Academic Editors: Dragan M. Djuric and Teruo Inoue
Background: Saline is still the most widely used storage and rinsing
solution for vessel grafts during cardiac surgery despite knowing evidence of its
negative influence on the human endothelial cell function. Aim of this study was
to assess the effect of DuraGraft©, an intraoperative graft
treatment solution, on human saphenous vein segments and further elaborate the
vasoprotective effect on rat aortic segments in comparison to saline.
Methods: Human Saphenous vein (HSV) graft segments from patients
undergoing aortocoronary bypass surgery (n = 15), were randomized to
DuraGraft© (n = 15) or saline (n = 15) solution before
intraoperative storage. Each segment was divided into two subsegmental parts for
evaluation. These segments as well as rat aortic segments stored in
DuraGraft© underwent assessment of vascular function in a
multichamber isometric myograph system in comparison to Krebs-Henseleit solution
(KHS), a physiologic organ buffer solution. Results: Potassium-Chloride
(KCL)-induced contraction depicted a tendency towards increase when treated with
DuraGraft© compared to saline preservation of HSV segments (23.02
Coronary artery bypass grafting (CABG) continues to be the “gold standard” for
patients with complex multivessel coronary artery disease because of the superior
long-term outcome. In the modern era of arterial revascularization, saphenous
vein grafts (SVGs) still remain the most often used conduits for CABG worldwide
[1, 2]. As widely known the graft patency is influenced by multiple factors. One
of these is intimal hyperplasia progressing to vein-graft disease and graft
failure [3]. Various aspects and procedures have been studied and published with
fundamental improvements like pedicled harvesting technique, no touch methods and
of course external stenting of the vein graft [4, 5]. However, one topic was
neglected for a long period but currently gains more and more attention [6]. The
type of graft storage, flushing and rinsing solution that is intraoperatively
used to store the conduits can largely influence endothelial integrity and vessel
function [7]. Since the moment of grafting is the last time when the surgeon can
influence the “auto-transplanted” vessel, every measure and care should be
taken to ensure the best possible long-term outcome. This includes the
intraoperative storage and flushing solution. The benefits of meticulous surgical
handling, training, experience and various precautions during harvesting are
redundant if the procedure is interfered by using the incorrect storage solution.
In line with that, a subsection of the PREVENT IV trial demonstrated that using a
buffered solution resulted in lower vein graft failure rates when studied by
angiography at 12 to 18 months after surgery and showed generally
a better clinical outcome compared to non-buffered acidic saline (pH value of
5.5) or autologous whole blood solutions (AWB) [8]. Since AWB is only beneficial
as long as inside the intact circulation and harmful once outside e.g. in the
operative setting, due to an alkalytic pH (above 8) as a result of CO
The current study compared the impact of intraoperative preservation of SVGs from patients undergoing CABG in a specific ionically balanced storage solution (DuraGraft®, Maryzyme Inc, Jupiter, FL, USA) versus saline solution in the isolated organ bath testing system. DuraGraft®, is also a pH-balanced physiological salt solution containing L-glutathione, L-ascorbic acid, L-arginine and other additives that protect the graft from the damaging effects of ischemia and handling during CABG. DuraGraft® is a CE marked intra-operative graft storage solution and currently approved in Europe and several other global health care systems.
Since this is currently the only specific storage solution DuraGraft® was the target solution in this study to be compared to the still most widely used solution saline. In addition further characterization of the impact of saline on vascular endothelial function in rat aortic segments and the direct influence on human umbilical vein endothelial cells (HUVECs) was undertaken to specify initial findings.
The study was approved by the local Ethics Committee Nr. EK-20-219-1020 of the City of Vienna/Austria and registered as observational study by ClinicalTrials.gov. under the number NCT04614077.
