Academic Editors: Yoshiaki Kaneko and Konstantinos P. Letsas
Recent versions of evidence-based guidelines on the management of atrial fibrillation (AF) have been published by the European Society of Cardiology (ESC) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS), the American College of Cardiology, American Heart Association, and the Heart Rhythm Society (AHA/ACC/HRS), and the Canadian Cardiovascular Society/Canadian Heart Rhythm Society (CCS). As all societies refer to the same multicentric and usually multinational studies, the similarities undoubtedly outweigh the differences. Nonetheless, interesting differences can often be found in details, which are usually based on a different assessment of the same study, the availability of data in relation to the publication date and local preferences and availabilities of certain cardiovascular drugs. The following article aims at lining out these similarities and differences.
Evidence-based guidelines on the management of atrial fibrillation (AF) are published and updated to integrate new scientific evidence for multiple years by the large cardiologic societies. In 2020, the European Society of Cardiology (ESC) published the third and current version of recommendations for diagnosis and management of atrial fibrillation in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) [1]. The latest cumulative guidelines from the American College of Cardiology, American Heart Association, and the Heart Rhythm Society (AHA/ACC/HRS) were published in 2014 [2] and underwent a focused update in 2019 [3]. The most recent guidelines on atrial fibrillation were published by the Canadian Cardiovascular Society/Canadian Heart Rhythm Society (CCS) in late 2020 [4].
This article aims at comparing these international guidelines for the management of atrial fibrillation and to identify both similarities and differences. Since all three guidelines largely refer to the same multicentric and usually multinational studies, the similarities undoubtedly outweigh the differences. Nonetheless, interesting differences can often be found in details, which are usually based on a different assessment of the same study, as for example the RACE-II trial [5]. In addition, the recent EAST-AFNET 6 trial [6] is only included in the most recently published CCS guideline due to its publication date. Furthermore, especially when comparing recommendations on drug treatment there are differences between the guidelines, which can be traced back to both common local practice and the local availability of certain medications.
Focusing on the structure of the guidelines, all three guidelines have an almost textbook-like character and, in addition to their recommendations for action, also offer a deeper understanding of atrial fibrillation and insight into the decision-making of the respective authors. However, the Focused Update of the AHA guidelines from 2019 [3] does not have this character, but rather represents a short summary of the instructions, especially for the topics of stroke prophylaxis and rhythm control.
In the ESC and AHA guidelines the level of recommendation and evidence is presented in the ACCF/AHA methodology, primarily in form of tables. While the CCS guideline uses the GRADE system mainly by offering short recommending texts.
In clinical practice, atrial fibrillation should be classified by episode duration and persistence. All three guidelines distinguish between paroxysmal, persistent, long-standing persistent, and permanent AF (“PPP”-pattern), whereas ESC guidelines further define first-diagnosed AF (Supplementary Table 1).
Although AF duration is a classification suggested by the three guidelines and relatively easy to define at first glance, this classification bares some shortcomings to be mentioned in the following: (1) the anamnestic AF pattern does not necessarily correlate with the AF burden in ECG monitoring [7], (2) AF duration alone does not predict the risk of AF recurrence or the chance to maintain sinus rhythm, respectively [8]. (3) Furthermore, the “PPP”-pattern neither gives mechanistic insights into the underlying pathophysiology in the individual patient nor points it out treatment options and implications.
As a consequence, the CCS and ESC guidelines further classify AF based on pathophysiological or etiological aspects (CCS) or a multidimensional model including clinical and structural aspects (ESC). The CCS guideline subdivides AF into “primary” and “secondary” AF. AF is defined as “primary AF” when it “represents an established pathophysiological course” and “secondary AF” refers to AF in the setting of reversible or self-limited factors. Secondary AF is further subcategorized into “reversible” and “provoked” AF based on the AF susceptibility and the risk of recurrence.
