†These authors contributed equally.
Academic Editors: Brian Tomlinson and Takatoshi Kasai
Background: Quantitative flow ratio (QFR) is a novel
angiography derived fractional flow reserve (FFR) technique. However, its
diagnostic accuracy has only be validated in native coronary lesions but not in
vessels after bioresorbable scaffold (BRS) implantation. This study aims to
evaluate the diagnostic accuracy of residual QFR in coronary vessels immediately
post-BRS implantation. Methods: This is a retrospective, two center, validation cohort study. 73 stable
angina patients who received at least one de novo lesion of an
everolimus eluting stent (EES)/BRS implantation with subsequent residual FFR
assessment were screened. Patients with aorta-ostial stenoses,
bridge vessels at the distal segment of targeted vessels, acute coronary
syndrome, previous coronary artery bypass grafting, age
Percutaneous coronary intervention (PCI) is
an effective technique to relieve myocardial ischemia [1]. Fractional flow
reserve (FFR), an invasive technique using adenosine infusions, has been the gold
standard to evaluate the functional significance of coronary artery lesions [2].
FFR
These studies demonstrated the feasibility and accuracy of QFR in assessing
the severity of coronary stenoses. Diagnostic accuracy of residual QFR correlated
well with post-PCI FFR [9]. To further
evaluate the effect of post-PCI, QFR was developed for invasive FFR
approximation. In clinical practice, the success of a PCI is
based solely on the post PCI angiographic assessment. Post-PCI physiologic
assessments showed that up to 30% of patients had an FFR
With the development of PCI, the concept of non-implantation is deeply rooted in
the philosophy of cardiologists. BRS is a novel poly-L-lactic acid
(PLLA)—based scaffold [10], whose beam is completely
absorbed within 3–5 years after implantation. The thickness of BRS is 170
The aim of this study is to evaluate the diagnostic accuracy of residual QFR in coronary vessels immediately after BRS implantation. We assessed the relationship between residual QFR and FFR in a cohort of patients post-EES or -BRS implantation.
Patients who were referred for PCI and then for invasive FFR measurement
following stent implantation were included in this study. Patients were excluded
if they had aorta-ostial stenoses, bridge vessels at the distal
segment of the targeted vessel, acute coronary syndrome (ACS), previous coronary
artery bypass grafting (CABG), age
We conducted a retrospective, two center, validation cohort study from June 2019
to July 2020. During the study period, 105 patients underwent residual FFR
measurements post-PCI as shown in Fig. 1. 6 patients were excluded for high FFR
drift; 4 patients were excluded for aorta-ostial stenoses, 3 patients were
excluded for bridge vessels at the distal targeted vessel, 5 patients were
excluded for ACS, 2 patients were excluded for previous CABG, 2 patients were
excluded for age
 Fig. 1.
Fig. 1.Study flow chart. 105 patients underwent residual FFR measurements post-PCI. After screening for enrollment and exclusion criteria, 73 lesions from 73 patients were eventually enrolled into this study.
Angiographic projections were performed with biplane systems. All FFR
measurements were performed with a pressure wire and a monitor (St. Jude Medical,
Minn, USA). A bolus of 200
Details concerning the QFR calculation have been reported previously [11]. At
least two adequate contrast-filled angiographic projections with
Continuous variables were reported as mean
In total, 73 vessels in 73 patients were included in the study. The baseline characteristics of all patients are shown in Table 1. 17 patients had diabetes mellitus and 40 patients had hypertension. 22 patients were smokers. 55 patients had a prior PCI. None of the patients had prior ACS, CABG or chronic obstructive pulmonary disease (COPD). 51 patients had been implanted with everolimus-eluting stents (EES) and 22 patients had been implanted with bioresorbable scaffold (BRS).