Saphenous vein segment remnants were collected from 15 CABG patients after their informed consent. Patient’s details are presented in Table 1. Within 15 patients undergoing aortocoronary bypass surgery, saphenous vein graft segments were randomized to DuraGraft© (n = 15) or heparinized saline (B Braun AG, Melsungen, Germany) (n = 15) solution before intraoperative storage. Each 2 cm long segment of human saphenous vein (HSV) was divided into two 1 cm long parts for twofold evaluation. In total n = 28 HSV segments were collected and n = 23 segments/conditions were used for endothelial-dependent and endothelial-independent vasorelaxation assessment. Special care was taken to exclude patients with concomitant diseases or medical treatment that could interfere with the outcome, testing methods with special focus on vessel wall reactivity and further pathophysiological vascular conditions.
| Patients characteristics and cardiovascular risk factors | |
| patients, n/ (female %) | 15/ (18%) |
| Age, median (years) | 66.9 |
| Body mass index (BMI), median | 27.2 |
| Hypertension, n (%) | 10 (67%) |
| Dyslipidemia, n (%) | 15 (100%) |
| Diabetes mellitus (any), n (%) | 7 (46.7%) |
| Smoker active, n (%) | 7( 46.7%) |
| Chronic renal insufficiency, n (%) | 4 (26.7%) |
| Dialysis, n (%) | 4 (26.7%) |
| Pulmonary hypertension, n (%) | 3 (20%) |
| Chronic obstructive pulmonary disease, n (%) | 4 (26.7%) |
| Atrial fibrillation, n (%) | 3 (20%) |
| Peripheral artery disease, n (%) | 4 (26.7%) |
| Central artery disease, n (%) | 3 (20%) |
| Prior percutaneous intervention, n (%) | 4 (26.7%) |
| Previous myocardial infarction, n (%) | 6 (40%) |
| Heart valve disease, n (%) | 2 (13%) |
| Preoperative medication | |
| Nitrates, n (%) | 0 (0%) |
| Calcium blockers, n (%) | 3 (20%) |
| Beta blockers, n (%) | 10 (67%) |
| Renin-angiotensin system inhibitors, n (%) | 2 (13%) |
| Diuretics, n (%) | 4 (26.7%) |
| Aspirin | 15 (100%) |
| Statins | 15 (100%) |
| n, numbers of patients; % indicates percentage. | |
The following inclusion criteria were applied:
Age between 18–80 years.
Planned CABG operation.
Suitable vein grafts with the absence of blow outs, varicous veins or previous stripping.
Exclusion criteria:
Age
Emergency CABG.
Preoperative myocardial infacrtion
Re- operation.
Prior PCI
Ejection fraction (EF)
Severe organ dysfunction (any malignancy, sepsis
Pregnant women were not included.
Any disease of the lower veins.
Any vasculitis.
Hemoglobin A1c (HbA1c) levels
The segments of human saphenous vein were harvested in open technique. All patients underwent preoperative ultrasound scanning of the vein segments and varicose veins (outer diameter above 3.5 mm) were excluded. Special care was taken not to stretch by brisk handling or to touch the vessel frankly during the harvest procedure, therefore vein grafts were only harvested by experienced surgeons.
Human Saphenous vein segments were cut out in 20 mm pieces, carefully pressure
controlled flushed with 10 mL (mL, NaCl or DuraGraft®) at room
temperature and placed in the solution they were assigned to (NaCl or Duragraft),
any contact with any other substance was fully avoided. There was no mixture of
the substances. Flushing, storage and testing was undertaken only with the given
solution (NaCl or DuraGraft®). Each 20 mm long vein segment was
divided into two study samples accounting for a total of 28 samples, as two
sub-segments had to be excluded. The segments were then put immediately into
pre-oxygenated (45 minutes oxygenation time) DuraGraft© or saline
solution at normal room temperature and transferred to the laboratory in a
sterile isolated box. The segments were kept under these conditions for a total
of 60 minutes and then put into cold and oxygenated (5% CO
To further test the potential vascular protective effects of DuraGraft©, segments of the abdominal aorta were used from Sprague Dawley rats. Male adult Sprague-Dawley rats (12–14 weeks old, body weight of 350–380 gram; Department for Laboratory Animal Science and Genetics, Himberg, Austria) were used. The experimental protocol was approved by the Ethics Committee for Laboratory Animal Experiments at the Medical University of Vienna and the Austrian Ministry of Science and Research (BMWF-66.009/0023-WF/V/3b/2016) and conforms with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) [12, 13]. Briefly, rats were anaesthetized by intraperitoneal injection of a mixture of Xylazine (4 mg/kg; Bayer, Leverksen, Germany) and Ketamine (100 mg/kg; Dr E. Gräub AG, Switzerland), heparin was injected (iv. femoral artery) and the abdominal aorta was collected as described previously [12].