The latest ESC guidelines introduce a newly developed 4S-AFscheme to achieve a structured characterization of the individual patient in view of stroke risk, symptom severity, severity of AF burden, and substrate severity (Supplementary Fig. 1). This holistic approach of AF classification is only partially based on established scores and is not validated but points out possible assessment tools and emphasizes that “the most appropriate descriptors of AF domains are yet to be defined” [1]. Up to now, there is no unified classification of different patients in the 4S-AF scheme, but it helps to line out and clarify the most important aspects in AF management and motivates health care providers and scientists to validate diagnostic tools for further classification.
The AHA and the CCS guidelines both distinguish between valvular and non-valvular AF. According to publication of the latest CCS guideline and the AHA update of 2019, non-valvular AF is defined as “AF in the absence of moderate-to-severe mitral stenosis or a mechanical heart valve” [3, 4]. The ESC guidelines do not use this subclassification anymore but nevertheless address it when discussing anticoagulation, as all three guidelines are recommending a vitamin K antagonist (VKA) as the drug of choice only in the setting of moderate-to-severe mitral stenosis or in patients with mechanical heart valves. This is due to the limited data on anticoagulation with non-vitamin K antagonist oral anticoagulants (NOAC) in patients with mitral stenosis [9] and the observed increased thromboembolic and bleeding risks under NOACs in patients with mechanical valves [10].
As asymptomatic AF is known to be an independent risk factor for stroke and other arterial thromboembolic events [11, 12], the subject of AF screening to detect asymptomatic AF in patients at high risk of stroke has come into focus.
While the AHA guidelines do not address this subject, the ESC and CCS guidelines offer a section on AF screening (Supplementary Table 2).
The CCS guidelines recommend opportunistic screening for AF in patients of
Furthermore, new screening technologies employing photoplethysmography or single-lead ECG recordings, got into the market. These technologies are addressed in the CCS and ESC guidelines, whereat both guidelines line out their effectiveness especially in population at high AF risk, although a 12-lead-ECG recording remains mandatory to confirm the diagnosis of AF. Notably the topic is not mentioned in the AHA guidelines.
While all guidelines line out the importance of AF-related symptoms, especially for the decision for rhythm control strategies, only the CCS and ESC guidelines recommend specific AF-symptom scales that evaluate the influence of AF-related symptoms on daily life and activities, as presented in Supplementary Table 3. To capture AF related symptoms more precisely, the CCS guidelines recommend the EQ-5D as a generic quality of life (QoL) assessment tool and the AFEQT questionnaire as a disease specific tool.
