†These authors contributed equally.
Academic Editors: Brian Tomlinson and Takatoshi Kasai
Heart failure (HF) is the end stage of several cardiovascular diseases with high mortality worldwide; however, current chemical drugs have not beneficial effect on reducing its mortality rate. Due to its properties of multiple targets components with multiple targets, natural products derived from traditional Chinese medicine (TCM) have exerts unique effects on the amelioration of the clinical symptoms of HF, yet, TCM is not widely used in the clinic since the potential therapeutic targets have not been fully investigated. Therefore, in this review, we briefly summarized the pathophysiological mechanism of HF and reviewed the published clinical evaluations of TCM and natural products from Chinese herbs to treat HF. Then, the therapeutic potential and the underlying mechanisms by which the natural products from Chinese herb exert their protective effects were further summarized. We concluded from this review that natural products from Chinese herbs have been shown to be more effective in treating HF by targeting multiple signaling pathways, including anticardiac hypertrophy, antifibrotic, anti-inflammatory, antioxidative and antiapoptotic activities. However, the major limitations of these compounds is that there are a lack of large scale, multicenter, randomized and controlled clinical trials for their use in treatment of HF, and the toxic effects of natural products from Chinese herbs also needed further investigation. Despite these limitations, further clinical trials and experimental studies will provide a better understanding of the mechanism of natural products from Chinese herbs and promote their wide use to treat HF.
Heart failure (HF) is a complicated syndrome in which ventricle filling or ejection is impaired due to structural or functional cardiac disorders [1]. Globally, there are more than 37.7 million individuals with HF, and almost 50% of HF patients die within 5 years after diagnosis; thus, the mortality rate of HF exceeds that of many cancers [2]. From a population-based study reported in 2021, the age-standardized prevalence and incidence of HF were 1.10% (an estimated 15 million HF patients) in China [3]. According to treatment guidelines, the main drugs used to treat HF in the clinic are angiotensin receptor blockers (ARBs) and mineralocorticoid receptor antagonists (MRAs). Although there has been a significant improvement in the number of hospital admissions, the 5-year mortality has not been reduced [4]. Indeed, there are some novel drugs used to treat HF in clinical trials, such as vericiguat (an oral soluble guanylate cyclase stimulator) [5], finerenone (a third-generation mineralocorticoid receptor antagonist) [6, 7] and ferric carboxymaltose [8], however, not all of them have an apparent effect on the risk of cardiovascular death. According to one Phase IIb trial, finerenone had a beneficial effect on reducing HF mortality, however, whether the high occurrence of malignant arrhythmia and hyperkalemia caused by finerenonen still needs to be further verified [7]. Moreover, prolonged use of the above mentioned chemical drugs may result in severe side effects, such as electrolyte depletion and hypotension [9]. Therefore, natural products from Chinese herbs have been considered an alternative therapeutic strategy for the treatment of HF that is less expensive and associated with fewer side effects.
Chinese herbs have been used as complementary therapy to treat HF for a long history [10]. Traditional Chinese medicine (TCM) combined with Western medicine treatment has been shown to improve the quality of life of HF patients [11]. In China, there are many patent Chinese drugs (where the active ingredients were extracted from these herbs and made into the form of capsules, pills or injections by modern therapeutic techniques) and Chinese formulae (several herbs in different amounts are prescribed together by physicians and boiled and concentrated using a rotary evaporator according to the Standard Operation Procedure of the Chinese Pharmacopoeia) used to treat patients with HF; however, their mechanisms of action, complex pharmacokinetics and therapeutic targets have not been fully investigated. Therefore, TCM is often criticized or even rejected by Western scientists. In this review, we will summarize the pathogenesis of HF and clinically evaluate common Chinese formulae and patent Chinese drugs. Furthermore, putative molecular mechanism of these natural products of traditional Chinese medicine will also be reviewed with respect to current research surrounding their use in treating HF.
HF is characterized by a decrease in cardiac output. According to the guidelines
of American College of Cardiology Foundation (ACCF)/American Heart Association
(AHA), HF with reduced ejection fraction (HFrEF) is defined as an ejection
fraction
During HF, the heart usually increases in size, which results in pathological cardiac hypertrophy. Pathological hypertrophy is characterized by contractile dysfunction and causes cardiac structural remodeling [15]. According to a previous study, calcium abnormalities and changes in gene expression play important roles in the development of cardiac hypertrophy [16] (Fig. 1).
 Fig. 1.
Fig. 1.A schematic of the major signaling pathways involved in cardiac
hypertrophy, impaired antioxidant systems and fibrosis progression in the failing
heart. First, calcium homeostasis imbalance is induced by multiple channels. The
release of Ca
Calcium homeostasis imbalance has been observed in HF and plays a major role in
its progression [17]. There are two major receptors in the sarcoplasmic reticulum
(SR) membrane, type 2 ryanodine receptor (RyR2) and sarcoendoplasmic reticulum
calcium ATPase (SERCA), both of are involved in regulating Ca
In addition to calcium abnormalities, the altered gene expression also
contributes to the progression of cardiac hypertrophy [26]. For instance, some
ligands, such as angiotensin (Ang) II, endothelin (ET)-1 and noradrenaline (NA),
are closely associated with the progress of pathological cardiac hypertrophy.