After cleaning, the segment was cut into 2 mm sections and mounted onto a
multi-chamber isometric myograph system (Model 620M, Danish Myo Technology,
Aarhus, Denmark). The chambers were filled with one the following solution: (1)
KHS (gold standard, positive control), (2) DuraGraft© and (3)
physiological saline (0.9%). Each individual chamber was further heated and
bubbled during the whole procedure. To determine the resting tension the AD
Instruments’ LabChart® DMT Normalization Module to mimic
physiological conditions was used with a target pressure of 100 mmHg. Segments
were allowed to equilibrate for 45 minutes and resting tension was continuously
adjusted during this period as described previously [11, 14]. Reference
contractions were elicited by hyperkaliaemic (124 mM, KCl) solution.
Precontraction of the aorta segments was achieved by Phenylephrine (PE, 1 nM–1
Human umbilical vein endothelial cells (HUVECs, Lonza, Basel, Switzerland) were
cultured in Medium 200 supplemented with Large Vessel Endothelial Supplement
(LVES), 10% foetal bovine serum (FBS) and 1% penicillin and streptomycin
solution and maintained at 37 °C and 5% CO
All chemicals were purchased from Sigma Aldrich (Sigma Inc. Burlington, MA, USA) unless otherwise specified. Preservation solutions for all experiments contained 10 units/mL unfractionated Heparin. DuraGraft© was purchased from Somahlution Inc, Jupiter, Florida, United States.
The contractile response was defined by the stress, which was calculated using the force generated by the vein rings. Vascular relaxation to Bradykinin and ACh was expressed as percentage of contraction to NE or PE, respectively. Differences in concentration-dependent relaxations induced by Bradykinine and ACh were analysed using two-way ANOVA followed by Bonferroni’s test when appropriate. Differences between multiple groups were analysed using one-way ANOVA followed by Bonferroni’s test. The number of experimental observations (n) refers to the number of vascular segments in respective experiments.
To demonstrate the good comparability of all cohorts, statistical testing for differences in baseline, procedural, and follow-up data has been performed. Depending on the variable’s distribution continuous data are either expressed as means and standard deviation (+/- SD) or median and were analyzed with one-way analysis of variance (ANOVA). Categorical variables are expressed in absolute numbers and percentages.
All data were expressed as mean
Statistical significance was accepted when p
We took maintenance of pH was vital to physiologic function and cellular viability of SVGs as given. In addition, it is important to state therefore that SVG segments after 30–60 minutes incubation with saline or DuraGraft© were transferred into Krebs solution and subsequently all measurements were performed in Krebs solution in this study.
Maximal contraction in response to KCl (124 mmol) in Krebs solution, showed a
tendency towards increase in contraction in DuraGraft© when
compared to normal saline preservation HSV (23.02
 Fig. 1.
Fig. 1.Effect of preservation solution on contractile responses in
HSV. (A) In response to the high K
To investigate the potential protective efficacy of DuraGraft© on
the vascular endothelium, we assessed the vascular reactivity of HSV segments in
patients planned for elective CABG. The HSV segments were stored in
DuraGraft© showed a significantly preserved endothelium-dependent
vasorelaxation in response to cumulative dosage of Bradykinin in comparison to
saline stored segments (Fig. 2A, p
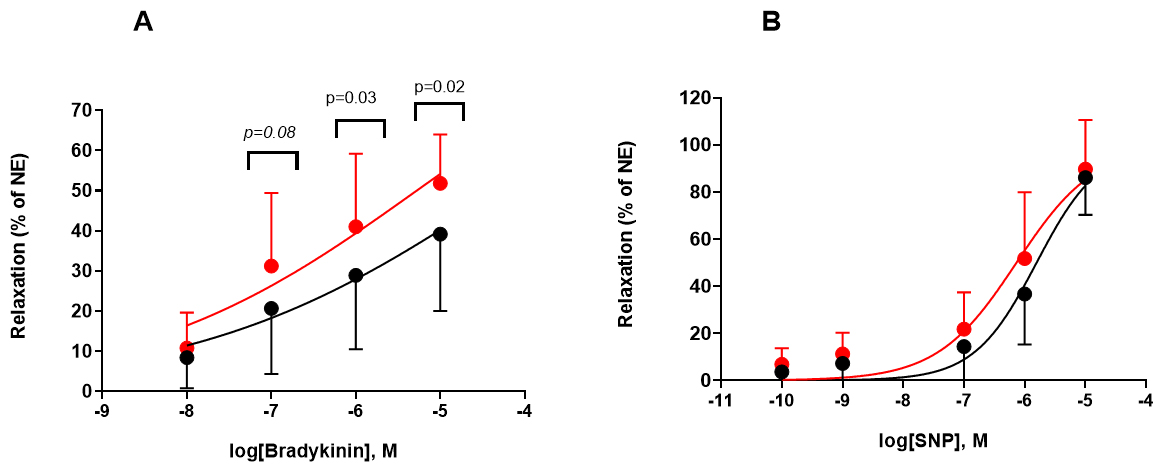 Fig. 2.
