Academic Editor: Giuseppe Santarpino
Background: Prediction of long-term mortality in patients with severe
symptomatic aortic valve stenosis undergoing transcatheter aortic valve
implantation (TAVI) is still challenging but of great impact with respect to the
selection of treatment strategy. Whereas most of the established scores address
perioperative risk and/or short-term mortality, the aim of our current study was
the integrative investigation of a multitude of patients’ characteristics
including novel biomarkers of cardiovascular remodeling with respect to their
value for the prediction of long-term mortality. Methods: In a first
subset of patients (n = 122, identification group) a wide range of baseline
characteristics were assigned to three clusters with 4 to 10 items each
(classical clinical parameters; risk assessment scores; novel biomarkers of
cardiovascular remodeling) and tested with respect to their predictive value for
one-year mortality. Thereby, a sum-score system (Jena Mortality Score, JMS) was
defined and tested in a larger collective of TAVI patients (n = 295, validation
group) with respect to one- and two-year mortality prediction. Results:
In the identification cohort, binary logistic regression analysis, with one-year
mortality as dependent variable and the items per cluster as cofounders, revealed
atrial fibrillation (Afib; odds ratio [OR] 7.583, 95% confidence interval [95% CI]: 2.051–28.040, p = 0.002), clinical frailty
scale (CFS; OR 2.258, 95% CI: 1.262–4.039, p = 0.006) and Tissue-Inhibitor
of Metalloproeinase-1 (TIMP-1; OR 1.006, 95% CI: 1.001–1.011, p = 0.019) as
independent predictors of one-year mortality. These 3 parameters were integrated
into a simplified sum-score as follows: presence of Afib (no = 0, yes = 1);
dichotomized CFS (1 to 4 = 0; 5 to 9 = 1); TIMP-1 range (cut-off value 187.2
ng/mL; below = 0, above = 1). The resulting sum-score (JMS) ranged from 0 to 3.
By binary logistic regression analysis in the validation cohort with one- and
two-year mortality as dependent variable and Society of Thoracic Surgeons (STS)
score (STS), staging of extra-valvular cardiac damage (stage), presence of high
gradient aortic stenosis (HGAS), EQ visual analogue scale score (EQ-VAS) and JMS
as cofounders, besides STS score, only JMS could be proven to serve as
independent predictor of both, one-year (OR 1.684, 95% CI: 1.094–2.592, p =
0.018) and two-year (OR 1.711, 95% CI: 1.136–2.576, p = 0.010) mortality.
After dichotomization of patients into a low-risk and a high-risk group according
to JMS, Kaplan-Meier survival analysis displayed a significant survival benefit
for the low-risk group after one and two years (p
Transcatheter aortic valve implantation (TAVI) as an alternative for surgical aortic valve replacement (SAVR) for the treatment of severe symptomatic aortic valve stenosis (AS) has rapidly developed in the last decade and represents the treatment of choice in patients of 75 years or older, irrespective of surgical risk [1, 2, 3, 4, 5]. Prediction of long-term mortality in patients undergoing TAVI is still challenging but of great impact with respect to the selection of the appropriate treatment strategy (SAVR versus TAVI), especially in the low- or moderate risk group between 70 and 75 years of age. Current guidelines and consensus papers claim the evaluation of these patients in the frame of a heart team to recommend the optimal treatment for each individual patient [6, 7]. Thus, the availability of risk scores, which are at once reliable and easy to perform in daily practice, are of great clinical interest. Currently, the majority of risk scores used for TAVI patients are borrowed from surgical mortality prediction tools, which are known to overestimate short-term mortality in TAVI patients, especially in the elective setting [8, 9, 10]. The most commonly used scores are EuroScore II and the Society of Thoracic Surgeons Predicted Risk of Mortality model (STS-PROM) [6, 11, 12]. Motivated by the above-mentioned limitations of the surgical scores, a variety of TAVI specific mortality prediction models have been proposed in the last years, e.g., the survival post TAVI score (STT), the OBSERVANT score or the FRANCE-2 score [13, 14, 15]. A common problem of these scores is the fact that they were mainly derived from the very early TAVI collectives, which were characterized by an extremely high (prohibitive) or high surgical risk. Thus, their transferability to present-day TAVI patients, which summarize elderly patients of all surgical risk categories, is very limited and the scores have not performed satisfactory in external validation studies [10].
