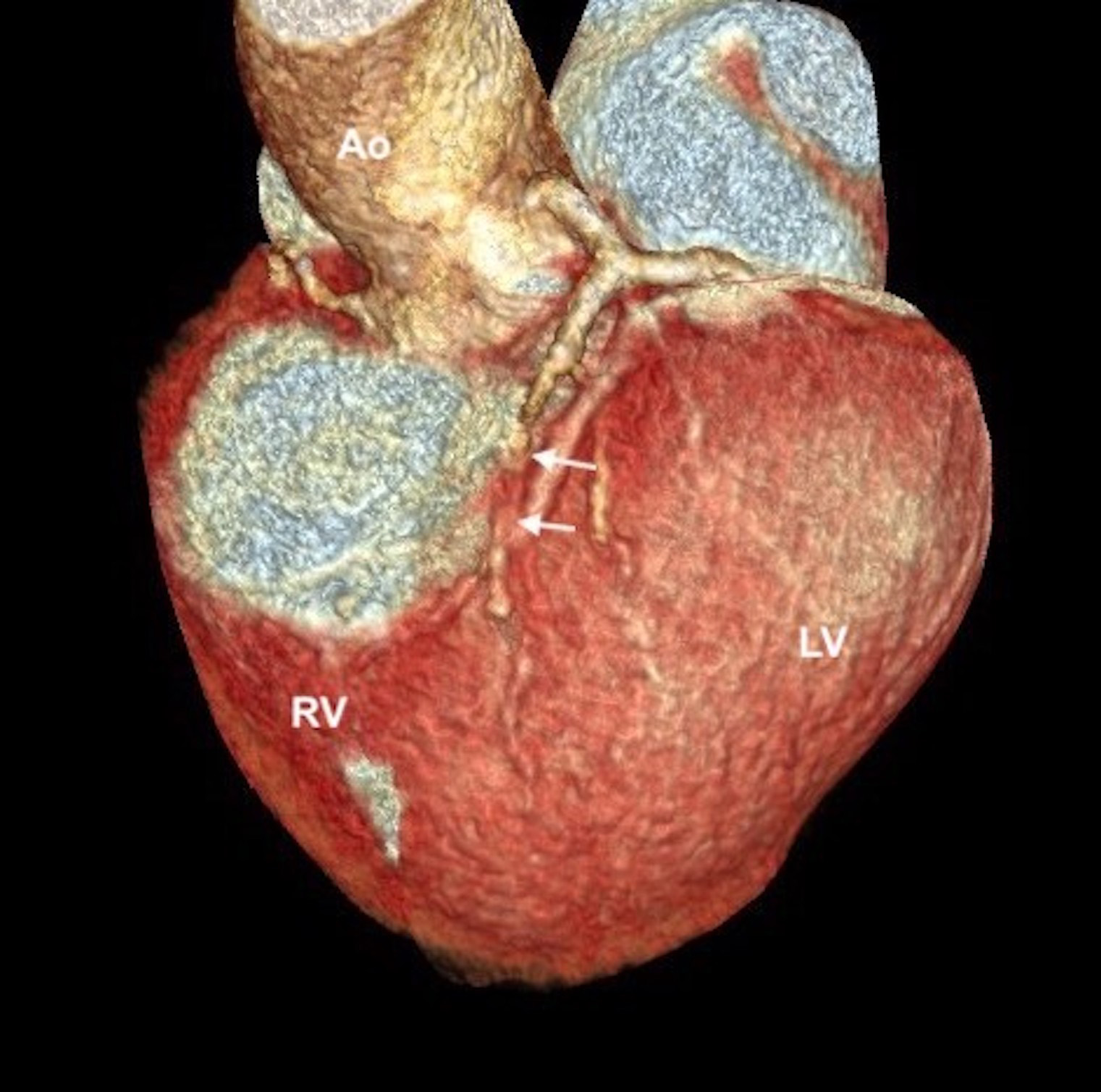 Fig. 6.
Fig. 6.Computed Tomography of patient 3 showing a sub-occlusive stenosis in the mid-LAD (arrows). Ao, Aorta; LV, Left Ventricle; RV, Right Ventricle.