Fig. 2.Endothelial dependent and independent vasorelaxation-saphenous
vein grafts. Effects of NaCl (black) and DuraGraft® (red) on
vascular reactivity in the saphenous vein grafts. (A) Vein rings were
precontracted with NE and relaxed with the cumulative dosages of Bradykinin. The
Bradykinin response is expressed as percentage of the maximum NE response and
baseline tension. (B) Sapneouse vein were precontracted with NE and relaxed with the
cumulative dosages of sodium nitroprusside (SNP). The SNP response is expressed as percentage of the
maximum NE response and baseline tension. Data are expressed as mean
In the next step, rat aorta segments were used as a model of normal vascular
tissue to further characterize and compare the vascular protective effects of
DuraGraft© solution. The myograph chambers were filled with KHS,
saline and DuraGraft© and the aorta segments from rat were
mounted. Aorta segments that were kept in DuraGraft© showed
comparable response to KCl (contraction; 20.97
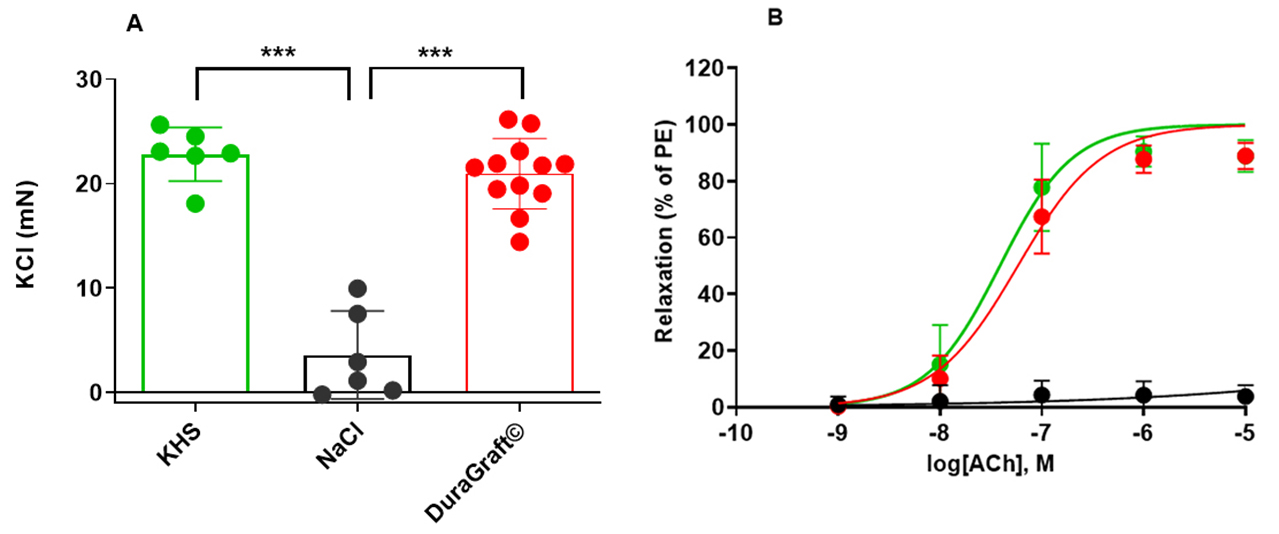 Fig. 3.
