Academic Editor: Jinnette D. Abbott
Objective: The study aims to evaluate the feasibility and
effectiveness of an individualized procedure for right ventricular outflow tract
(RVOT) reconstruction in pulmonary atresia with ventricular septal defect
(PA-VSD). Methods: RVOT was reconstructed using autologous pulmonary
artery tissue preserved in situ as the posterior wall and a bovine jugular vein
patch (BJVP) as the anterior wall in patients with PA-VSD (observation group).
The size of the BJVP made from a bovine jugular vein conduit (BJVC) was
individually calculated using a formula based on the child’s weight and the size
of the autologous pulmonary artery (the diameter of BJVC
D
Pulmonary Atresia with ventricular septal defect (PA-VSD) is a complex cyanotic congenital heart disease that accounts for approximately 2% of all congenital heart diseases with a high mortality rate. Per the Tchervenkov classification, PA-VSD can be divided into three types, given its inherent pulmonary artery development and source of pulmonary blood supply, namely, type A: Patent ductus arteriosus (PDA)-dependent PA-VSD with inherent pulmonary artery and no major aortopulmonary collateral arteries (MAPCAs); type B: PA-VSD with poor developed inherent pulmonary artery and significant major aortopulmonary collateral arteries (MAPCAs); and type C: PA-VSD with no inherent pulmonary artery, where MAPCAs is the only source of pulmonary blood supply. Different surgical strategies should be chosen for different situations, such as systematic-pulmonary shunt, confluence or closure of MAPCAs, a connection from RVOT to pulmonary artery (PA), and repair of VSD.
For PA-VSD that implements corrections from RVOT to PA, though varying surgical interventions over the years have been employed in treating the complex congenital anomaly, its prognosis presently remains poor [1]. In 1955, Lillehei first reported and established a surgical procedure by directly replanting the pulmonary artery to the RVOT [2]. Since then, the disease’s surgical technique and treatment effects have rapidly improved. Also, in 1965, Rastelli used a valveless extracardiac conduit to establish a connection between the right ventricle and the pulmonary artery, although the technique was without its setbacks, such as pulmonary regurgitation (PR) [3].
Hence, to improve the severe setbacks, Ross began reconstructing the RVOT with a homograft aortic valve in 1966 [4]. Ever since the improvements by Ross, several valved conduits have been used in the reconstruction of the RVOT, which advertently solved the issue concerning pulmonary regurgitation to an extent. However, anti-regurgitation effect is the most significant disadvantage for a simple pericardial patch or the Sung technique. Ideally, the patch should be readily available, easy to handle with growth potentials, recellularize, resist regurgitation with remodeling capabilities, and adequately coapt to suture lines for proper hemostasis and lower thrombogenicity [5]. Unfortunately, none of the available patches meet all the criteria above, particularly the growth potential [6].
Furthermore, given the disadvantages of these implanted conduits, such as poor extensions with growth and immune rejection [7, 8], there were high incidence rates of re-interventions following primary surgery. Thus, to improve existing concepts and techniques, the study attempted to preserve and make full use of autologous pulmonary tissue as the posterior wall of the newly built pulmonary artery, preserving the pulmonary artery’s growth potential. The study also aims to uniquely individualize the procedure depending on the patient’s weight and the size of the autologous pulmonary artery to achieve physiological blood flow.
Since 2009, the new procedure was performed for 22 patients at three tertiary institutions, namely the Second Xiangya Hospital of Central South University, Hunan Children’s Hospital, and Hunan Provincial People’s Hospital (Changsha, China). On the other hand, 25 PA-VSD patients underwent the traditional Rastelli procedure, where the BJVC was used to connect the right ventricle and the confluent pulmonary artery (Table 1).
| Observation group | Control group | p Value | |
| Gender (male/female) | 10/12 | 13/12 | |
| Age (month) | 11.82 |
13.16 |
0.155 |
| Weight (kg) | 9.30 |
9.51 |
0.530 |
| oxygen saturation | 74%–91% | 75%–90% | |
| Mcgoon index | 1.48 |
1.50 |
0.648 |
| Diameter of main pulmonary artery (mm) | 5.23 |
5.33 |
0.690 |
| Diameter of whole MAPCAs (mm) | 2.46 |
3.24 |
0.214 |
| Diameter of PDA (mm) | 3.90 |
4.22 |
0.142 |
All patients had severe cyanosis, and their upper and lower limb skin oxygen
saturation ranged from 74% to 91% at rest. The echocardiography (scanner:
Philips EPIQ 7C and probe: S8-3, Amsterdam, The Netherlands) by two experienced
sonographers showed hypertrophy of the right ventricle (RV) and enlarged right
atrium, VSD, and the absence of blood flow from the RV to the pulmonary artery.