Another unmet clinical need, in particular when talking about moderate or low surgical risk patients, is the missing appropriateness of TAVI specific scores to predict long-term mortality reflecting patients’ survival beyond the procedure. Thus, reliable prediction of one- or even two-year mortality would be of great interest for the individual patient as well as the health care system [10, 16, 17].
To reach the goal of defining a risk prediction model fulfilling all these requirements, one has to expand conventional scoring parameters by the integrative implementation of further prognostic determinants derived from, e.g., individual frailty assessment, imaging modalities or circulating reflectors of cardiovascular tissue remodeling. Especially the latter aspect was largely neglected in the past causing that those novel biomarkers, which have been shown to be prognostic in representative cohorts, are not implemented in TAVI risk prediction tools yet [10, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27].
The aim of our current preliminary study was the integrative investigation of a multitude of patients’ characteristics including frailty assessment and novel biomarkers of cardiovascular remodeling with respect to their value for the prediction of long-term mortality in real-life patients undergoing TAVI.
The Jenaer Aortenklappenregister (JAKR), established in 2016, prospectively includes all patients that undergo transcatheter aortic valve implantation (TAVI) at the University Hospital Jena. In this study, we investigated two patient cohorts with the aim to, first, identify novel markers for mortality risk prediction and define a simple sum-score system (identification cohort, n = 122) and second, validate it in a larger study population (validation cohort, n = 295). All patients included gave written informed consent for participation and the local ethics committee of the University Hospital Jena has approved the study (registration number: 4815-06/16). Detailed clinical, laboratory, functional and imaging analysis according to local standard operating procedures were performed in adherence to the principles of the current version of the Declaration of Helsinki and good clinical practice guidelines. Structured patient follow-up was carried out at 6 weeks, 6 months, 12 months and 24 months according to the JAKR protocol. In principle, all patients undergoing TAVI and agreed to participate were consecutively included in the study. Due to potential extra-cardiovascular sources of the serum biomarkers determined by Enzyme linked Immunosorbent Assay (ELISA), the following conditions were excluded: active malignant or autoimmune disease, hyperthyroidism, infections or systemic intake of corticosteroids.
For the assessment of patients’ health status, the EuroQol visual analogue scale (EQ-VAS; between 0 and 100 points) was used as a component of the EuroQol questionnaire (EQ-5D-5L), which is an accepted tool for the standardized simple generic Quality of Life (QoL) assessment already used in TAVI patients [28, 29, 30]. The Clinical Frailty Scale (CFS), known to predict death or the need for institutional care, was applied to assess clinical frailty of the patients by assigning the following categories: very fit (1), well (2), managing well (3), vulnerable (4), mildly frail (5), moderately frail (6), severely frail (7), very severely frail (8) and terminally ill (9) [31].
In the frame of the JAKR study protocol, blood samples were taken from all study
participants by standard venous puncture. The collection tubes were centrifuged
within 20 min after withdrawal and serum was transferred into special low binding
tubes (Protein LoBind, Eppendorf AG, Hamburg, Germany), snap frozen in liquid
nitrogen to reduce artificial protein degradation and finally stored at –80
Statistical analyses were performed using IBM SPSS statistical software, version
25.0 (IBM SPSS Statistics for Windows. Armonk, NY, USA). Data are expressed as
mean/median
For the identification of independent predictors for one- and two-year mortality, multivariate regression analysis was performed by using a binary logistic model (backward elimination method: Wald). One- or two-year mortality was defined as the dependent variable.
In the identification cohort, (n = 122), a wide range of baseline
characteristics were assigned to three clusters with 4 to 10 items each. For each
of the three clusters, a multivariate analysis was performed by using one-year
mortality as dependent variable and the following items as cofounders. For
cluster 1 (classical clinical parameters/comorbidities), New York Heart
Association (NYHA) functional class, coronary artery disease (CAD), peripheral
artery disease (PAD), diabetes mellitus (DM), chronic obstructive pulmonary
disease (COPD), atrial fibrillation (Afib), presence of pacemaker (PM) and
glomerular filtration rate (GFR) were included as cofounders. The items for
cluster 2 (risk assessment scores) were EQ-VAS (0 to 100), CFS (1 to 9), STS
score (%) and staging classification of extra-valvular cardiac damage (stage 0
to 4) and for cluster 3 (novel biomarkers of cardiovascular remodeling) MMP-9,
TIMP-1, B
In each cluster, one parameter could be identified to significantly increase
one-year mortality risk in the identification cohort (Afib, CFS and TIMP-1).