Fig. 3.Contractile response and endothelial dependent
vasorelaxation-rat aorta. (A) Aorta segments that were kept in
DuraGraft© or Krebs-Henseleit solution (KHS) showed comparable response to KCl (contraction;
20.97
Fig. 4C displays the morphology of HUVECs cultured in control medium (M200
Medium). The cells were aliquoted and kept in one of the following conditions:
(1) control group (M200 Medium), (2) KHS, (3) physiological saline and (4)
DuraGraft© solution for 30 minutes or 60 minutes. Then the cell
viability was evaluated by XTT assay. Saline treatment for 30 or 60 minutes
markedly inhibited cell viability (Fig. 4A,B compared to control, KHS and
DuraGraft©, respectively (p
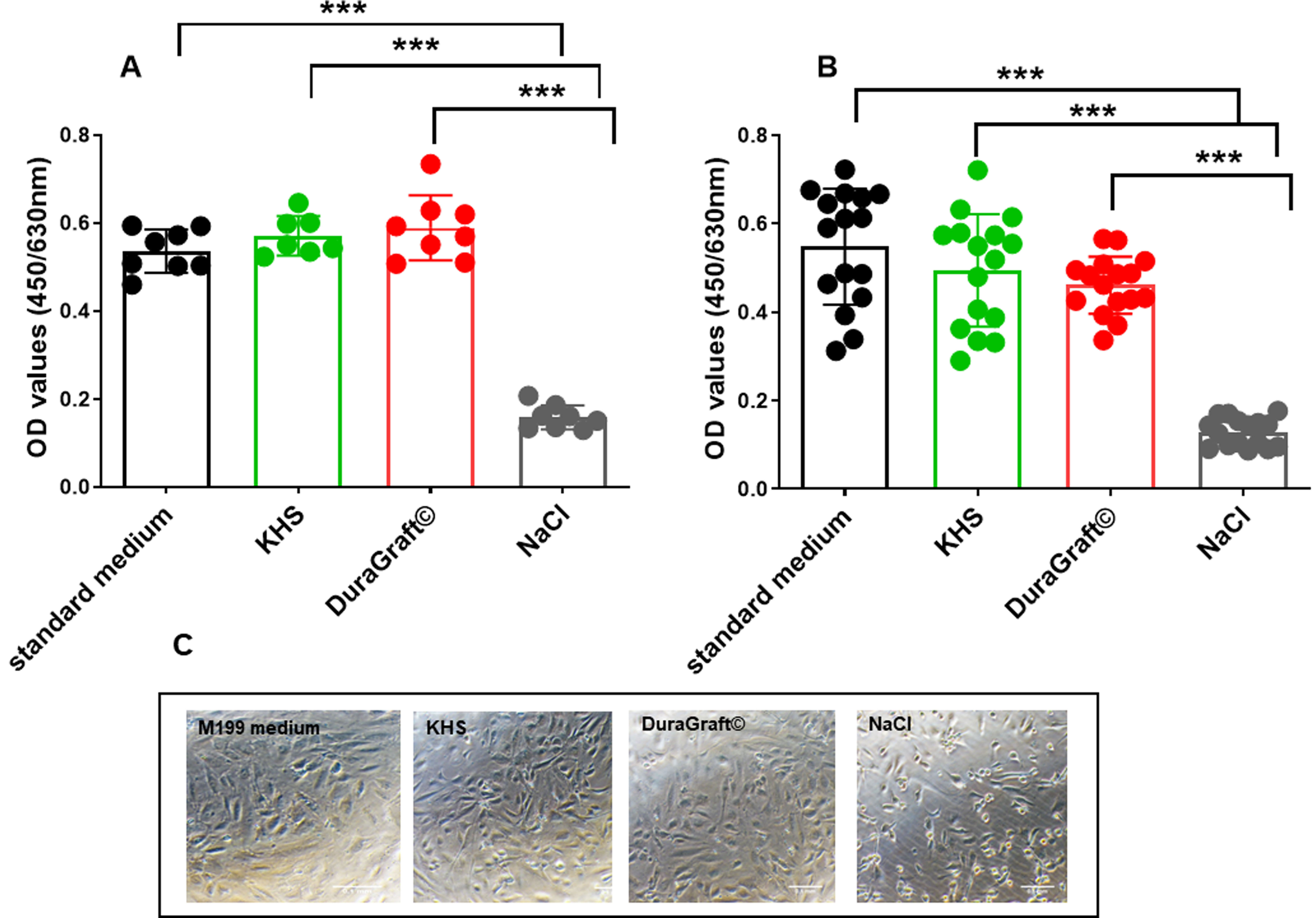 Fig. 4.