Furthermore, the CCS and ESC guidelines include non-cardiac AF symptoms such as the increased prevalence of depression in AF patients.
Stroke prevention remains the central part of AF management and a major part of all three clinical practice guidelines.
To weigh the benefit of anticoagulation against the potential bleeding risk,
stroke-risk scores were introduced to identify patients at high risk for stroke.
To this end, the ESC and AHA guidelines recommend the
CHA
The CCS guidelines recommend the CHADS-65 score, which generally recommends
anticoagulation in patients aged
| ESC AHA/ACC/HRS | CCS | |||
| Risk factors | Points awarded | Definitions | Risk factors | Definitions |
| C | 1 | Congestive heart failure | C | Congestive heart failure |
| Symptoms of heart failure, or objective evidence of moderate to severe LV dysfunction, or HCM | ||||
| H | 1 | Hypertension | H | Hypertension |
| or on antihypertensive therapy | ||||
| A | 2 | Age |
A | Age |
| D | 1 | Diabetes mellitus | D | Diabetes mellitus |
| Treatment with oral hypoglycaemic drugs and/or insulin or fasting blood glucose | ||||
| S | 2 | Stroke | S | Previous stroke |
| Previous Stroke, transient ischemic attack, or thrombembolism | or transient ischemic attack | |||
| V | 1 | Vascular disease | ||
| Angiographically significant coronary artery disease, previous myocardial infarction, peripheral artery disease, or aortic plaque | ||||
| A | 1 | Age 65–74 yrs. | ||
| Sc | 1 | Sex category (female) | ||
| Indications for anticoagulation | ||||
| ESC | AHA/ACC/HRS | CCS algorithm | ||
| OAC recommended in AF pts. with a CHA |
For patients with AF and a CHA |
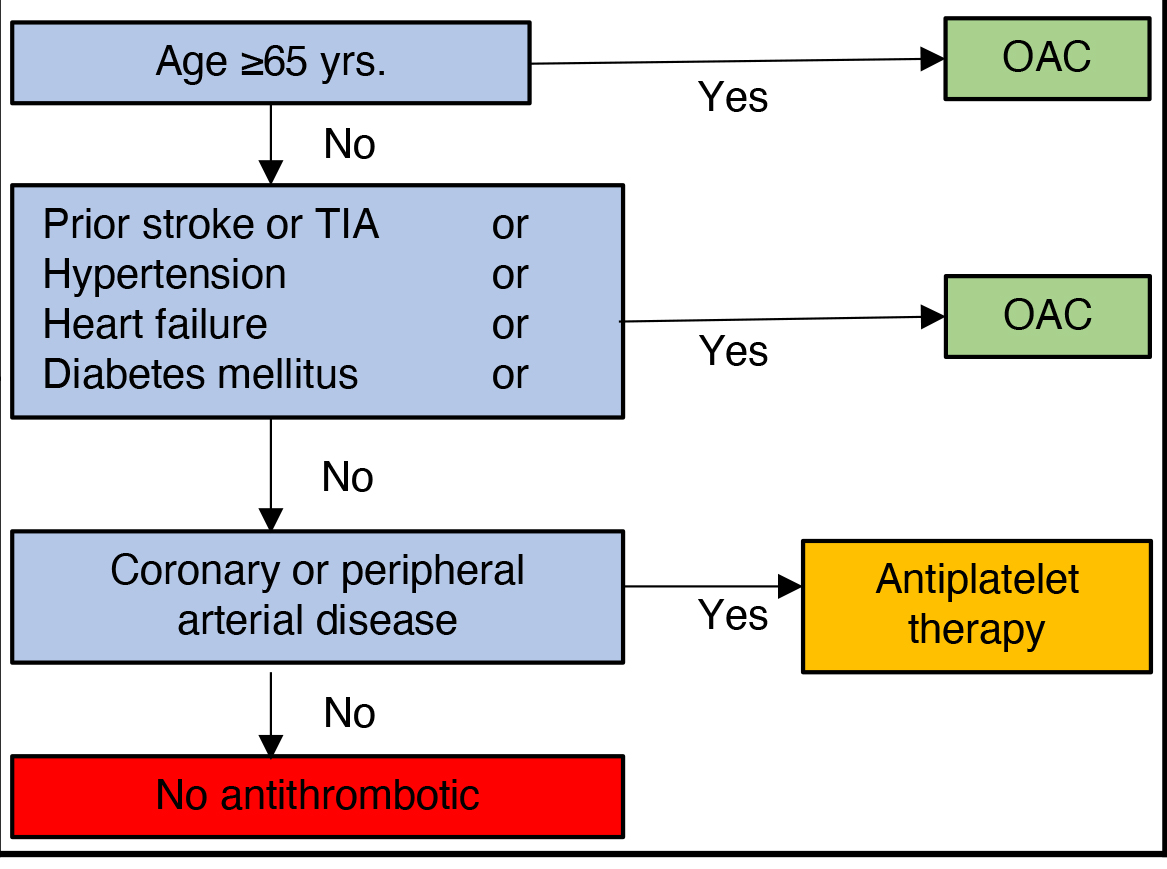 | ||
| Class: I | Class: I | |||
| OAC should be considered in AF pts. with a CHA |
For patients with AF and a CHA |
|||
| Class: IIa | Class: IIb | |||
| AF, atrial fibrillation; HCM, hypertrophic cardiomyopathy; OAC, oral anticoagulation; TIA, transient ischemic attack; yrs., years. Levels of recommendation as presented in the ESC and AHA guidelines. | ||||
The application of these different risk assessment tools results in different treatment recommendations as demonstrated in the following examples (Fig. 1). A 70-year-old male patient, without comorbidities would have a clear indication for anticoagulation in the CCS guidelines, anticoagulation should be considered (IIa) in the ESC guidelines and anticoagulation may be considered (IIb) in the AHA guidelines. In contrast, a 50-year-old female patient with peripheral artery disease would receive sole antiplatelet therapy in accordance with the CCS guidelines, while ESC and AHA guidelines imply different levels of recommendation for effective oral anticoagulation (IIa: ESC; IIb: AHA).