Basing on these findings, a simplified sum-score system was defined as follows:
presence of Afib (no = 0, yes = 1); dichotomized CFS (1–4 = 0; 5–9 = 1); TIMP-1
range (cut-off value: 187.2 ng/mL; below = 0, above = 1). The resulting
sum-score, denominated as Jena Mortality Score (JMS), ranged from level 0 to 3.
The one-year mortality rates per JMS level were calculated in the identification
cohort and a multivariate analysis was performed as described including all
parameters showing a p-value
In a next step, to confirm our findings in a larger patients’ collective, JMS
was tested with respect to its predictive value for one- and two-year mortality
in the validation cohort (n = 295). Therefore, survivors and non-survivors after
one and two years were compared with respect to a wide range of clinical,
laboratory, functional and imaging parameters including TIMP-1 serum levels using
the Mann–Whitney-U test. Then, multivariate regression analysis was performed,
again by using a binary logistic model (backward elimination method: Wald), with
one- or two-year mortality as dependent variable and all parameters showing a
p-value
Finally, Kaplan-Meier survival analysis including log-rank test as well as receiver operating characteristic (ROC) analysis was performed to verify differences between high and low-risk patients according to JMS compared with STS score with respect to both, one- and two-year mortality. Comparison of area under the curve (AUC) values was performed by DeLong method using MedCalc statistical software (Version 20.023; MedCalc Software Ltd, Ostend, Belgium).
Baseline characteristics of the identification cohort, which represented a
typical TAVI collective with a mean STS score of 4.8%, are given in Table 1. The
one-year mortality rate was 14%. By performing binary logistic regression
analysis with one-year mortality as dependent variable and the items per cluster
as cofounders as described in the material and methods section, Afib (cluster 1;
OR 7.583, 95% CI: 2.051–28.040, p = 0.002), CFS (cluster 2; OR 2.258, 95% CI:
1.262–4.039, p = 0.006) and TIMP-1 (cluster 3; OR 1.006, 95% CI:
1.001–1.011, p = 0.019) could be identified to significantly increase
long-term mortality risk. These 3 parameters were integrated into a simplified
sum-score (JMS) as follows as described. As cut-off value for TIMP-1, the median
of the 122 patients, which was 187.2 ng/mL, was chosen. The resulting JMS value
ranged from 0 to 3. The mortality rates per score level in the identification
group were 0% for JMS 0, 8.8% for JMS 1, 22.2% for JMS 2 and 33.3% for JMS 3.
When performing binary logistic regression analysis including all parameters
showing a p
| Parameter | Identification cohort (n = 122) | Validation cohort (n = 295) | p-value | |
| Age (years; mean |
78.2 |
78.7 |