Computed tomography (CT) (Siemens Healthcare Corporation, Erlangen, Germany
cardiac imaging was used to confirm the diagnosis further and identify the main
pulmonary artery and its branches, patent ductus arteriosus (PDA), and MAPCAs. To
calculate the Mcgoon index (Mcgoon index
All patients underwent selective catheter angiography (catheters: Johnson Corporation, New Brunswick, New Brunswick, USA; vascular access: right femoral artery) to confirm the PA size and the source of pulmonary blood supply to exclude patients whose MAPCAs provided a single lobe blood flow. The patients denied any family genetic history of cardiovascular disease, and this study did not carry out systematic genetic tests. Consents from all patients and the Institutional Research Ethics Committee were obtained beforehand.
All patients underwent longitudinal sternotomy and a cardiopulmonary bypass
surgical procedure under moderate hypothermia (28–30
For individualized reconstruction of the RVOT, a BJVC of appropriate size was selected by a formula (Fig. 1, details described below), and then the BJVC was longitudinally incised along the valve leaflets junction to avoid damage to the leaflets. After thoroughly examining the valve leaflets, the BJVP was continuously sutured to the posterior autologous PA tissue and the incision on the RV. Keen attention should be paid when placing the valve leaflets of the BJVP at the supposed position of the native pulmonary valve. Following RVOT reconstruction, the autologous pulmonary artery tissue was preserved in situ as the posterior wall for the new pulmonary