These ligands can bind with G-protein-coupled receptors (GPCRs) to activate
downstream signaling molecules, including phospholipase C (PLC), Protein kinase C
(PKC) and mitogen-activated protein kinases (MAPKs), and then induce the
expression of certain hypertrophic genes, such as atrial natriuretic peptide
(ANP), B-type natriuretic peptide (BNP) and
Oxidative stress, defined as the excessive generation of reactive oxygen species (ROS), has been shown to play a critical role in the pathophysiology of HF [29, 30]. Normally, ROS are constantly formed in cells and removed by the antioxidant defense system; however, this balance is disturbed in HF. First, antioxidant capacity is impaired in HF; for example, the activities of superoxide dismutase (SOD) and other catalases are decreased [29]. In addition, ROS generation is enhanced in HF due to increased activity of certain enzymes, including NADPH oxidase (NOX), xanthine oxidase (XO), and uncoupled nitric oxide synthases (NOS) [31]. Therefore, we believe that oxidative stress in HF is not only induced by the enhancement of ROS generation but also by the impairment of the antioxidant defense within the heart (Fig. 1).
When excessive ROS are produced, myocardial cell injury is induced via multiple mechanisms (Fig. 1). First, ROS can impair the contractile machinery of cardiomyocytes by modifying the proteins that bind to multiple ion channels, such as calcium, potassium, and sodium channels [32]. Second, ROS can lead to contractile dysfunction by reducing myofilament calcium sensitivity and inhibiting the activity of SERCA [32]. Third, increased ROS production inversely results in further mitochondrial and energy metabolism dysfunction [29]. Fourth, ROS induce cardiac fibroblast proliferation, resulting in extracellular remodeling. Finally, prolonged excessive ROS production could cause DNA damage and lead to cell damage and death.
Cardiac fibrosis is characterized by the accumulation of extracellular matrix (ECM) in the myocardium and is an integral aspect of most pathological cardiac conditions, including HF [33]. In the healthy adult heart, cardiomyocytes are supported by ECM which is synthesized in cardiac fibroblasts. According to a previous study, the concentration of myocardial collagen increases by as much as 3- to 6-fold in HF patients [34]. Although the pathogenesis of cardiac fibrosis in HF has not been fully classified, there are two main related theories. One is that fibrotic changes are due to an accumulation of reactive collagen fibers in the interstitium and perivascular regions [35]. On the other hand, myocyte loss is suspected to stimulate replacement fibrosis to replace dead tissue. Finally, cardiac systolic and diastolic function are affected by collagen accumulation. A wide range of molecular signals are involved in this process (Fig. 1).
Transforming growth factor
In HF, the innate and adaptive immune systems in the heart are activated (Fig. 2). At the beginning of myocardial injury, the innate immune system is activated
to allow the heart to adapt to increased stress in the short term [46]. However,
this inflammatory response can become dysregulated, resulting in activation of
the expression of certain proinflammatory cytokines. According to a previous
study, TNF-
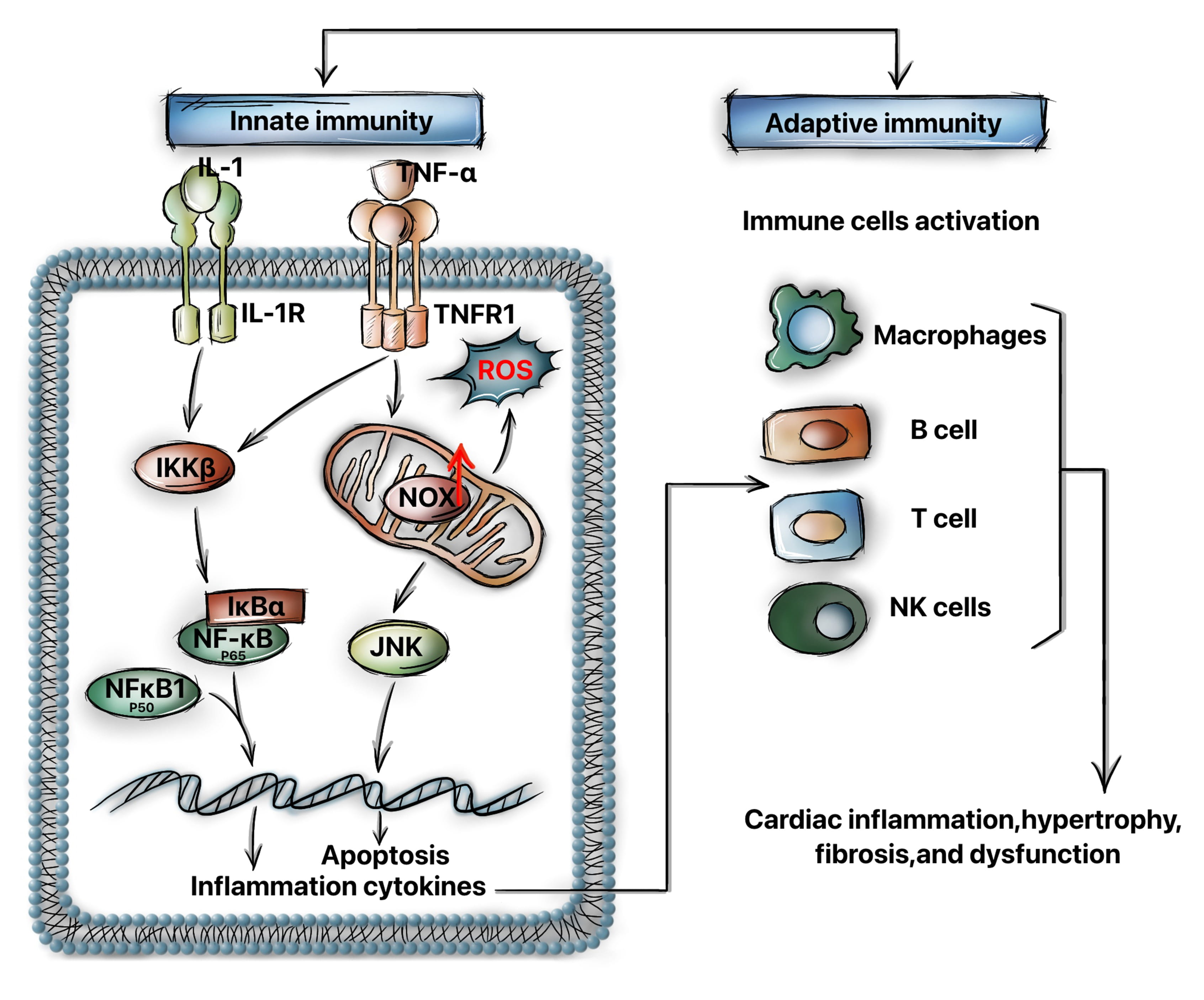 Fig. 2.