The use of rest MCE in the context of chest pain was evaluated in different studies either or both for WM and MP assessments (Table 1, Ref. [1, 2, 3, 4, 5, 6]). The studies that evaluated only MP were not included in our review.
| Study/Year | N° patients | Setting | Contrast agent | Contrast Infusion modality | Contrast echo modality for wall motion | WM and/or MP evaluation | Comparator/Endpoint |
| Rinkevich et al. 2005 [1] | 1017 | CP | Optison | Continuous infusion | Real-time | WM and MP | MACE |
| MI | |||||||
| Tong et al. 2005 [2] | 957 | CP | Optison | Continuous infusion | Real-time harmonic unknown MI | WM and MP | mTIMI score |
| Wei et al. 2010 [3] | 1166 | CP | Optison | Continuous infusion | Real-time | WM and MP | MACE |
| MI | |||||||
| Kalvaitis et al. 2006 [4] | 957 | CP | Optison | Continuous infusion | Real-time | WM and MP | Time/MACE |
| MI | |||||||
| Porter et al. 2013 [5] | 2014 | CP | Definity | Continuous infusion | Both real-time | WM and MP | MACE |
| MI | |||||||
| ACS, Acute Coronary Syndrome; CP, Chest pain of unknow origin with no ST-segment elevation at the ECG; MI, mechanical index; WM, wall motion; MP, myocardial perfusion; MACE, Major adverse cardiovascular events. Meta-analysis [6] was not included in the tab. | |||||||
Rinkevich et al. [1] studied the MCE in predicting events in pts with chest pain (CP) who presented to the emergency department (ED) with non ST-elevation at the ECG; in particular they analysed 1017 pts, assessing both regional WM and MP with MCE in addition to the standard ECG evaluation, with a mean follow-up of 7.7 months. Considering only rest WM data, which is the main purpose of the current review, 43 pts with normal WM had events and 249 events (85% of all events) took place in patients with rest WM abnormalities assessed with contrast.
On the multivariable Cox regression analysis, history of hypertension
(p = 0.028), ECG (p = 0.0001), WM (p
They concluded that early assessment of WM (and MP) on MCE added significant diagnostic and prognostic value to routine evaluation in pts presenting to the ED with suspected cardiac CP and no ST-segment elevation. There was no standard (without contrast) echocardiography included in the standard clinical comparison control, so that MCE for rest WM assessment was compared to clinical assessment and ECG only. Furthermore, Troponin assessment was not included in the study.
Tong et al. [2] compared WM and MP analysis with modified Thrombolysis
In Myocardial Infarction (TIMI) risk score (mTIMI, which is TIMI score not
including troponin levels) in 957 pts presenting to the ED with CP and a
nondiagnostic ECG. Cumulative pts outcomes were determined at three time points:
early (within 24 hours), intermediate (up to 30 days), and late (
Wei et al. [3] enrolled 1166 pts (cohort 1) with a validation cohort
(cohort 2) of 720 pts; all pts presented to ED with CP lasting 30 minutes or more
and there wasn’t any ST-segment elevation on the ECG. Wall motion (WM) and
myocardial perfusion (MP) were separately assessed by MCE. Any abnormality or ST
changes on ECG (odds ratio [OR] 2.5; 95% confidence interval [CI], 1.4–4.5,
p = 0.002, and OR 2.9, 95% CI, 1.7–4.8, p
Kalvaitis et al. [4] explored the effect of time delay of the use of
MCE in the ED. In particular 957 pts were enrolled, they presented to ED with CP
and no ST-elevation at the ECG and were divided into 4 quartiles depending on the
time between their last episode of CP and the MCE evaluation. Pts in quartile I
had MCE during ongoing CP (time delay of 0 minutes). The time delays in quartiles
II, III, and IV were 54
Wyrick et al. [7] analysed the cost-efficiency of MCE in 957 pts presenting to ED with CP and no ST-elevation at the ECG, but this analysis is most probably conducted on the same patient cohort studied by Kalvaitis et al. [4], so we did not include it in our review.