artery, while the BJVP, covering the autologous pulmonary artery and the RVOT, acted as the anterior wall. On the other hand, the same procedure was performed for the control group before RVOT reconstruction, but the autologous pulmonary artery was then removed, and a whole BJVC was applied to establish the connection between the RV and the PA (Fig. 2).
 Fig. 1.
Fig. 1.Illustration for formula derivation. The figure shows
cross-sections of the BJVC for reconstruction and the reconstructed PA. The
cross-sectional circumference of the BJVC L =
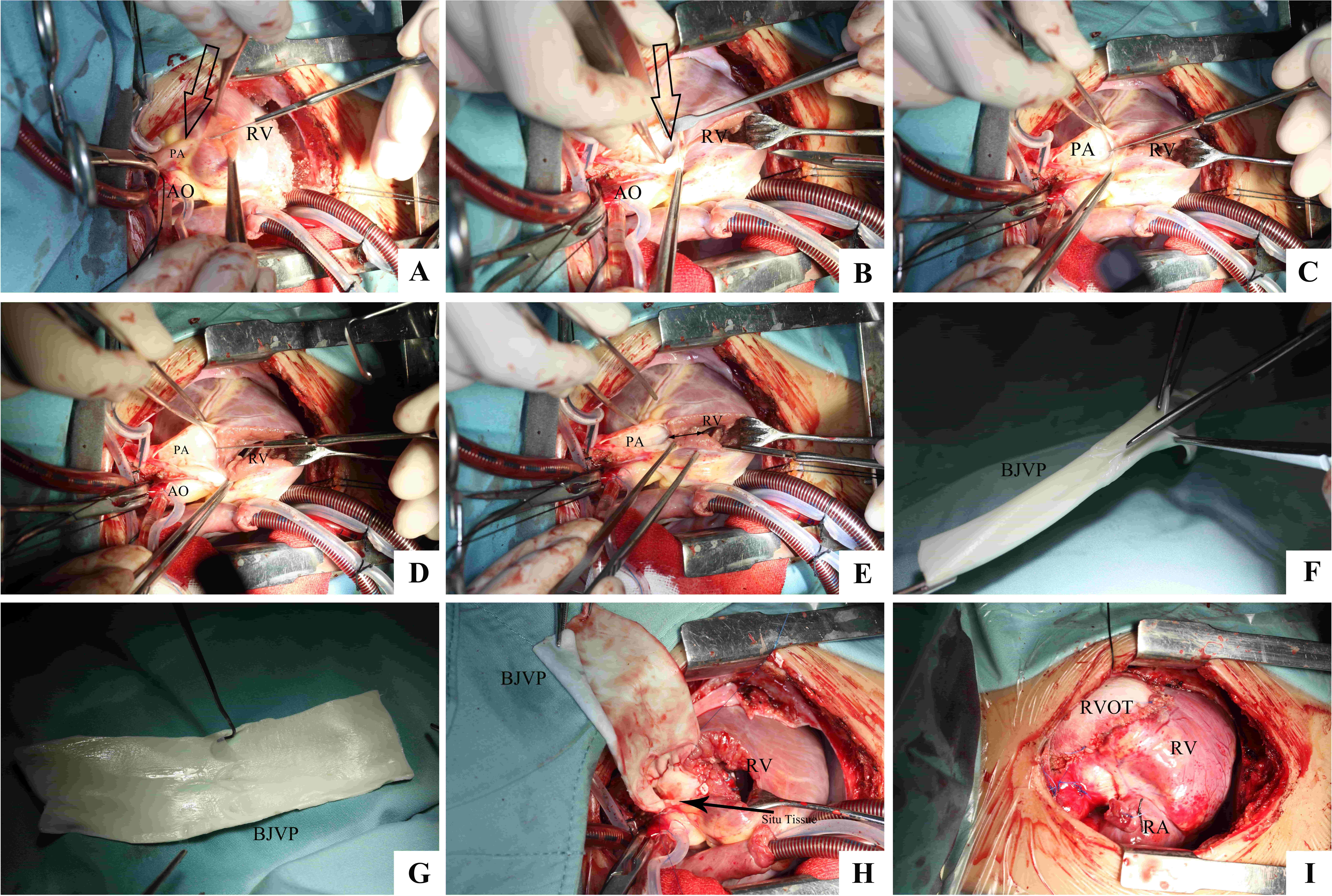 Fig. 2.
Fig. 2.Surgical procedure for pulmonary atresia. (A) Longitudinal incision of the RVOT. (B) The longitudinal incision was extended to the pulmonary confluence. (A–B) The muscular septum between the blind end of the PA and the RV is highlighted by a black arrow. (C) Incision of the muscular atresia of the PA. (D) Cut off muscular atresia tissu©. (E) The thick muscle tissue between the top of the RVOT and the PA is highlighted by a black arrow. (F) BJVC of appropriate size was selected using the formula and then longitudinally incised along the junction of the valve leaflets to avoid damaging the leaflets. (G) Thorough examination of the BJVP’s valve leaflets. (H) The BJVP was continuously sutured with the posterior situ autologous PA tissue and the incision of the RVOT. I: Complete RVOT reconstruction. (A–I) RVOT: right ventricular outflow tract; PA, pulmonary artery; BJVC, bovine jugular vein conduit; BJVP, bovine jugular vein patch; RV, right ventricle; RA, right atrium; and AO, Aorta.