Fig. 2.The innate and adaptive immune systems are activated in heart
failure. Acute injury, such as ischemia, pressure, or volume overload, can
induce the binding of certain proinflammatory cytokines (TNF-
On the other hand, the adaptive immune system provides a highly specific response that is mediated by certain cellular components, such as activated T lymphocytes, macrophages and NK cells. These cells were observed to be increased in number in histological heart specimens from patients with chronic HF [51]. These activated macrophages, T cells and B cells can trigger a sustained inflammatory response by stimulating cytokine production, ultimately resulting in cytotoxicity and tissue injury [43].
The loss of cardiomyocytes due to cell death, including apoptosis and necrosis,
has been implicated in HF [52, 53], while inhibition of cardiomyocyte death has
beneficial effects in the prevention of HF [54, 55]; therefore, blocking
cardiomyocyte death may be a new potential approach for the HF treatment. It was
reported that FasL is expressed in HF patients [56]; furthermore, suppression of
FasL has beneficial antiapoptotic effects and reduces the risk of death in HF
patients [57, 58]. On the other hand, the loss of mitochondrial cristae and
destruction of the inner mitochondrial membrane were observed in vivo in
animal models and in HF patients [59]. This process could mediate the cleavage of
Bid and promote cytochrome c release. Finally, the induction of mitochondrial
necrosis is triggered by the opening of the mitochondrial permeability transition
pore (mPTP) due to Ca
Considering the complex mechanisms of HF pathophysiology, it is clear that
multiple signaling pathways and processes are involved in the induction of HF.
More importantly, these processes do not function alone and are affected by each
other. For instance, calcium abnormalities not only induce cardiomyocyte
hypertrophy but also aggravate oxidative stress and increase ROS production. In
addition, some proteins, such as Ang II and TNF-
As mentioned above, Chinese herbs have been used for the effective management of HF for a long time. There are four different types of Chinese medicines that are being used in preclinical studies and in the clinic to treat HF, including TCM formulae, Chinese medicines, crude extracts of herbsand active components and compounds.
The herbs commonly used to treat HF are: Radix aconiti carmichaeli (Fuzi), Radix astragali (Huangqi), Panax ginseng (Renshen), Salvia miltiorrhiza (Danshen), etc. Chinese herbs have been successfully produced and made into the form of capsules, pills or injections, such as Qishenyiqi dripping pill (QSYQ), Danqi pill (DQP), Shengmai capsule, Shenmai injection, and Shengmai injection. On the other hand, several herbs in different proportions can be prescribed together. These decoctions prescribed by physicians include: Baoyuan tang, Fuzhengkangfu Decoction, Shengmai san, Xuefuzhuyu tang and Zhenwu tang, etc. Compared to herbal compounds, active components and compounds can easily be investigated by scientifically rigorous clinical evaluations and some have been approved by the Food and Drug Administration (FDA) [62]. The different types of Chinese medicine used in the clinic and their components and sources are listed in Table 1.