Fig. 4.Human Umbilical Vein Endothelial Cells-Viability. Effects of
standard medium, NaCl, DuraGraft® and Krebs-Henseleit solution (KHS) on HUVECs
viability after (A) 30 minutes and (B) 1 h incubation with the respective
conditions. (C) Representative images of the cell morphology under the respective
conditions after 1 h. Data are mean
Previous studies have already suggested that preservation in physiologic saline may harm vascular conduits and can accelerate the development of neointimal hyperplasia formation [3, 5, 6, 7, 8]. Still saline is one of the most or the most widely used solution for intraoperative graft storage or graft flushing besides AWB preparations or individual mixtures. Saline if non buffered has an acidic pH value of 5.5, the physiological pH of circulating blood is 7.32 pH. AWB becomes alkalotic once outside the humas circulation as described above. As Veres et al. [15] stated in 2015 storage with physiological saline and heparinized blood solutions is unable to protect the endothelium against cold ischaemia and warm reperfusion injury. Already in 2014, Harskamp et al. [8] examined the influence of the preservation solutions on vein graft failure using data from the PREVENT IV trial. Grafts were randomized to different groups of preservation solution consisting of saline, buffered saline and autologous whole blood. Grafts stored in buffered saline had significantly lower one-year vein graft failure rates compared to the other two groups, and were associated with a lower risk of five-year death, myocardial infarction and secondary revascularization, suggesting that intraoperative graft preservation is one of the key procedures in order to reduce graft failure risk. Despite these important clinical findings, heparinized normal saline is still widely used in coronary artery bypass grafting. Interestingly the first representative study was by O’Connell et al. [16] in 1974, conducted on the intima of arterial and not venous grafts. This study clearly demonstrated negative effects of normal saline (NS) on vascular endothelium and graft patency in a rabbit model. The topic of a specific graft storage or even treatment solution was neglected in cardiovascular research but gains in recent years more and more interest [6, 7, 17]. The data from the current study showed a clear positive effect for DuraGraft© as a representative for a specific solution. As presented in the results the HSV segments that were preserved with DuraGraft© showed significantly preserved endothelium-dependent vasorelaxation in response to cumulative dosage of Bradykinin when compared to saline preservation. The solution itself is buffered and upholding the cell metabolism due to preserving glucose levels but also reducing oxidative stress and amino acid (L-Argine) related vasodilatation [17]. The product is a relatively novel solution against endothelial-damage developed to efficiently protect the structural and functional integrity of the vascular endothelium. DuraGraft© is described as structural and functional endothelial stabilizer in aortocoronary bypass surgery, antioxidative, radical-scavenging, nitric oxide (NO)-synthetize-supporting, anticoagulant, isotonic structural and functional endothelial stabilizer for graft stabilization during venous and arterial aortocoronary bypass surgery. Saline does of course not provide any metabolism upholding elements and if not buffered has a direct damaging acidic effect on the endothelium. DuraGraft© alleviated in a recent study vascular function in vitro following ischemia- reperfusion injury [18]. These results although representing data from in vitro animal studies were in line with the findings of this study conducted on human saphenous vein segements.
Currently a prospective observational registry with DuraGraft© targeting at 3000 patients undergoing an isolated CABG procedure or a combined procedure with at least one saphenous vein grafts or one free arterial graft is finished: EU Multicenter Registry to Assess Outcomes in CABG Patients: Treatment of Vascular Conduits With DuraGraft [VASC]. Data on baseline, clinical, and angiographic characteristics as well as procedural and clinical events were and will be collected [3, 17, 19]. Because preservation in the buffered solution represented by DuraGraft© appeared to be superior to non-buffered saline in isolated rat aorta segments and relaxation in HSV, we concluded that maintenance of pH could be vital to physiologic function and cellular viability of HSV. Furthermore, a recent study by Tekin et al. [20] demonstrated that SVG stored in DuraGraft© had lower oxidative level and higher antioxidant capacity, both may contribute and partially explain the preservation of endothelial function as observed in another study by Szabó et al. [12]. In addition, it is important to state that SVG segments after 40–60 min (comparative time as in the Operating room) incubation with saline or DuraGraft© were transferred and then kept in KHS solution with all measurements performed under this conditions, suggesting DuraGraft© storage solution effectively alleviates endothelial dysfunction [21].