When autologous tissue was used as the posterior wall of the reconstructed
pulmonary artery, the diameter of the BJVC was decided by the formula (Fig. 1):
D
Patients were postoperatively followed up at one month, six months, and then
yearly after discharge. The pulmonary regurgitation jet width/annular ratio
detected by transthoracic echocardiography was used to assess PR severity after
RVOT reconstruction, and peak gradient across RVOT was used to assess its
stenosis. Ratios
Statistics: Data are expressed as the mean
Cardiopulmonary bypass time and aortic cross-clamp time were similar between the
two groups. There was also no difference between the two groups’ mechanical
ventilation time and ICU and postoperative residence time (Table 2). There was no
death during hospitalization and follow-up period. Postoperative echocardiography
showed no residual ventricular septal shunt, and all deformities were
satisfactorily repaired. No third-degree atrioventricular block occurred. The
level of skin oxygen saturation in the limbs at rest significantly improved
(
| Observation group | Control group | p Value | |
| Cardiopulmonary bypass time (min) | 101.72 |
99.95 |
0.473 |
| Aortic cross-clamp time (min) | 75.76 |
75.32 |
0.847 |
| Mechanical ventilation time (hour) | 69.68 |
68.36 |
0.786 |
| ICU residence time (day) | 6.20 |
6.50 |
0.327 |
| Postoperative residence time (day) | 14.32 |
14.84 |
0.380 |
| Pulmonary regurgitation (moderate and serious ) | 4 | 8 | 0.331* |
| Serious PR | 0 | 2 | 0.491* |
| transvalvular pressure gradient (more than 36 mmhg) | 0 | 8 | 0.004* |
| Re-intervention (case) | 0 | 5 | 0.0285# |
In the observation group, no patient had a transvalvular pressure gradient
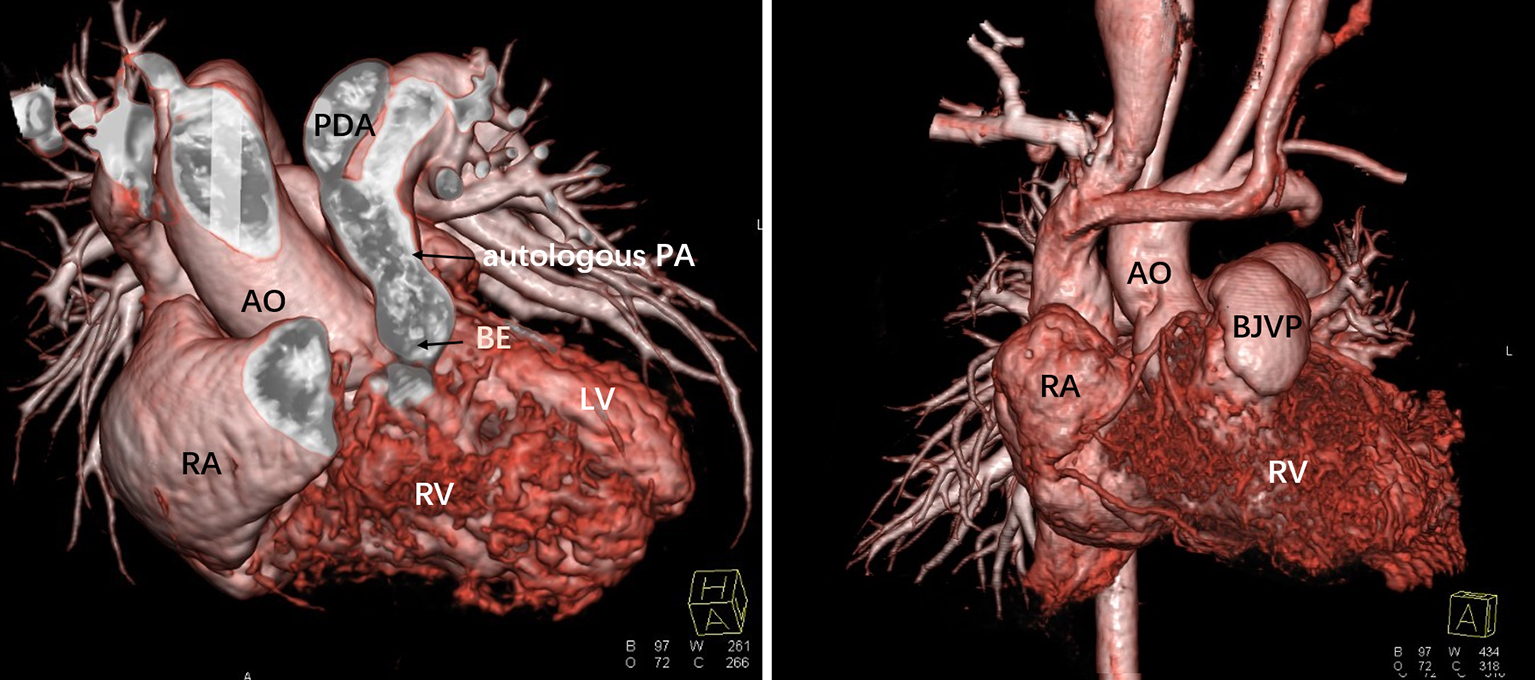 Fig. 3.