| Medicine form | Name | Dosage form | Prescription rights | Source | Major active compounds | Target disease |
| TCM formula | Baoyuan tang | Decoction | TCM Physicians | Panax ginseng, Radix astragali, Glycyrrhiza uralensis, Cinnamomum cassia | Astragaloside IV, Ginsenoside, etc. | Coronary disease, Congestive heart failure |
| Danshen Yin | Decoction | TCM Physicians | Salvia miltiorrhiza, Santalum album, Amomum villosum | Tanshinone IIA, Salvianolic acid B, etc. | Coronary disease | |
| Fuzhengkangfu Decoction | Decoction | TCM Physicians | Radix codonopsis, Radix saposhnikoviae, Rhizoma atractylodis macrocephalae, Poria, Radix morindae officinalis, Radix glycyrrhizae | Tangshenoside, Trametenolic acid, Arachic acid, etc. | Non small cell lung cancer, Heart failure | |
| Shengmai San | Decoction | TCM Physicians | Panax ginseng, Ophiopogon japonicas, Schisandra chinensis | Ophiopogon saponin, Ginsenoside, etc. | Coronary disease, Congestive heart failure | |
| Taohongsiwu Tang | Decoction | TCM Physicians | Angelica sinensis, Ligusticum chuanxiong, Paeonia lactiflora, Carthamus tinctorius, Radix rehmanniae preparata, Prunus persica | Polysaccharide, Iridoids, Ligustrazine, Carthamin, Paeoniflorin, Amygdalin, etc. | Coronary disease, Cerebral infarction | |
| Xuefuzhuyu Decoction | Decoction | TCM Physicians | Prunus persica, Angelica sinensis, Carthamus tinctorius, Radix paeoniae, Achyranthes bidentata, Ligusticum chuanxiong, Platycodon grandiflorus, Radix Bupleuri, Citrus aurantium, Rehmannia glutinosa, Glycyrrhiza uralensis | Carthamin, Amygdalin,Paeoniflorin, Triterpene saponins, Lgustrazine, Saikoside, Iridoids, Umbellifera lactone, etc. | Coronary disease, Congestive heart failure | |
| Zhenwu Tang | Decoction | TCM Physicians | Poria cocos, Paeonia lactiflora, Atractylodes macrocephala, Zingiber officinale Roscoe, Radix Praeparata | Ganodericacid, Paeoniflorin, Atractylol, Zingiberol, etc. | Cardiogenic edema heart failure | |
| Chinese medicine | Danqi Pill | Guttate Pills | Western medicine/TCM Physicians | Salvia miltiorrhiza, Panax notoginseng, Borneol | Borneol, Notoginsenoside Tanshinone IIA, etc. | Coronary disease,Angina pectoris |
| Danhong Injection | Injection | Western medicine/TCM Physicians | Salvia miltiorrhiza, Carthamus tinctorius | Carthamin, Tanshinone IIA, etc. | Coronary disease, Angina pectoris, Myocardial infarction | |
| Qili Qiangxin Capsule | Capsule | Western medicine/TCM Physicians | Radix astragali, Ginseng, Aconitum carmichaelii Debx, Salvia miltiorrhiza, Lepidium virginicum, Alisma orientalis, Polygonatum odoratum, Cinnamomum cassia Presl, Carthamus tinctorius , Periploca sepium , Citrus reticulata Blanco | Astragaloside IV, Ginsenoside, Aconitine, Tanshinone IIA, Alisol, Carthamin, Cinnamaldehyde, Hesperidin, etc. | Coronary disease, Congestive heart failure | |
| Qishen Yiqi Dripping Pill (QSYQ) | Guttate Pills | Western medicine/ TCM Physicians | Radix astragali, Salvia miltiorrhiza, Panax notoginseng, Dalbergia odorifera | Astragaloside IV, Tanshinone IIA, Duartin, Ginsenoside, etc. | Coronary disease, Angina pectoris | |
| Shenmai Injection | Injection | TCM Physicians | Panax ginseng, Ophiopogon japonicus | Ginsenoside, Ophiopogonin, etc. | Coronary disease, Viral myocarditis | |
| Shengmai Injection | Injection | TCM Physicians | Panax ginseng, Ophiopogon japonicas, Schisandra chinensis | Ginsenoside, Ophiopogonin, Schizandrin, etc. | Myocardial infarction,Chronic heart failure | |
| Crude extract of herb | Di’ao XinxuekangCapsule | Capsule | Western medicine/TCM Physicians | Dioscorea panthaica | Steroidal saponins | Coronary disease, Chronic heart failure, |
| Huangqi Injection | Injection | TCM Physicians | Radix astragali | Astragaloside IV (AS-IV) | Viral myocarditis | |
| Active component and compound | Adoniside | Tablet | Western medicine/TCM Physicians | Adonis vernalis | Adoniside | Acute and chronic heart failure |
| Astragaloside IV (AS-IV) | Tablet | Western medicine/TCM Physicians | Radix astragali | Astragaloside IV(AS-IV) | Chronic heart failure | |
| Digitoxin | Tablet/Injection | Western medicine/TCM Physicians | Digitalis lanata | Digitoxin | Coronary disease Chronic heart failure | |
| Tanshinone IIA | Injection | TCM Physicians | Salvia miltiorrhiza | Tanshinone IIA | Acute myocardial infarction, severe heart failure | |
| Ibopamine | Tablet | Western medicine/TCM Physicians | Plectranthus barbatus | Ibopamine | Heart failure | |
| Ouabain | Tablet | Western medicine/TCM Physicians | Strophanthus gratus | Ouabain | Coronary disease Congestive heart failure |
Although TCMs in China do not undergo the same rigorous testing procedures as Western drugs, a large number of randomized controlled trials were carried out to assess the efficacy of TCMs to treat HF. Some of these TCMs have already been put into clinical evaluations.
Di’ao Xinxuekang capsule is extracted from the rhizomes of Dioscorea panthaica. A randomized control trial showed that the proportion of angina pectoris patients was significantly reduced after 20 weeks of treatment with Di’ao Xinxuekang capsule [63]. Furthermore, a randomized clinical trial demonstrated that Di’ao Xinxuekang capsule seemed to have a better protective effect on HF patients than isosorbide dinitrate [64].