0.829 | |
| Female (%) | 54.1 | 55.3 | 0.534 | |
| STS (%) | 4.8 |
4.8 |
1.000 | |
| NYHA |
64.8 | 73.6 | 0.024 | |
| CAD (%) | 61.5 | 60.4 | 0.840 | |
| PAD (%) | 10.7 | 12.0 | 0.700 | |
| Diabetes (%) | 45.1 | 49.2 | 0.816 | |
| COPD (%) | 25.4 | 28.3 | 0.545 | |
| Afib (%) | 44.3 | 48.6 | 0.418 | |
| PM (%) | 16.4 | 15.8 | 0.882 | |
| GFR (mL/min; mean |
52.1 |
52.6 |
0.883 | |
| BNP (pg/mL; mean |
863.5 |
913.9 |
0.861 | |
| LVEF (%; mean |
55.7 |
57.6 |
0.136 | |
| AS subtype (%) | 0.760 | |||
| HGAS | 73.8 | 71.8 | ||
| LGAS | 14.8 | 17.8 | ||
| PLFLGAS | 11.5 | 10.5 | ||
| Stage 0–4 (%) | 0.185 | |||
| 0 | 3.3 | 3.1 | ||
| 1 | 18.2 | 12.6 | ||
| 2 | 49.6 | 50.9 | ||
| 3 | 19.0 | 21.5 | ||
| 4 | 9.9 | 11.9 | ||
| STS score, Society of Thoracic Surgeons score; NYHA, New York Heart Association; CAD, coronary artery disease; PAD, peripheral artery disease; COPD, chronic obstructive pulmonary disease; Afib, atrial fibrillation; PM, pace maker; GFR, glomerular filtration rate; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction; AS, aortic stenosis; HGAS, high gradient aortic stenosis; LGAS, low gradient aortic stenosis; PLFLGAS paradoxical low flow low gradient aortic stenosis; stage, stages of extra-valvular cardiac damage according. | ||||
With respect to the biomarkers of cardiovascular remodeling measured by ELISA
and included in cluster 3 as described in Material and Methods, the median values
| Biomarker | Identification cohort | Survivors | Non-survivors | p value |
| (n = 122) | after 1 year (n = 105) | after 1 year (n = 17) | (survivors vs. non-surviors) | |
| MMP-9 (ng/mL) | 400 |
405 |
369 |
0.618 |
| TIMP-1 (ng/mL) | 187 |
182 |
287 |
0.053 |
| B |
784 |
755 |
920 |
0.656 |
| ET-1 (ng/mL) | 2.5 |
2.5 |
3.2 |
0.190 |
| NGAL (ng/mL) | 137 |
137 |
140 |
0.819 |
| ED-A |
10.5 |
10.7 |
9.6 |
0.399 |
| ED-B |
4.1 |
4.3 |
2.9 |
0.482 |
Baseline characteristics of the validation cohort, which again represented a typical TAVI collective with a mean STS score of 4.8%, are given in Table 1. The mortality rate was 18% after one and 23% after two years.
Binary logistic regression analysis with one- and two-year mortality as
dependent variable and STS score (STS), staging of extra-valvular cardiac damage
(stage), presence of high gradient aortic stenosis (HGAS), brain natriuretic
peptide (BNP), EQ visual analogue scale (EQ-VAS) and JMS as cofounders was
performed. The cofounders were defined as all parameters not contributing to the
implemented scores and showing a p-value
As a result, besides STS score, only JMS could be proven to serve as independent
predictor of both, one-year (JMS: OR 1.626, 95% CI: 1.053–2.509, p = 0.028;
STS score: OR 1.180, 95% CI: 1.052–1.325, p = 0.005) and two-year (JMS: OR
1.649, 95% CI: 1.093–2.488, p = 0.017; STS score: OR 1.253, 95% CI:
1.106–1.419, p
The one-/two-year mortality rates per score level in the validation cohort were 1.75/2.62% for JMS 0, 3.93/4.80% for JMS 1, 6.11/7.86% for JMS 2 and 6.55/7.86% for JMS 3.
After dichotomization of patients into a low- and high-risk group according to
JMS and STS score (JMS: 0 or 1 = low-risk, 2 or 3 = high-risk; STS score:
 Fig. 1.
Fig. 1.Kaplan-Meier survival analysis for JMS and STS scoredisplaying a significant survival benefit for the low-risk groups according after one and two years. (a) JMS. (b) STS score.
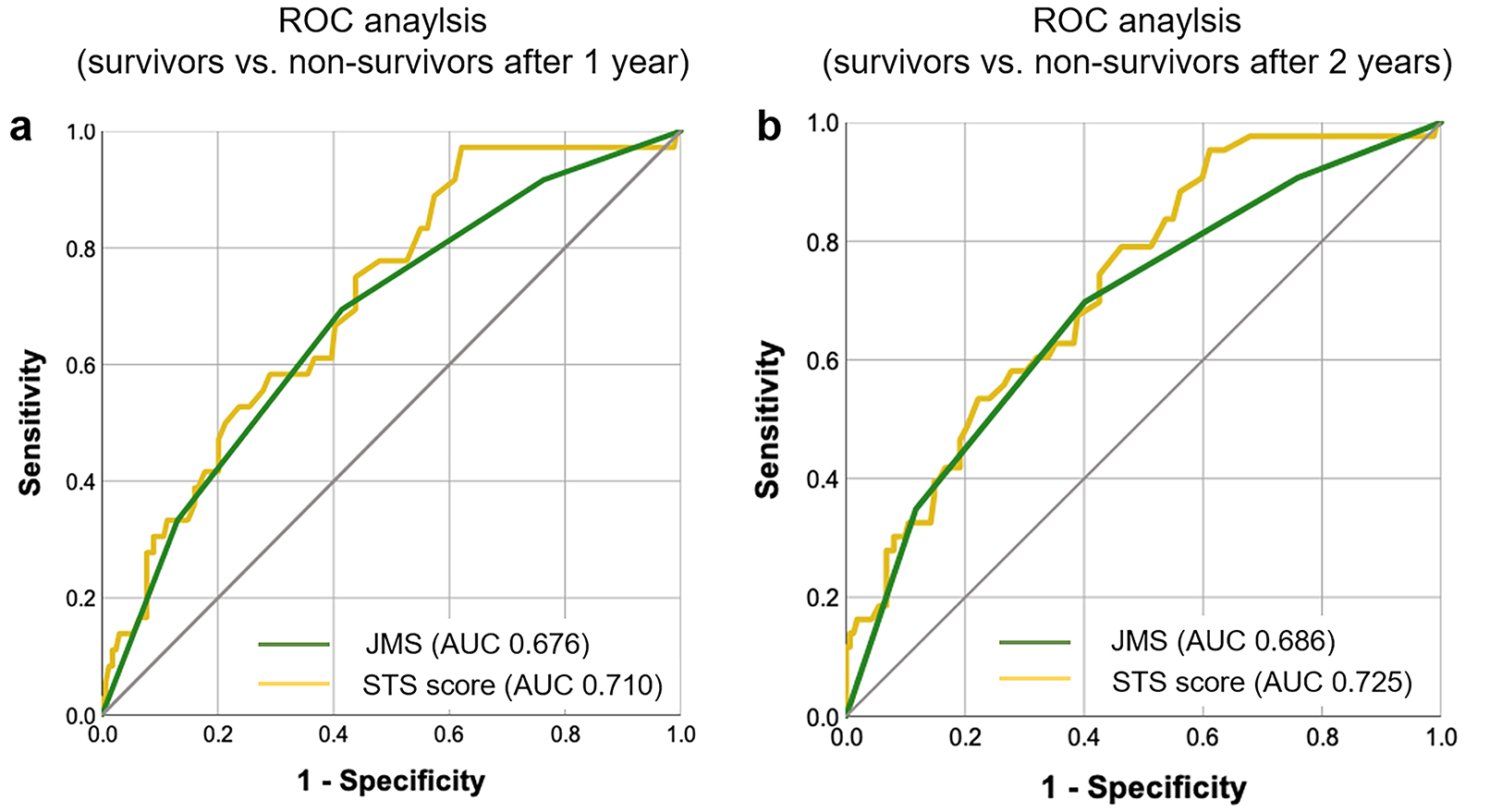 Fig. 2.
Fig. 2.ROC analysis for the discrimination of survivors from non-survivors revealing an AUC for the JMS of 0.676 and for the STS score of 0.710 after one year and an AUC for the JMS of 0.686 and for the STS score of 0.725 after two years. (a) and (b) results after one year.
With explicit focus on the serum levels of TIMP-1, which have been already
reported above as part of JMS, the following observations are of value to be
reported separately. When comparing survivors and non-survivors after one and two
years, there are significantly increased TIMP-1 concentrations in the
non-survivors (p
 Fig. 3.
Fig. 3.Baseline TIMP-1 concentrations in survivors and non-survivors after one and two years showing significantly increased levels in non-survivors for both time-points. (a) Results after one year. (b) After two years.
The newly introduced Jena Mortality Score (JMS) for the prediction of long-term mortality after TAVI entails not only classical clinical, laboratory or imaging parameters but, for the first time also a novel biomarker of cardiovascular extracellular matrix accumulation (ECM) and fibrosis. Thus, it represents an innovative approach possibly contributing to a more precise risk assessment facilitating patient-centered heart-team decision making as recommended by current guidelines [34]. Mortality prediction in TAVI patients is a remaining challenge, especially when focusing on long-term outcomes. This situation is due to the fact that most available scores were developed and validated in SAVR patients to predict short-term, e.g., 30-day, mortality. Therefore, their use in TAVI patients leads to an overestimation of 30-day mortality, at least in the elective setting [8, 9, 35]. Although, several TAVI specific scores have been proposed in recent years, their additive value in daily clinical practice is limited since most scores focus on 30-day mortality and underperform in external validation cohorts [13, 15, 36]. Especially for the prediction of long-term mortality, only small cohorts have been the objects of recent studies. While there were some efforts to develop TAVI risk prediction models for one-year mortality, there are no data for longer survival periods, e.g., two years after TAVI.