| Total (73) | |
| Age (years) | 65.24 |
| Male | 53 (70.7%) |
| BMI |
24.64 |
| Creatinine | 79.76 |
| eGFR |
87.99 |
| Lp (a) | 11.25 (5.38, 32.50) |
| LDL-C | 1.99 |
| NT Pro BNP | 55.00 (26.00, 129.00) |
| HbA1c (%) | 6.22 |
| LVEF |
68.49 |
| Diabetes | 17 (22.7%) |
| Hypertension | 40 (53.3%) |
| Smoking | 22 (29.3%) |
| Prior MI |
0 |
| COPD |
0 |
| Prior CABG |
0 |
| Prior PCI |
55 (73.3%) |
| EES |
51 (70.7%) |
| BRS |
22 (29.3%) |
In total, 73 coronary arteries were analyzed. The mean residual QFR and FFR
values was 0.91
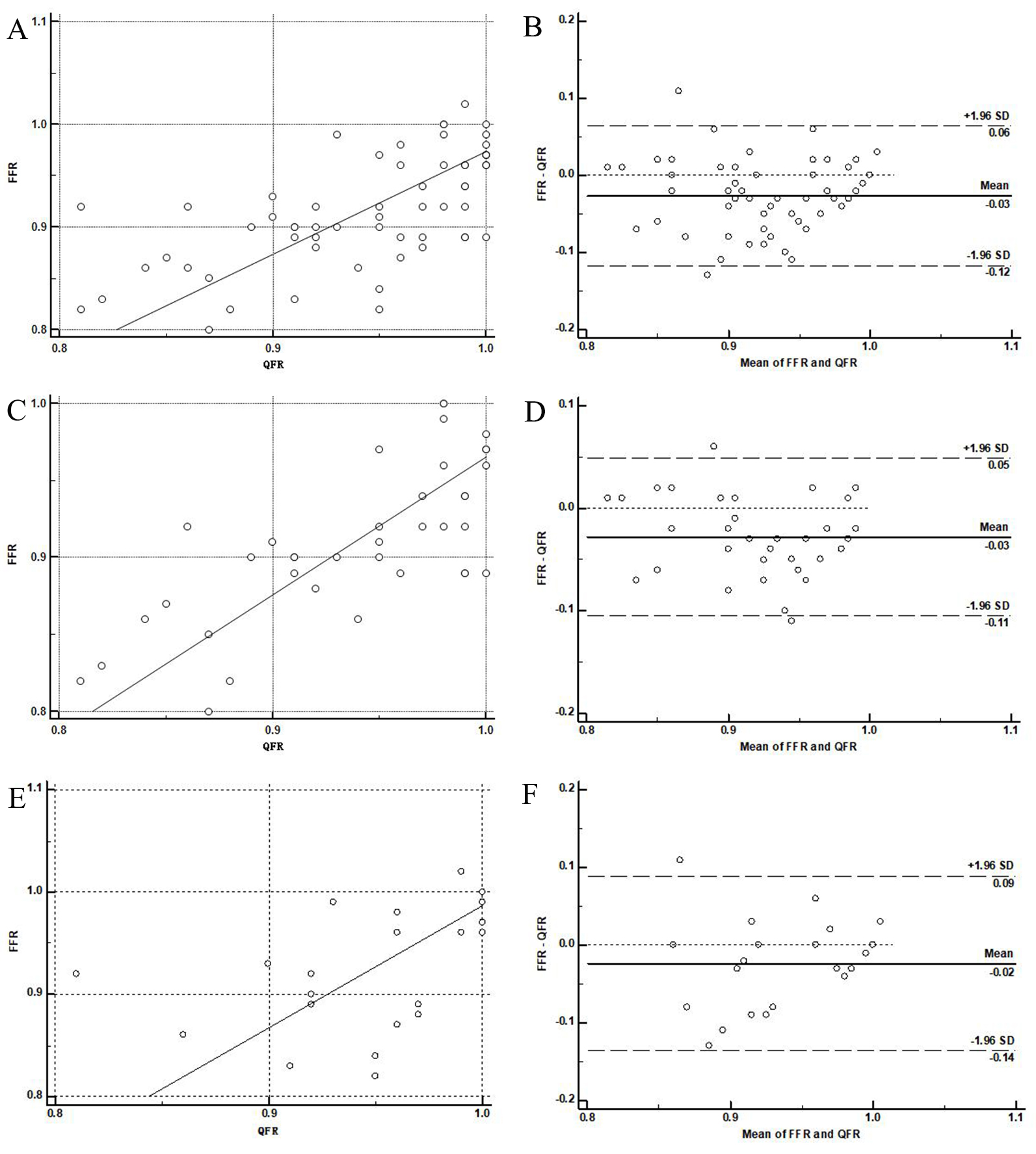 Fig. 2.