QSYQ, a patented Chinese prescription, has been applied for the integrative treatment of HF patients. A randomized clinical trial demonstrated that 12 months of QSYQ treatment had effects similar to those of aspirin for the treatment of myocardial infarction [65].
Qili qiangxin capsule was approved for the treatment of HF in 2004. It contains extract of 11 Chinese herbs, as shown in Table 1. In a clinical trial, N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels were downregulated by Qili qiangxin capsule and its performance was superior compared with the placebo [66].
Huangqi injection is an extract of Radix astragali that is also commonly used to treat chronic HF in the clinic. It was found that Huangqi injection has a beneficial effect on improving heart function, such as increased left ventricular ejection fraction (LVEF) and decreased stroke volume (SV) levels [67]. On the other hand, it was found that the expression levels of sIL-2R, IgG and IgA were reduced in congestive HF patients after Huangqi injection treatment which suggested that immune function was also rescued [68].
Shenmai injection is extracted from Panax ginseng. A double-blind, multicenter study showed that the Shenmai injection group demonstrated a significant improvement in heart function compared with the placebo group [69]. On the other hand, Shenmai injection also improved left ventricular diastolic function (LVDF) in chronic HF patients [70]. Finally, Shenmai injectionreduced the expression levels of plasma neuropeptide Y (NPY), endothelin (ET) and atrial natriuretic polypeptide (ANP) to improve the cardiac function in HF patient [71].
Shengmai injection is extracted from Panax ginseng, Ophiopogon japonicas and Schisandra chinensis. It was reported that Shengmai injection could improve heart function in HF patients and inhibit myocardial cell apoptosis by reducing the death receptor of soluble tumor necrosis factor related apoptosis inducing ligand (TRAIL) [72]. The levels of plasma P-selectin, von Willebrand factor (vWF) and D-dimer in HF patients were also reduced after Shengmai injection which suggests that cardiac function was improved [73].
Digitoxin, a traditional Chinese medicine monomer extracted from Digitalis lanata Ehrh (Maohua Yangdihua) has been commonly applied in the treatment of HF patients. Several double-blind placebo-controlled trials have proven the efficacy of digoxin in the treatment of HF [74, 75, 76]. From the PROVED trial, a significant worsening of exercise duration occurred in patients on placebo medication when compared with the digoxin group. Moreover, the left ventricular ejection fraction was increased in the digoxin group and decreased in the placebo group [74]. A randomized, placebo-controlled clinical trial enrolled 3505 patients and revealed that digitoxin could reduce the risks of mortality and hospital admission for HF patients [75]. On the other hand, it was shown that the differences in chronic HF scores during the digoxin and digitoxin periods were not significant [76]. In addition to digitoxin, other natural products extracted from Digitalis lanata, including lanatoside A/C, were used to treat HF in clinical trials [77, 78].
Tanshinone IIA is a potent pharmacological compound extracted from Salvia miltiorrhiza (Danshen). Tanshinone IIA has a similar therapeutic effect on myocardial infarction as alprostadil; however, the cardiac functions of all patients who were treated with Tanshinone IIA injection were significantly improved [79]. As a derivative of Tanshinone IIA, sodium Tanshinone IIA sulfonate (STS) also has a cardioprotective effect. In a randomized clinical trial, 101 myocardial infarction patients were immediately assigned to receive STS or saline control, and 6-months of treatment with STS inhibited the damage to the infarcted myocardium by significantly reducing the level of neutrophils [80].
Astragaloside IV(AS-IV) is the main component of Radix Astragali (Huangqi) which is widely used among patients with chronic HF in the clinic [81]. Alleviation was shown in 15 congestive heart failure patients, and their exercise capability was reinforced after AS-IV injection [82]; however, there have been no direct clinical trials to explore the cardioprotective effect of adoniside.
Ibopamine is extracted from Plectranthus barbatus. A double-blind placebo-controlled study showed that ibopamine inhibited neurohumoral activation in patients with mild to moderate chronic HF [83].
The pharmacological mechanisms by which these natural products treat HF have been explored in numerous studies [84]. Experimental studies have demonstrated that natural products from Chinese herbs have anticardiac hypertrophy, antioxidant, antifibrotic, antiinflammatory and antiapoptotic properties (Table 2, Ref. [85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135]). As discussed above, these biological pathways are interconnected, and these natural products may function in a multicomponent and multitarget manner.