 Fig. 1.
Fig. 1.Illustrative example of different decision regarding anticoagulation in selected patients between the ESC, AHA/ACC/HRS and CCS guidelines. AF, atrial fibrillation; OAC, oral anticoagulation; PAD, peripheral arterial disease; yrs., years, levels of recommendation as presented in the ESC and AHA guidelines.
Furthermore, aortic plaque is not included in the CHADS-65 score, although previous studies have indicated independent stroke-risk associated with aortic plaques [16].
We think that the cumulative nature of the CHA
Aspirin alone is not suitable for stroke prevention in AF according to the ESC,
CCS guidelines. In the previous ESC and in the AHA guidelines from 2014 aspirin
could be considered in borderline indications (CHA
All guidelines do only recommend aspirin in combination with OAC in certain clinical settings, as for example post-PCI (see below) and after LAA occluder implantation.
All three guidelines prefer NOACs over VKA for anticoagulation in patients eligible for NOACs. The preference for NOACs is based on their non-inferiority regarding stroke prevention [18, 19, 20, 21] and simultaneously significantly reduced bleeding risk with NOACs compared to VKA [22, 23]. The criteria for dose reduction of NOACs differ between the guidelines with a trend to stricter dose reduction criteria in the CCS guidelines as presented in Supplementary Table 4.
Left atrial appendage occlusion (LAAO) is considered an alternative to oral anticoagulation in AF patients at medium or high risk of stroke with contraindications for long-term oral anticoagulation according to all three guidelines (Supplementary Table 5). As a result of the limited data comparing LAAO with NOACs and the increased stroke risk under LAAO compared to VKA, the recommendations for LAAO are rather weak (level of recommendation: IIb in ESC and AHA guidelines). The indication for surgical occlusion is differing between the CCS and the ESC and AHA guidelines. Compared to ESC and AHA guidelines, the CCS guidelines do only recommend surgical occlusion or exclusion in patients with contraindications for long-term oral anticoagulation. This recommendation must be questioned in the light of the LAAOS III trial [24], which did not enter the guidelines due to its publication date.
The ESC, CCS, and AHA guidelines emphasize the risk of cardioversion-associated stroke (Supplementary Table 6). Therefore, all three guidelines recommend an anticoagulation period of at least three weeks prior to cardioversion. In case of an unplanned cardioversion, all guidelines agree that cardioversion can be performed after transoesophageal echocardiography (TOE) for thrombus exclusion.
However, the guidelines differ regarding their recommendation of cardioversion
of new-onset AF without TOE: The ESC guidelines allow cardioversion without TOE
or previous OAC within 48 hours of AF-onset. The AHA guidelines do not clearly
comment on TOE in AF patients with AF duration less than 48 hours; but from the
context, cardioversion can be performed without TOE after initiation of OAC in
patients with AF onset of less than 48 hours. The CCS guidelines are giving
different recommendations depending on AF-duration and individual risk of stroke.
If AF-onset is
With regard to anticoagulation post cardioversion, the guideline recommendations only differ for low-risk patients.
The ESC gives a IIb-recommendation (may be omitted) for anticoagulation in
low-risk patients (CHA
The CCS recommendations are mainly based on the data from the FinCV [27],
ClevelandClinic-Study [28], and the ANTIKogulation registry [25] while the ESC
guidelines line out the low thromboembolic risk after cardioversion of AF lasting
Based on previous studies all guidelines emphasize the increased risk for stroke after AF catheter ablation (Supplementary Table 6). All guidelines suggest that OAC should be initiated prior to catheter ablation. The ESC and CCS guidelines incorporate recent studies [31, 32, 33] and advise against interruption of OAC or bridging with heparin for ablation. The former AHA guidelines of 2014 do not give clear recommendations for the management of OAC periprocedural and the subject was not revived in the recent 2019 update.