Fig. 2.Pearson’s correlation. (A) and agreement (B) between QFR and FFR in 73 patients. Pearson’s correlation (C) and agreement (D) between QFR and FFR in EES group. Pearson’s correlation (E) and agreement (F) between QFR and FFR in BRS group.
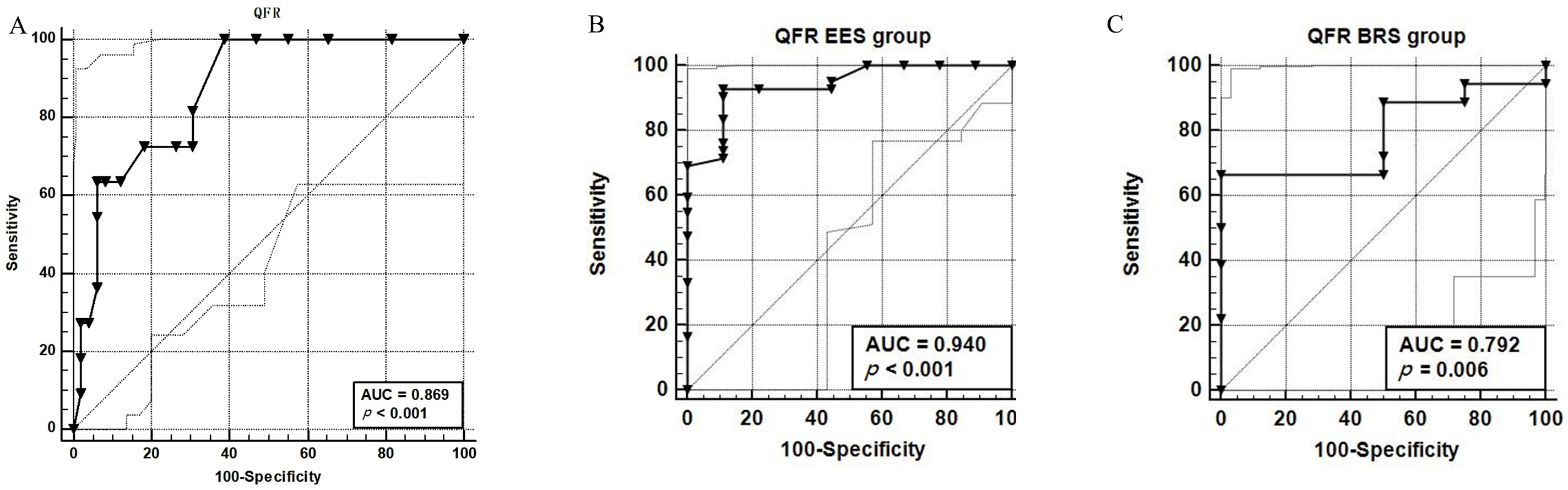 Fig. 3.
Fig. 3.Area Under Receiver-operating characteritic curve for QFR. ROC
for QFR to detect an invasive FFR of
Our study revealed equal diagnostic accuracy of residual QFR in coronary vessels immediately after BRS implantation compared with residual FFR. The residual QFR showed a moderate correlation and agreement with residual FFR for the detection of cutoff 0.86 in post-BRS patients. In addition, the physiologic QFR indices were evaluated using FFR as a reference standard. Importantly, residual QFR showed moderate correlation and certifying performance for invasive FFR, regardless of the types of implanted stents. Residual QFR in the BRS group showed a moderate agreement and correlation compared with the EES group.