| Property | Natural products | Targets or pathways | Type of study | References |
| Inhibiting hypertrophy | Zhenwu Tang | p-ERK1/2↓, p-p38↓, p-JNK↓ and BNP↓ | Constricting the abdominal aorta in Rat | [85, 86] |
| Danhong injection | p38 and NF-κb pathway↓→ANP and BNP↓ | ISO in H9C2 and rat | [87] | |
| Shengmai injection | AMPK signaling pathway↑ | Angiotensin II in primary cardiomyocyte | [88] | |
| Digitoxin | Na |
Coronary artery ligation in rats | [89] | |
| Tanshinone IIA | Voltage-gated Ca |
Systolic blood pressure in rat | [90] | |
| Store-operated Ca |
Hypoxia in smooth muscle cells | [91] | ||
| MAPKs and IGF-2R pathway ↓ | Angiotensin II in H9C2 cells | [92] | ||
| Astragaloside IV(AS-IV) | CaSR/PLC/PKC |
Ligaturing LAD in rats | [93] | |
| Nrf2 ↑→TBK1/PI3K/AKT activity↓ | Aortic banding in mice | [94, 95] | ||
| TLR4/NF-кB↓ | ISO in rat | [96] | ||
| Anti-oxidation | Shengmai San | MDA↓ | Ligaturing LAD in rat | [97] |
| Danqi Pill | HIF-1 |
Ligaturing LAD in rat, OGD/R-induced H9C2 cells | [98] | |
| Shengmai injection | AKT and ERK1/2→SOD ↑ | H2O2 in n primary cardiomyocyte | [99] | |
| Tanshinone IIA | GPx↑→SOD ↑, MDA↓ | H2O2 in macrophages | [100] | |
| Nrf2↑ | H2O2 in H9C2 cells | [101] | ||
| NADPH oxidase↑ | Ligaturing LAD in rats | [102] | ||
| Astragaloside IV(AS-IV) | NOXs ↓→SOD ↑ | ApoE−/− mice, ISO in primary rat | [103, 104] | |
| MDA, CPK, LDH ↓ | H/R in cardiomyocyte | [105] | ||
| NADPH oxidase-ROS-NF-κB ↓ | H2O2 in HUVECs | [106] | ||
| Ouabain | PI3K/Akt↑→NO ↑ | Ligaturing LAD in rats | [107] | |
| NADPH oxidase↑ | Primary cardiomyocytes | [108] | ||
| Anti- fibrosis | Danshen formulae | NOX2/ROS/p38→Collagen I↓ | ISO in rat | [109] |
| Shengmai San | Collagen I, Collagen III↓ | Myocardial infarction (MI) in rat | [110] | |
| Xuefuzhuyu Decoction | TGF- |
Spontaneously hypertensive rats | [111] | |
| Qishen Yiqi Dripping Pill (QSYQ) | RAAS↓→MMP-2,MMP-9, | Ligaturing LAD in rats | [112] | |
| Collagen I, Collagen III↓ | ||||
| Shengmai | Collagen I, Collagen III↓, TGF- |
Doxorubicin injection in rat | [113] | |
| Tanshinone IIA | NADPH oxidase↑→collagen I, | Ligaturing LAD in rats | [102] | |
| collagen III, TGF‑ |
||||
| NO↑, eNOS phosphorylation↑ | Angiotensin II in rat cardiac fibroblasts | [114] | ||
| NOX↓ | LPS in mice | [115] | ||
| Astragaloside IV(AS-IV) | TRPM7 ↓→Collagen ↓ | ISO in rats; Hypoxia or ISO in fibroblasts | [116] | |
| ROS‑mediated MAPK↓ | ISO in rat | [105] | ||
| ROS↓→cardiotrophin‑1↓→Collagen ↓ | ISO in cardiac fibroblasts | [117] | ||
| Anti-inflammtion | Danqi pill | PLA2, COX-2, NF-κB↓ | Ligaturing LAD in rat | [118] |
| HIF-1 |
Ligaturing LAD in rat | [98] | ||
| TXB2↓, PGI2↑ | Ligaturing LAD in rat | [119] | ||
| Qishen Yiqi Dripping Pill (QSYQ) | NF-κB↓, JAK1↓ and AKT↓ | Ligaturing LAD in rats | [112] | |
| Danhong injection | NF-κB↓→TNF- |
Ligaturing LAD in rat | [120] | |
| Tanshinone IIA | TLR4-NF-κB↓ | Ligaturing LAD in mice | [121] | |
| SIRT1↑→TNF- |
TAC in rat | [122] | ||
| Astragaloside IV(AS-IV) | TLR4/NF-κB ↓ | ISO in rats | [96, 123] | |
| NF-кB signaling ↓, PI3K/AKT↑→TNF- |
LPS in mice | [124] | ||
| Ouabain | IL-6↓, TNF-alpha↓ | LPS in mice | [125] | |
| Anti-cardiomyocyte death | Fuzhengkangfu decoction | Bcl-2↑, cleaved caspase-3↓ | Doxorubicin in H9C2 | [126] |
| Qili Qiangxin Capsule | Fas↓, cleaved caspase-3↓ | Ligaturing LAD in rat | [127] | |
| Shengmai injection | ER stress↓ →caspase-12↓ | Doxorubicin in rat | [128] | |
| Tanshinone IIA/ | Bcl-X↓, cleaved caspase-3↓ | Abdominal aortic coarctation in rat, Doxorubicin in cardiomyocyte | [129, 130] | |
| Nrf2↑→p38 and mTOR signaling↓ | TAC in mice | [131] | ||
| AMPA↑→Bax, cleaved caspase-3↓ | LAD in rat, H2O2 in H9C2 | [132] | ||
| Astragaloside IV(AS-IV) | Calpain-1 and ROS ↓→Bcl-2/Bax↑, MMP ↑ | ISO in rats or H9C2 cells | [133] | |
| PI3K/AKT↑ | Doxorubicin in cardiomyocyte | [134] | ||
| Senp1↓→Bcl-2/bax↑, cleaved caspase-3↓ | LAD in mice | [135] | ||
| AKT, protein kinase B; AMPK, AMP-activated protein kinase; ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; CaSR, calcium-sensing receptor; COX-2, cyclooxygenase 2; ERK1/2, extracellular signal-regulated kinase 1/2; GPx, glutathione peroxidase; HIF-1 | ||||
Although it was reported that Zhenwu Tang could reduce the weight and volume of the heart and improve cardiac function, the mechanism is not clear [85]. Another study showed that Zhenwu Tang delayed ventricular hypertrophy by suppressing the expression of BNP, p-extracellular signal-regulated kinase 1/2 (ERK1/2), p-p38 and p-JNK [86].