Fig. 3.Comparison of preoperative and postoperative cardiac CT images. BJVP, bovine jugular vein patch; RV, right ventricle; RA, right atrium; LV, left ventricle; AO, Aorta; PA, pulmonary artery; PDA, Patent ductus arteriosus; BE, blind end of the pulmonary artery.
PA-VSD, a rare cyanotic congenital heart disease and a significant cause of neonatal cyanosis and hypoxemia in children, accounts for approximately 2% of congenital heart diseases. Pathological malformation of PA-VSD includes VSD, pulmonary atresia, PDA, and MAPCAs. Previously, for patients with inherent pulmonary arteries who can undergo one-stage radical surgery, a modified Rastelli procedure with various conduits was used to reconstruct the connection between the RV and the pulmonary artery confluence.
A variety of materials can be used for RVOT reconstruction, as described in our previous series study [12, 13, 14]. However, pediatric patients’ options are limited to homograft, bovine jugular vein conduit, and valved conduits sewn by hand using pericardium or expanded polytetrafluoroethylene (ePTFE) due to size limitations. Homograft is currently a preferred pulmonary valve substitute for RVOT reconstruction due to its good operability during surgery, excellent valve function, and unnecessary anticoagulation. BJVC with a tri-leaflet valve and a sinus structure is also a common option with a 12–22 mm range of products. However, the lack of donors has significantly limited homograft’s clinical application, and their long-term prognoses are not satisfactory.
The rate of freedom from re-intervention due to graft failure after 10-years for homograft and BJVC is around 69%–85% [15, 16, 17, 18, 19] and 59%–70% [18, 19, 20, 21], respectively, which also includes study reports of older children and adults. For young children, especially patients younger than 2-years old, the probability of freedom from re-intervention for 10-years is only 40%–60% [15, 18, 22, 23, 24, 25]. Hand-sewn blood vessel grafts may have superior performance in freedom from re-intervention than other conduits [21, 26, 27, 28, 29], but it lacks the potential for expansion alongside somatic growth and suturing the valve leaflets requires skill. The use of valved conduits indeed solves the problem of pulmonary regurgitation to an extent; however, none have solved the issues concerning poor expansion alongside somatic growth and anastomotic stenosis. Thus, built on previous concepts and the desire to improve existing treatment options, the present study attempted to preserve and make full use of autologous pulmonary tissue as the posterior wall of the newly built pulmonary artery, thereby preserving the growth potential of the pulmonary artery and thus relieving the anterior anastomotic stenosis caused by graft calcification. Moreover, the widest diameter of the autologous pulmonary artery is always located at the pulmonary confluence, where acquired distal conduit stenosis at the suture line is the most typical indication for conduit-specific reinterventions [30]. Therefore, it is safe to authoritatively say the risk of postoperative stenosis with non-circular graft anastomosis can significantly be decreased or avoided given the features and method mentioned above.
Though a small gap exists between the posterior autologous pulmonary artery and the anterior BJVP, given that the autologous pulmonary artery is always small, it will not cause severe pulmonary valve regurgitation. However, if present, the pulmonary regurgitation caused by the gap is deemed acceptable, as reflected in the cohort herein, where only four cases (18%) with moderate regurgitation were observed during the follow-up period (Fig. 4). In addition, it can also effectively prevent pulmonary valve regurgitation and help protect RV function.
 Fig. 4.
Fig. 4.Kaplan-Meier curves. Freedom from re-intervention during the follow-up period of the two groups with significant differences.
Though enveloped in controversy, an oversized pulmonary trunk is meant to reduce the risk of relative stenosis and adapt to the rapid somatic growth of children. Interestingly, Askovich Bojana et al. [31] thought that rapid somatic growth might be one of the several reasons for conduit failure in children. Thus, large allografts may fail due to external conduit compression by the closed sternum, valve distortion, insufficiency with sternal compression, or distal PA distortion from the oversized conduit [31, 32, 33]. Simon J. Sonntag et al. [34], via image-based fluid dynamic simulations for shear wall stress (SWS) analysis, suggested that choosing a prosthesis size that will cause high SWS and an associated intimal reaction, possibly leading to stenosis, can defeat the benefit of having a typically larger orifice area directly after implantation.