On the other hand, all guidelines agree that postinterventional OAC should be continued for at least eight weeks in all patients and indication for continuing OAC depends on the individual risk of stroke and is not influenced by ablation-success. A randomized-controlled trial to evaluate the need for OAC after successful catheter ablation is still ongoing [34].
All three guidelines address the subject of anticoagulation in combination with antiplatelet therapy in patients with percutaneous coronary intervention (PCI) (Supplementary Table 7). While the ESC and CCS guidelines discriminate between PCI for acute coronary syndrome (ACS) and elective PCI in the setting of chronic coronary artery disease (CCS), the AHA guidelines only give a recommendation on PCI in ACS.
In general, all guidelines recommend the combination of antiplatelet therapy with oral anticoagulation. The ESC and CCS guidelines recommend all eligible NOAC over VKA for combination with antiplatelet therapy mainly based on the AUGUSTUS [35] and RE-DUAL PCI study [36], whereas the AHA guidelines only mention VKA, rivaroxaban, and dabigatran in combination with antiplatelets without giving a preference for a certain substance class.
For the combination of rivaroxaban with antiplatelet therapy, the CCS guidelines
recommend a dose reduction of rivaroxaban to 15 mg (o.d.) while the ESC
guidelines limit this recommendation exclusively for patients at high bleeding
risk, defined as HAS-BLED score
The most controversial and ambiguous subject in this field is the use of triple
therapy (dual antiplatelet therapy plus anticoagulation) and its duration. The
ESC and CCS guidelines suggest triple therapy after PCI in ACS patients for 1 day
to 1 month (CCS guideline) or
All guidelines emphasize the different treatment options for AF in terms of rate control and rhythm control.
The ESC, CCS, and AHA guidelines do not significantly differ in patient criteria for a rhythm control strategy and mainly symptomatic, younger patients with limited comorbidities and recent-onset AF are considered suitable candidates. Furthermore, all guidelines recommend rhythm-control strategies in patients who failed a rate control regimen and patients with AF-induced tachymyopathy (Table 2).
| Indications for rhythm control | ||
| ESC | AHA/ACC/HRS | CCS |
| Favouring rhythm control: | Favouring rhythm control: | Favouring rhythm control: |
| • Younger age | • Symptoms under rate control | • Recently diagnosed ( |
| • First AF episode or short history | • Failed rate control | • Highly symptomatic or significant QoL impairment |
| • Arrhythmia induced cardiomyopathy | • Younger age | • Multiple recurrences |
| • Normal to moderate increased LAVI/atrial conduction delay (limited atrial remodeling) | • Arrhythmia induced cardiomyopathy | • Difficulty to achieve rate control |
| • No or few comorbidities/heart disease | • First AF episode | • Arrhythmia induced cardiomyopathy |
| • Rate control difficult to achieve | • Patient’s choice | |
| • AF precipitated by a temporary event | ||
| • Patient’s choice | ||
| AF, atrial fibrillation; QoL, Quality of life, LAVI, Left atrial volume-index. | ||
Due to its most recent publication date, solely the CCS guidelines incorporate the results from the EAST-AFNET 4 study [6], which demonstrated positive effects of rhythm over rate control regardless of patient’s symptoms and recommend that “a rhythm control strategy (should) be considered for most stable patients with recent-onset AF” [4].