According to previous study, the mass of the left ventricle (LV) and the thickness of the LV end-systolic posterior wall (LVPWs) in isoproterenol (ISO)-induced rats were reduced after Danghong injection which was associated with a reduction in atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) [87]. On the other hand, Danghong injection significantly attenuated cardiac hypertrophy by suppressing cell area enlargement and downregulating ET-1-induced brain natriuretic peptide (BNP) and cardiac muscle troponin T (TNNT2) protein expression [136]. In addition, it was reported that Shengmai injection could improve the cell-survival rate and reduce myocardial cell hypertrophy via activation of the AMP-activated protein kinase (AMPK) signaling pathway [88].
The intracellular calcium level is an important hypertrophic messenger and there
are many Ca
Tanshinone IIA has the ability to open ATP-sensitive K
AS-IV improved cardiac diastole in chronic HF by decreasing the expression of
protein kinase C-
As discussed above, oxidative stress plays a key role in the development of HF.
It was reported that YiQiFuMai powder injection (YQFM), a traditional Chinese
medicine prescription redeveloped based on Sheng-Mai-San, could decrease the
content of malondialdehyde (MDA) [97]. Moreover, Danqi Pill protects against HF
induced myocardial infarction by improving myocardial glucose metabolism,
mitochondrial oxidative phosphorylation and biogenesis, which is associated with
regulation of the hypoxia-inducible factor-1alpha (HIF-1
According to Chen et al. [137], Tanshinone IIA reduces the level of MDA and elevates the level of SOD, which is consistent with our previous study [138]. Furthermore, Tanshinone IIA can prevent oxidative stress injury by increasing glutathione peroxidase (GPx) activity and mRNA levels [100]. Although there is no direct evidence for the underlying mechanism, some studies have reported that Tan IIA can induce the expression of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a redox-sensitive transcription factor, to attenuate oxidative stress injury via increased transcription of certain antioxidant genes, including SOD [101]. Finally, a recent study showed that Tanshinone IIA reversed the decreases in superoxide dismutase activity and malondialdehyde and the increases of superoxide anions and NADPH oxidase activity in both HF rats and Ang IItreated cardiac fibroblasts [102].
It was reported that AS-IV treatment could attenuate oxidative stress via
increasing the activity of SOD and reducing nicotinamide adenine dinucleotide
phosphate oxidase (NOX) levels [103]. On the other hand, AS-IV could markedly
enhanced cell viability and reduced MDA content and lactate dehydrogenase (LDH)
activity in cardiomyocytes a hypoxia/reoxygenation (H/R) model [104]. AS-IV could
also block ROS production in ISO-treated primary rat cardiac fibroblasts which
might be associated with inhibiting nicotinamide adenine dinucleotide phosphate
(NADPH) oxidase-NF-
Ouabain is an endogenous cardiac glycoside produced in the central nervous system that can also be extracted from the seeds of Strophanthus gratus [139]. It was reported that ouabain could decrease the vascular reactivity of aortic rings and increase nitric oxide production via a PI3K/Akt-dependent pathway in HF rats [107]. On the other hand, ouabain could also stimulate mitochondrial NADH production to attenuate the oxidative metabolic state [108].
Several TCM formulae have been shown to have anti-fibrotic effects. Isopropyl
3-(3,4-dihydroxyphenyl)-2-hydroxylpropanoate (IDHP), a metabolite of Danshen
formulae, attenuates cardiac fibrosis and decreases collagen I synthesis via a
NOX2/ROS/p38 pathway [109]. Additionally, a recent study showed that a Shengmai
San-derived herbal improved the conduction velocity and homogeneity and decreased
left atrial positive fibrosis areas and the expression of type I and III collagen
in myocardial infarction (MI)-induced HF rats [110]. Finally, a study showed that
Xuefuzhuyu decoction treatment decreased cardiac fibrosis induced by hypertension
and inhibited the expression of TGF-
QSYQ exerted an anti-fibrotic effect in AMI rat models by reducing matrix
metalloproteinase-2 (MMP-2), MMP-9, collagen I and collagen III by inhibiting the
renin-angiotensin-aldosterone system (RAAS) [112]. Other studies confirmed that
QSYQ had definite effect on myocardial fibrosis via the TGF
According to a previous study, Tan IIA administration markedly reversed the
increases of expression levels of collagen I, III, TGF
According to a previous study, AS-IV inhibited transient receptor potential melastatin 7 (TRPM7) protein expression and blocked hypoxia-induced fibrosis to attenuate fibrosis of the heart tissue [116]. On the other hand, AS-IV significantly inhibited ISO-induced fibrosis by blocking MAPK activation in rat cardiac fibroblasts [105]. Finally, cardiac fibroblast proliferation and type I collagen synthesis were effectively abrogated by cardiotrophin-1 (CT-1) small interfering RNA. This result suggested that AS-IV could also prevent cardiac fibrosis by inhibiting CT-1 upregulation [117].