Considering the growth potential of the pulmonary artery by this method and the possible adverse effects of a large pulmonary artery, the study individually planned the diameter of the pulmonary artery following reconstruction per the weight of the patient using a formula. A larger patch will increase the gap between the autologous pulmonary artery and the BJVP, which will reduce the anti-regurgitation function of the valve leaflets on the patch (Fig. 5). Since the reconstructed pulmonary artery size in the cohort herein is identical to standard physiological needs, the pulmonary artery hemodynamic performance and blood flow (wall shear stress) should be in line with physiology, hence a stable fluid hemodynamic. It is also suitable and adaptive for the expected growth of the autologous pulmonary artery. In addition, it can also reduce the space-occupying effect caused by the sizeable reconstructed artery and avoid a mismatch between the pulmonary artery and its branches that may result in distortion.
 Fig. 5.
Fig. 5.Diagram of the procedure for pulmonary atresia. (A) Thorough anatomical examination of the RVOT and PDA (B) Longitudinal incision from the RV to the PA via the pulmonary BE while reserving the autologou©iththe septum between the BE and the RV. The width of the autologous PA was measured using a ruler. (C) A suitable BJVP was selected using the formula mentioned above and then continuously sutured with the autologous PA in situ posteriorly. (D) Completed RVOT reconstruction. PDA, Patent ductus arteriosus; BE, blind end of the pulmonary artery. BJVP: bovine jugular vein patch. (A–D) All cardiopulmonary bypasses are removed.
Finally, the study herein is without its limitations. Although the short-term outcome is good, the main issue of long-term competence needs to be addressed. Also, the series is relatively small with a retrospective study type. And, this study did not carry out systematic genetic tests.
For PA-VSD that implements corrections from RVOT to PA, though varying surgical interventions over the years have been employed in treating the complex congenital anomaly, its prognosis presently remains poor. Given the disadvantages of implanting conduits that most commonly used at present, such as poor extensions with growth and immune rejection, there were high incidence rates of re-interventions following primary surgery. This study preserved and made full use of autologous pulmonary tissue as the posterior wall of the newly built pulmonary artery, preserving the pulmonary artery’s growth potential, and uniquely individualized the procedure depending on the patient’s weight and the size of the autologous pulmonary artery to achieve physiological blood flow. The mid-term outcomes of this study indicates that it is adequate for treating PA-VSD.
BJVC, bovine jugular vein conduit; BJVP, bovine jugular vein patch; d-Ao, diameter of descending aorta; d-LPA, diameter of left pulmonary artery; d-RPA, diameter of right pulmonary artery; ePTFE, expanded polytetrafluoroethylene; MAPCA, major aortopulmonary collateral arteries; PA, pulmonary artery; PA-VSD, pulmonary atresia with ventricular septal defect; PDA, patent ductus arteriosus; PR, pulmonary regurgitation; PS, pulmonary stenosis; RVOT, right ventricular outflow tract; RV, right ventricle; SWS, shear wall stress; VSD, ventricular septal defect.
MW drafted the manuscript. CF and JY designed the study. MW, JL, CDI, WC, PH, MT, XW, CW, KX and CF collected data and revised the manuscript. MW, JL and WZ were responsible for the analysis of data and project administration. All authors have read and agreed to the published version of the manuscript.
The study protocol was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University, Changsha, China. Written informed consent was provided preoperatively by the patient relatives.
We would like to express our gratitude to all the peer reviewers for their opinions and suggestions.
This work was supported by Hunan Provincial Technology Innovation Guidance Program-Clinical Medical Technology Innovation Guidance Project (No. 2017SK50501 to WZ).
The authors declare no conflict of interest.
Written informed consent was obtained from the patients’ legal guardian(s) for publication of this study and any accompanying images.
Data are available from the corresponding author upon reasonable request.