When focusing on rate control targets, the results of RACE II [5] and AFFIRM
[37] are integrated into all three guidelines in different ways (Table 3). The
ESC guidelines recommend a lenient rate-control strategy with target heart rates
| Rate control | |||||
| ESC | AHA/ACC/HRS | CCS | |||
| • |
• Resting heart rate |
• |
|||
| • | 1st line drugs when LVEF ( -betablockers -verapamil -diltiazem |
• Resting heart rate |
• 1st line drugs when LVEF ( -betablockers -verapamil -diltiazem (Calcium-antagonists could be favorable for long-term therapy) |
||
| • 1st line drugs for rate control: -betablockers -nondihydropyridine calcium channel antagonist |
|||||
| • AV-node ablation should be considered in patients without effective rhythm control who are not eligibile for LA ablation. | • 1st line drugs when LVEF ( -betablockers |
||||
| • 1st line drugs when LVEF ( |
• Patients with preexcitation and AF should not be treated with digoxin, non- dihydropyridine calcium channel antagonists, or intravenous amiodarone as they may result in ventricular fibrillation | • Digoxin might be considered in older or sedentary individuals with permanent AF alone or in combination with betablocker | |||
| • AV-node ablation should be considered in patients without effective rhythm control who are not eligibile for LA ablation. | • In CRT-patients 100% biventricular pacing should be targeted. In cases of biventricular pacing |
||||
| • AV-node ablation should be considered in patients without effective rhythm control who are not eligible for LA ablation. | |||||
| AF, atrial fibrillation; bpm, beats per minute; Ca-Ant., calcium channel antagonists; CRT, Cardiac resynchronization therapy; LA, left atrial; LVEF, Left ventricular ejection fraction. | |||||
Furthermore, possible beneficial effects of calcium-antagonists over betablockers in patients with preserved ejection fraction [38, 39] are lined out in the CCS guidelines.
The comparative RATE-AF study [40] on bisoprolol vs. digoxin on the quality of life of patients with permanent AF was not included in the three guidelines due to its recent publication date.
In the setting of AF with preexcitation, the AHA and CCS guidelines warn against possible AV conduction slowing medications, with the risk of ventricular fibrillation, a subject which is not included in the ESC guidelines.
Lastly, all guidelines recommend AV-node ablation in case of unachievable rate control and unsuccessful conversion to sinus rhythm, especially in elderly patients and in CRT patients with subtherapeutic biventricular pacing. The ESC and AHA guidelines differ in the recommended lower tracking rate after AV-node ablation, whereas the AHA guidelines recommend 90–100 bpm and the ESC guidelines recommend 70–90 bpm to lower the risk of sudden bradycardia-triggered proarrhythmia [41].
All three guidelines include recommendations on cardioversion, while the mode of cardioversion (electrical vs. pharmacological) should be chosen based on patient characteristics, possible side effects, and hemodynamic stability.
The ESC guidelines incorporate data from the RATE-7-trial [42] and therefore offer a watch and wait approach for stable patients with AF and a duration of less than 48 hours.
Electrical cardioversion. The value of electrical cardioversion is mostly its timesaving character especially in the setting of hemodynamic instability.
The preferred mode of electrical cardioversion differs between the guidelines: while the ESC and AHA guidelines recommend an anterior-posterior electrodeposition, the CCS guidelines do not give a recommendation as they evaluate the study results as heterogenous [43, 44, 45].
6.2.2.1 Pharmacological cardioversion
In the setting of hemodynamic stability, pharmacological cardioversion can be performed by intravenous or oral application of different antiarrhythmic drugs. All three guidelines mainly refer to the same drugs and the evaluation of the different drugs is mostly homogeneous. However, they differ in certain details or the local availability of drugs (Supplementary Table 8).
All guidelines agree that amiodarone is not the first drug of choice in patients eligible for other drugs due to its side effects and its delayed effectiveness.
The ESC and AHA guidelines recommend flecainide or propafenone for cardioversion in eligible patients (no LV-dysfunction, no structural heart disease, no signs of coronary artery disease, etc.) and consider ibutilide, especially for cardioversion of atrial flutter [46], but also outline its possible proarrhythmic side effects [47]. In contrast, intravenous flecainide or propafenone is not available in Canada and therefore not recommended in the CCS guidelines, while intravenous magnesium is recommended to reduce ibutilides possible torsadogenic properties [48, 49].