According to a previous study, the Danqi pill downregulated the expression of
phospholipase A2 (PLA2), cyclooxygenase 2 (COX-2) and NF-
Furthermore, QSYQ attenuated myocardial fibrosis by reducing the expression of
the angiotensin type 1 receptor (AT1), increasing the expression of AT2 in the
RAAS pathway and suppressing the levels of NF-
Tanshinone IIA has been used in TCM for the treatment of a variety of
inflammatory and cardiovascular disorders. According to a previous study, Tan-IIA
can attenuate LADinduced myocardial ischemia injury by inhibiting the expression
of certain proinflammatory cytokines (IL-1
AS-IV has a strong effect against inflammation. AS-IV treatment inhibited the
highly activated inflammatory response by suppressing the TLR4/NF-
Ouabain was found to protect against LPS-induced inflammation in mice and
decrease the expression levels of IL-6 and TNF-alpha in vivo [125];
however, a recent study demonstrated that ouabain could induce NLRP3 inflammasome
activation as well as subsequent IL-1
According to our study, Fuzhengkangfu decoction ameliorated DOX-induced H9C2
cell apoptosis and increased the Bcl-2/Bax ratio which might be associated with
inhibiting the loss of mitochondrial membrane potential [126]. Qili qiangxin
capsule inhibited cardiomyocyte apoptosis in ischemic heart tissues by
suppressing the expression of Fas and caspase-3 [127]. QishenYiqi Dripping Pill
also efficiently improved cardiac function by inhibiting cardiac apoptosis via
the
Several studies have reported that Tan IIA plays a critical role in attenuating cardiomyocyte apoptosis in models of HF [129, 130]. Specifically, Tan IIA can protect cardiomyocytes from doxorubicin-induced apoptosis by reducing the levels of cleaved caspase-3 and cytosolic cytochrome c, which are associated with the activation of AKT signaling pathways and suppression of ROS generation [130]. Indeed, our previous study showed that Tan IIA can increase the activity of SOD [146]. Although the underlying mechanism is not clear, it might be associated with an increase in Nrf2 gene transcription. It has been demonstrated that Tan IIA can increase Nrf2 expression to reduce myocardial apoptosis and that this effect is significantly dampened in cardiac-specific Nrf2 knockout mice [131]. Tan IIA can also upregulate AMPK expression and downregulate mammalian target of rapamycin (mTOR) expression to inhibit apoptosis [132]. Finally, Tan IIA can reduce apoptosis via inhibition of endoplasmic reticulum stress [146].
It was found that the apoptosis rate was reduced by AS-IV treatment which was associated with increasing Bcl-2 levels, decreasing the expression of calpain-1 and improving the integrity of the mitochondrial structure and the mitochondrial membrane potential (MMP) in ISO-induced hypertrophic cardiomyocytes; moreover, AS-IV also increased the expression of mitochondrial superoxide dismutase (mito-SOD) [133]. Furthermore, AS-IV significantly inhibited apoptosis via the PI3K/Akt signaling pathway in DOX-induced cardiotoxicity [134]. Finally, AS-IV improved the cardiac function of HF mice by decreasing the levels of ROS and H2O2 in the myocardium, suppressing the decrease in mitochondrial membrane potential and decreasing myocardial cell apoptosis by decreasing the expression of Bcl-2 and increasing the expression of cleaved caspase-3 and Bax. These functions were mediated by inhibiting the increased expression of small ubiquitin-like modifier (SUMO)-specific protease 1 (Senp1) [135].
As discussed above, HF is associated with multiple risk factors. Therefore, multitargeted treatments may be more effective in treating HF than therapy against a single target. However, chemical drugs do not have ideal therapeutic effects according to recent clinical studies. The combined application of herbal and chemical drugs for the treatment of HF is emerging as a trend in modern medicine. Natural products from Chinese herbs have been shown to have anticardiac hypertrophy, antifibrotic, anti-inflammatory, antioxidative and antiapoptotic properties. However, there are several limitations to these findings. First, most of the evidence did not come from clinical studies, and there is a lack of large scale, multicenter, randomized and controlled clinical trials for the use of these drugs to treat HF. Second, it has been shown that at high concentrations, some natural products have toxic effects on embryonic development [147]. The systemic and organ-specific toxic effects and the minimally toxic dose of these natural products remain to be investigated. Finally, HF is caused by multiple factors. A large part of the published literature has focused on examining the effects of natural products on one or a few aspects of HF. Despite these limitations, further clinical trials and experimental studies will provide a better understanding of the mechanism of natural products from Chinese herbs to promote the development of these natural products from Chinese herbs for the treatment and prevention of HF.
LHX, LYC and GYG wrote the manuscript with support from YW, YZX and YGZ; LHX LYC and GYG revised the manuscript under the guidance of YW, YZX and YGZ. All authors read and approved the final manuscript.
Not applicable.
We would like to thank the key medical discipline (Cardiovascular medicine) of Hangzhou for supporting references material during the preparation of this manuscript.
This research was funded by the Science and Technology Program of Traditional Chinese Medicine in Zhejiang Province (2022ZZ028).
The authors declare no conflict of interest.