Despite the availability of various studies investigating the value of novel biomarkers of cardiovascular remodeling for improving the diagnosis or estimating the prognosis of AS patients [22, 25, 26, 37], to our best knowledge, none of these markers has ever been implemented into a long-term mortality risk prediction model after TAVI. Thus, the JMS presented here, might fill this gap by integrating circulating levels of TIMP-1, which is a well-known biomarker of cardiovascular ECM accumulation and fibrosis [22, 38, 39, 40].
When interpreting the results of the current study, it has to be clearly mentioned that the number of patients included in both, the identification as well as the validation cohort, is limited and that we cannot provide external validation since both cohorts were derived from the Jenaer Aortenklappenregister (JAKR), which includes patients from only one center.
Nevertheless, when comparing JMS versus STS score derived low- versus high-risk patients with respect to one- and two-year survival, JMS derived risk class performed better in our study. This goes in line with recent reports on the limited value of STS score-based risk assessment in TAVI populations, especially with respect to long-term outcomes [10].
Interestingly, a very recent meta-regression analysis of 8 randomized trials investigating TAVI in high-, intermediate- and low-risk patients, impressively demonstrated that there was no association between STS score and hazard ratio for two-year mortality and thereby questions the usefulness of surgical risk-scoring in TAVI patients in general [41].
In contrast to most studies investigating TAVI specific risk assessment models, which aimed to predict one-year mortality [16, 17, 42], JMS, described by us, is even capable to predict two-year mortality. However, its validity in larger external cohorts has still to be proven, which represents a clear limitation of our study. Of note, to overcome this issue will remain challenging since TIMP-1 serum levels are not part of routine laboratory diagnostics and not available for retrospective analyses. On the other hand, the development of a TIMP-1 point-of-care test should be very easy to realize since it is already available for a variety of other cardiac biomarkers, e.g., Troponin T (TnT), brain natriuretic peptide (BNP), D-dimer or even novel markers like heart-type fatty acid-binding protein (H-FABP) [43]. Once available, assessment of JMS would be not only very simple but could also be performed extremely fast at bedside. This might represent a great advantage compared to nearly all the other available scores, e.g., the complex STS score, which takes, depending on the physician’s experience, at least several minutes. However, it will take certain efforts in the future to somehow implement TIMP-1 measurement in clinical routine for risk assessment of AS patients.
Besides TIMP-1, JMS also implies the presence of Afib and the CFS, which are both very well-known mortality predictors in TAVI patients [10, 44]. In that context, especially frailty assessment scores for TAVI risk evaluation facilitating heart-team decision making have been recommended frequently in the literature [45].
Moreover, a great advantage of JMS is the fact that it has been developed in TAVI patients. In contrast, the STS score, which has been widely used for risk assessment in TAVI patients over the last decade, is derived from SAVR patients [12]. Against that background, we would like to suggest integrating both scores in risk assessment during heart team evaluation, especially in patients between 70 and 75 years, in which current ESC guidelines place special emphasis on individualized decision making between SAVR and TAVI [34].
Taken together, we suggest a simple and fast to perform risk-prediction model to estimate long-term mortality after transfemoral TAVI performed in the elective setting. Although, external validation remains a mandatory future task, we would like to place special emphasis on the incorporation of novel biomarkers of cardiovascular tissue remodeling into TAVI risk assessment scores.
JMS, including TIMP-1 as a novel biomarker of cardiac extracellular matrix accumulation and fibrosis, could serve as a simple tool to assess long-term mortality risk after TAVI and might thereby contribute to a more precise stratification of individual risk. A great advantage is its simplicity and, compared to many other scores, extremely short performance time.
LB and MF designed the research study. LB, KG, MD, SMW, PCS and MF performed the research. LB, KG, CJ and MF provided help and advice on the ELISA experiments. LB, MF, AP, CJ and PCS analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All patients included gave written informed consent for participation and the local ethics committee of the University Hospital Jena has approved the study (registration number: 4815-06/16).
The authors would like to thank Annett Schmidt for excellent technical assistance.
This work was supported by funding from the Foundation “Else Kröner-Fresenius-Stiftung” within the Research Program “Else Kröner-Forschungskolleg AntiAge”.
The authors declare no conflict of interest.