As the next step further characterization to compare the vascular protective
effects of DuraGraft© solution on rat aorta segments as a model
system of healthy vascular tissue was conducted. The aorta segments that were
kept in DuraGraft© showed comparable response to
Potassium-Chloride and endothelium dependent relaxation solution. In contrast,
the aorta segments that were stored in saline showed significantly impaired
vasoconstriction and vasorelaxation when compared to KHS and
DuraGraft© preservation. In line with the initial findings,
DuraGraft® alleviates vascular dysfunction following ischemia and
reperfusion injury by reducing nitro-oxidative stress and the expression of
intercellular adhesion molecule-1 (ICAM-1), without leukocytes engagement in the
rat model [12]. Furthermore, the study by Pachuk et al. [7] in 2019
displayed in pig mammary arteries results exactly in line with the data above for
an animal model set up for healthy vessel segments. Loss of HSV graft-cell
viability was observed as early as 15 minutes post-exposure to saline whereas
viability was maintained up to 5 hours’ exposure to DuraGraft©.
Histological analyses performed on pig mammarian artery (PMVs) demonstrated
endothelial damage in PMVs stored in saline. Cytotoxicity assays demonstrated
that saline-induced microscopically visible cell damage occurred within 60
minutes [22, 23]. In line with these findings, cell viability was evaluated in
HUVECs by XTT assay in this study, saline (30 and 60 minutes) treatment markedly
inhibited cell viability when compared to the control KHS and
DuraGraft©, respectively (p
The study was limited by its single center design and patient’s numbers in terms of the HSV samples. Although specific care was taken to avoid any influence on the vessel and endothelium in terms of preparation or transport some undetectable risk factors or patient’s details might be present but not obvious at the time of hospitalization. In addition, animal study experiments confirm that DuraGraft© is as efficient as KHS in respect of vascular reactivity, and superior than saline. However, we have not stored the segments of aorta (rat) either in saline or that DuraGraft© prior to performing vascular reactivity assessment and performed on isolated aortic not on venous segments. However, we do not anticipate a difference between the artery and the venous segment in respect to vascular protection by Duragraft©. This study represented a momentum snapshot of the influence and further longterm data is urgently needed to confirm the protective effects. The author WB is participating in the European Multicenter Trail VASC as national PI. Nevertheless, the protective effect of DuraGraft© was demonstrated on human specimens in this study.
Saline is still the most widely used storage and flushing solution for vessel grafts during cardiac surgery. Saline is clearly not beneficial for the human endothelium whilst DuraGraft© being a representative for a specific storage and treatment solution demonstrated a positive effect. This study and the current results call for the stop of saline as vascular storage and graft flushing solution and for the use of a specific agent instead.
AK—Conceptualization; Data curation; Formal Analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing. PLS—Conceptualization; Investigation; Methodology; Project administration; Supervision; Validation; Writing – review & editing. CD—Conceptualization; Writing – review & editing. ZA—Conceptualization; Writing – review & editing. DG—Conceptualization; Writing – review & editing. IC—Conceptualization; Writing – review & editing. SF—Conceptualization; Writing – review & editing. MG—Conceptualization; Writing – review & editing. BKP—Conceptualization; Investigation; Methodology; Project administration; Supervision; Validation; Writing – review & editing. BW—Conceptualization; Investigation; Methodology; Project administration; Supervision; Validation; Writing – review & editing.
The study was approved by the local Ethics Committee Nr. EK-20-219-1020 of the City of Vienna/Austria and registered as observational study by ClinicalTrials.gov. under the number NCT04614077.
We acknowledge the efforts of all colleagues who have worked and helped in the operation theatre and laboratory on the development of this manuscript. Special thanks to all the patients as well as Mrs. Shalett Mathew and Ibrahim Aykac (Center for Biomedical Research. Medical University of Vienna) to perform HUVEC experiments and created the visual abstract, respectively. We thank Mrs Sabrina Rohringer (Center for Biomedical Research. Medical University of Vienna) for providing us the HUVECs.
The study was supported by institutional funds and the Karl Landsteiner Society of Cardiac Research/ KH North Vienna, and the funds were directly awarded to the corresponding and last author Dr. Winkler Bernhard who was in charge of the study as PI.
The authors declare no conflict of interest. Attila Kiss is serving as one of the Guest editors of this journal. We declare that Attila Kiss had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Dragan M. Djuric and Teruo Inoue.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