There have been numerous efforts to detect the clinic significance of coronary
stenoses to determine the need for PCI. Although many non-invasive tests to
assess myocardial ischemia are available, previous studies reported a low
diagnostic yield [8]. Post-PCI physiologic assessment in complex lesions showed
many patients had FFR
QFR is a method derived from angiography, which calculates the number of
3-dimensional quantitifying coronary angiography (3D QCA) and TIMI (thrombolysis
in myocardial infarction) frames without the expense of wire instrumentation or
the need for coronary artery hyperemia. A good correlation and agreement were
observed between QFR and FFR in the FAVOR Pliot Study [5]. The
FAVOR II China Study assessed the diagnostic accuracy of QFR
prior to FFR measurement [7]. Compared with diameter stenoses (DS)
Although QFR is not currently the gold standard for evaluating myocardial
ischemia, QFR is now the most studied angiography-derived FFR technology. Thus,
QFR may provide more accurate information on the hemodynamic significance of a
coronary stenosis prior to PCI. QFR has not been well validated with FFR in
evaluating post-PCI interventions. No studies has been reported on whether QFR is
comparable to FFR in patients with residual coronary artery stenoses post-PCI. In
this study, we found that QFR showed a good correlation and agreement with FFR in
post-PCI both in the EES and BRS groups. This study verifies the improved
diagnostic accuracy of QFR with FFR following PCI. The
correlation and agreement between residual QFR and FFR were
0.63, p
We found moderate correlation and agreement in BRS group. Our study is unique in
that it included BRS, the latest generation of coronary stents. QFR and FFR had
moderate correlation (r = 0.45, p = 0.04) and agreement (–0.02
According to the European Society of Cardiology (ESC) guidelines, FFR is
recommended to identify hemodynamically significant coronary lesions [16]. FFR
has many limitations in clinical practice. The use of QFR in the catheter
laboratory is very feasible [17]. When computed by specialized technicians, the
average QFR calculation time is only 4.36
Visual assessment alone is known to be inaccurate in determining the hemodynamic significance of residual coronary stenoses post-BRS implantation. Additional functional testing, such as FFR increases procedure time and may lead to increased X-ray exposure. Our study showed that residual QFR was similar in accuracy to residual FFR in estimating residual coronary stenoses post-BRS implantation, which decreased operating time and radiation exposure.
This study was limited by its retrospective analysis. Determination of residual coronary stenoses would be more accurate when performed on prospectively selected angiographic projections with high image quality. The selection of angiographic images may result in a superior diagnostic accuracy of residual QFR and a further increase in the proportion of patients that could be correctly deferred from invasive FFR referral post-PCI. Our patient cohort is a real-world representation of the patients that are referred for invasive FFR. Finally, selection bias cannot be excluded due to the retrospective design of this study.
Residual QFR shows a moderate correlation and good agreement with FFR. Residual QFR assessment after BRS implantation is feasible, and has a moderate correlation and agreement with residual FFR. QFR may be a promising tool similar to FFR to evaluate post-BRS effect.
QFR, quantitative flow ratio; FFR, fractional flow reserve; EES, everolimus eluting stent; BRS, bioresorbable scaffold; PCI, percutaneous coronary intervention; ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; ROC, receiver operator characteristic; COPD, obstructive pulmonary diseases; 3D QCA, 3-dimensional quantifying coronary angiography; DS, diameter stenosis; ESC, European Society of Cardiology.
Conceptualization—ZL and CJ; methodology—JZ and JH; software—JH; validation—GF and CJ; formal analysis—ZL; investigation—ZL and JZ; resources—ZL and GF; data curation—ZL; writing - original draft preparation—ZL, GF and CJ; writing - review and editing—ZL and CJ; visualization—CJ; supervision—CJ; project administration—CJ; funding acquisition—ZL and CJ. All authors have read and agreed to the published version of the manuscript.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Sir Run Run Shaw Hospital Affiliated to Zhejiang University School of Medicine (approval number: 20200917).
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