Porter et al. [5] compared patient outcome after stress real-time MCE
(RTMCE), using very-low mechanical index, versus conventional stress echo with
low mechanical index and harmonic imaging (CSE). Outpatient and inpatient
subjects admitted for chest pain with normal or equivocal troponin underwent
exercise or dobutamine stress echocardiography and were randomized prospectivey
to either RTMCE or CSE. For CSE they used definity contrast when the delineation
of the endocardial border was not adequate (63% of the studies). 2014 pts were
evaluated with a mean follow-up of 2.6 years. At peak stress it was observed more
frequently an abnormal RTMCE then an abnormal CSE (p
Finally, Qian et al. [6] made a meta-analysis about prognostic value of resting MCE evaluating both WM and MP. Seven studies met criteria, including 3668 patients. When patients had abnormal MP and WM, the relative risk (RR) to predict MACE was 6.1 (95% CI, 5.1–7.2) and 14.3 (95% CI, 10.3–19.8) for death/non-fatal myocardial infarction compared to patients with normal MP and WM. This was true also for patients with abnormal MP and WM in comparison with abnormal WM and normal resting MP to predict MACE (RR, 1.7; 95% CI, 1.5–1.9) and death/non-fatal myocardial infarction (RR, 2.2; 95% CI, 1.8–2.7) when compared to abnormal WM with normal resting MP.
Contrast agents in conjunction with very low MI contrast real-time imaging increase the accuracy of WM assessment, both through endocardial border enhancement [10, 11, 12, 13, 14, 15] and by simultaneously providing collateral information on MP (MP defects always precede WM abnormalities) [16, 17, 18, 19, 20, 21, 22, 23], which in turns enhances the visual capability to detect a WM abnormality, if present. This can be particularly helpful in cases of poor acoustic windows, as well as in cases of difficult evaluation of the anterior wall and of the apex. In this not-unusual context, MCE could be of great interest in the routine evaluation of wall motion in pts with chest pain of uncertain origin. Indeed, our cases were paradigmatic examples of how very-low-MI MCE setting could reveal WM abnormalities not obvious at the first evaluation at the TTE without contrast. MCE in these cases changed the clinical management of these pts moving up the way for revascularisation.
In fact, the importance and usefulness of MCE for better rest WM assessment in the evaluation of CP has been only partially demonstrated in the studies reported above, in terms of risk stratification, diagnostic and prognostic impact as well as cost-efficiency. Most such studies were actually performed several years ago, most were single-centre, most used echocardiography machines not anymore commercially available (as it is also the case for the contrast media used) and they used impractical long continuous infusion of contrast. Furthermore, no study compared the usefulness of an enhanced evaluation of WM by MCE with the standard evaluation of WM by standard echocardiography (with no contrast), which is probably the most compelling practical clinical issue.
This could be of great interest in the context of the ED for the evaluation of CP of unknown origin, even in the current era of high-sensitivity troponins, beyond the already defined applications of MCE in stress-echocardiography [24, 25, 26, 27].
Indeed, we think that there remain many grey-cases in the daily routine practice, in which MCE could play a key role in detecting chest pain subtended by previously unknown CAD. For example, in pts without significant ECG modifications or in whom high sensitivity troponins show only borderline increase (still below the upper limit) or have no clearly significant delta. In such cases the more sensitive evaluation of WM powered by MCE could add diagnostic information, above all in pts with severe CAD but apparently normal WM at standard echocardiography.
In conclusion, more and contemporary studies are warranted to confirm the usefulness of MCE in pts with CP despite the availability of high sensitivity troponins; as shown in the reported cases, we believe that MCE, through the detection of otherwise apparently and falsely normal WM could play an important role in the detection of underlying CAD as a cause of acute/subacute CP admitted to the ED.
The small number of cases (3) obviously does not allow the authors to provide any information on the overall accuracy of their method, including sensitivity, specificity, and predictive accuracy.
SS and NG performed the echocardiograms of the clinical cases presented; SS, NG and DT selected the images and the video for the paper; SS, NG and DT performed the review of the pertinent literature. All authors contributed to editorial changes in the manuscript and read and approved the final manuscript.
Not applicable.
We would like to express our gratitude to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest. Nicola Gaibazzi is serving as one of the Guest editors of this journal. We declare that Nicola Gaibazzi had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Ezra Abraham Amsterdam.