The ESC guidelines state the effectiveness of vernakalant whereas the CCS guidelines do not expect it to be significantly more effective than amiodarone or ibutilide based on previous data [50, 51].
6.2.2.2 Long-term antiarrhythmic drug therapy
In patients with symptomatic AF, antiarrhythmic drugs can be used to reduce symptomatic AF recurrence. All three guidelines collectively recommend the guidance of antiarrhythmic drug therapy based on drug’s safety in the individual patient and the patient’s symptom relief or reduction of symptomatic AF-burden and not by sole drug efficacy (Supplementary Table 8).
Possible and frequently recommended drugs are the class I antiarrhythmic drugs
flecainide and propafenone, which are limited to patients without severe
structural or ischemic heart disease, without severe conduction disorder. They
are preferably combined with betablockers to reduce the risk of atrial flutter
with 1:1 conduction. While all three guidelines emphasize ischemic heart disease
as a possible contraindication, the CCS guidelines clearly recommend an ischemia
risk assessment in patients
For amiodarone the three guidelines acknowledge the significant amount of possible severe side effects, by which it has mostly become a second choice in selected cases. Nonetheless, the ESC and AHA guidelines agree that the risk of proarrhythmia in spite of significant QT prolongation is rare [52, 53].
Dronedarone is a possible alternative to amiodarone, with overall reduced side effects compared to amiodarone, but the ESC and AHA guidelines and presumably most physicians recognize dronedarone as “less effective” compared to amiodarone. In previous randomized trials [54, 55] dronedarone showed reduced cardiovascular morbidity in patients with paroxysmal and persistent AF and further comorbidities, but increased mortality in patients with heart failure, heart failure with reduced ejection fraction (HFrEF), and permanent AF, which resulted in the recommendations in all guidelines as shown in Supplementary Table 8. As a result of the profound data regarding dronedarone it might be considered a first-choice antiarrhythmic drug for suitable patients according to the ESC guidelines.
Sotalol is mostly, due to its I
Long-term antiarrhythmic drug therapy with disopyramide is mentioned in the ESC and AHA guidelines. Both guidelines are evaluating disopyramide as a possible treatment alternative in patients with vagal AF [52] or patients with hypertrophic cardiomyopathy as disopyramide is able to reduce LV outflow tract obstruction [59], but leads to significantly increased mortality [60].
6.2.3 Catheter ablation
Catheter ablation for AF is discussed in detail in all three guidelines as a possible option to achieve rhythm control in certain patients. Predominantly, symptomatic patients after an ineffective or impossible antiarrhythmic treatment regimen are considered suitable for catheter ablation (Table 4). The ESC and AHA guidelines prefer catheter ablation in patients with paroxysmal AF, while patients with persistent AF are also suitable candidates for catheter ablation especially in the absence of risk factors for AF recurrence.
| Catheter ablation of atrial fibrillation | |||||
| ESC | AHA/ACC/HRS | CCS | |||
| Indications for catheter ablation | Indications for catheter ablation | Indications for catheter ablation | |||
| • Symptomatic atrial fibrillation | • Rhythm-control desired and paroxysmal atrial fibrillation refractory to at least 1 class I or III AAD | • Recommended in symptomatic atrial fibrillation with desired rhythm-control who remain symptomatic after AAD trial | |||
| • Catheter ablation after failed AAD: -paroxysmal AF (IA) -persistent AF without risk factors for AF recurrence (IA) -persistent AF with risk factors for AF recurrence (IB) |
• Some pts. with persistent AF | • First-line therapy in selected symptomatic pts. | |||
| • Initial strategy in paroxysmal AF after profound risk consideration | • Success rate higher when performed earlier | ||||
| • May be considered in long-standing persistent AF refractory to at least 1 class I or III AAD | |||||
| First line catheter ablation -paroxysmal AF (IIaB) -persistent AF (IIbC) |
• Might be reasonable in selected HFrEF pts. to improve survival and reduce hospitalization for heart failure | ||||
| • Recommended in AF-related tachymyopathy independent of symptoms | |||||
| • Selected HFrEF pts. to improve survival and reduce hospitalization for heart failure | |||||
| Ablation technique | |||||
| • Complete electrical isolation of pulmonary veins in all AF-ablation procedures | • No recommendations regarding the technique of AF ablation | • PVI is the “cornerstone” of all AF ablation procedures | |||
| • Cavo-tricuspid isthmus isolation (CTI) may be considered in pts. with history of typical atrial flutter or onset of typical atrial flutter during PVI | |||||
| • Additional lesions beyond PVI may be considered but are not well-established | |||||
| AF, atrial fibrillation; AAD, antiarrhythmic drug; CTI, Cavo-tricuspid isthmus isolation; HFrEF, Heart failure with reduced ejection fraction; pts., patients; PVI, pulmonary vein isolation. Levels of recommendation as presented in the ESC and AHA guidelines. | |||||
Furthermore, all guidelines include the results of the CASTLE-AF trial [61] and consider catheter ablation as a suitable treatment option in patients with symptomatic AF and HFrEF to improve survival and reduce hospitalizations for heart failure.
A difference can be found between AHA and CCS guidelines regarding the interpretation of the results of the CABANA trial [62], in which pulmonary vein isolation (PVI) was not superior to antiarrhythmic drugs in the reduction of death, stroke, and cardiac arrest, when focusing on the intention-to-treat analysis. But, as mentioned in the CCS guidelines, PVI was superior in the as-treated analysis.
Furthermore, the CCS guidelines point out upcoming new data on PVI in heart failure with reduced and preserved ejection fraction from the RAFT-AF study [63], which has closed enrollment.
The ESC and CCS guidelines recommend pulmonary vein isolation in all AF-ablation procedures, whereas the data for left atrial ablation techniques beyond PVI (e.g., additional linear ablations, rotors, fragmented activity, ectopic activity, etc.) are sparse and these procedures are not generally recommended.
The latest guidelines on the management of atrial fibrillation show great agreement in most aspects. Nevertheless, differences can be found in detail. To sum up, the ESC and CCS guidelines recently introduced different concepts for AF classification, further differences between the three guidelines can be found in the recommendations on stroke prophylaxis, the indications and targets for rate control and the patients eligible for rhythm control concepts.
AAD, antiarrhythmic drug; ACS, acute coronary syndrome; AF, atrial fibrillation; AFEQT, Atrial Fibrillation Effect on QualiTy-of-Life questionnaire; AFL, atrial flutter; Bpm, beats per minute; b.d., bis in die (twice a day); Ca-Ant., calcium channel antagonist; CCS, chronic coronary syndrome; CRT, Cardiac resynchronization therapy; CTI, Cavo-tricuspid isthmus isolation; CV, cardiovascular; EQ-5D, EuroQol-5D; HCM, hypertrophic cardiomyopathy; HFrEF, Heart failure with reduced ejection fraction; HFpEF, Heart failure with preserved ejection fraction; HOCM, Hypertrophic obstructive cardiomyopathy; i.v., intravenous; INR, International normalized ratio; LA, left atrial; LAA, left atrial appendage; LAAO, left atrial appendage occlusion; LAVI, Left atrial volume-index; LV, left ventricle; LVEF, Left ventricular ejection fraction; NOAC, Non-vitamin K antagonist oral anticoagulant; NYHA, New York Heart Association; o.d., omni die (once daily); OAC, oral anticoagulation; PAD, peripheral arterial disease; PCI, Percutaneous coronary intervention; pts., patients; PVI, pulmonary vein isolation; QoL, Quality of life; TdP, Torsade de pointes tachycardia; TIA, transient ischemic attack; TOE, Transoesophageal echocardiography; VKA, Vitamin K antagonist; yrs., years.
JW—literature research and writing; CE and GF—literature research and corrections; LE—initiation, literature research and corrections.
Not applicable.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
The APC was funded by the “Open Access Publication Fund” of the University of Muenster.
The authors declare no conflict of interest.
