†These authors contributed equally.
Academic Editor: Alessandro Cataliotti
Background: Acute kidney injury (AKI) is a relatively common complication after surgery for type A acute aortic dissection (ATAAD) and is associated with a poor prognosis. Preclinical models suggest that toll-like receptor 4 (TLR4) may participate in the pathogenesis of AKI. However, the correlation of serum TLR4 and post-operative AKI has not been studied in ATAAD patients. This study aimed to explore the possibility of using serum TLR4 levels to predict AKI and 30-day mortality in patients undergoing ATAAD surgery. Methods: A prospective, single-center cohort study was conducted and enrolled a total of 64 patients undergoing ATAAD surgery. The level of serum TLR4 was measured and compared before and within 24 hours after the completion of surgery. Results: Thirty-five (54.7%) patients developed AKI, including 7 (10.9%) diagnosed with severe AKI (Kidney Disease Improving Global Outcomes (KDIGO) stage 3). TLR4 levels at 0-hour,1-hour, 3-hour, and 6-hour after intensive care unit (ICU) admission were significantly different between patients with or without AKI. Further analysis showed that the difference was most significant at 0-hour after ICU admission which corresponded to an area under the curve (AUC) of 0.886 (95% confidence interval (CI), 0.800 to 0.973). For severe AKI, the AUC of TLR4 was the highest with 0.923 (0.852 to 0.995) at 1-hour after ICU admission. TLR4 levels before surgery and at 0-hour, 1-hour, as well as 3-hour after ICU admission were significantly different between survivors and non-survivors. Furthermore, we found that the serum level of TLR4 upon ICU admission could be used to predict the 30-day mortality with AUC of 0.805 (0.648 to 0.962). Conclusions: Serum TLR4 levels can be used as a biomarker to predict the occurrence of AKI and 30-day mortality in patients undergoing ATAAD surgery. Clinical Trial Registration Number: ChiCTR2200057197.
Toll-like receptor 4 (TLR4) is an important pattern recognition receptor mainly
expressed on renal tubular epithelial cells and vascular endothelial cells that
mediates the nuclear factor (NF)-
Acute type A aortic dissection (ATAAD) is a life-threatening condition that is associated with high mortality, not only due to the disease itself, but also due to surgical related major complications [12, 13, 14, 15]. AKI is a relatively common and severe complication of ATAAD surgery and is often associated with poor prognosis. AKI may develop in up to 20% to 67% of all patients who undergo ATAAD surgery [16, 17], which can decrease long-term survival and quality of life, even though the dissection has been successfully repaired [17]. Therefore, early identification of patients with a high risk for developing AKI after ATAAD surgery would help to improve their immediate and long-term prognosis.
Conventional markers such as serum creatine (sCr) level and urinary output can be affected by many factors during the postoperative period, and therefore have limited value in the early diagnosis of AKI [18, 19]. Novel biomarkers, such as cystatin C, have showed less sensitivity for AKI than sCr levels [20]. Comprehensive measurements such as renal resistive index, have been proposed to be a useful tool to predict AKI after ATAAD surgery but might be influenced by heart rate and mean arterial pressure [21].
Therefore, we conducted a prospective study to evaluate the value of TLR4 levels at different time points to determine the early diagnosis of AKI in ATAAD patients who underwent reparative surgery and their association with 30-day mortality.
70 adult patients who were diagnosed with ATAAD by enhanced computed tomography (CT) and received surgery within 14 days of disease onset were enrolled in this prospective, observational single-center study. The study was conducted between December 29 2021 and April 25 2022. As shown in Fig. 1, patients on renal replacement therapy (RRT) before surgery (n = 3) and who died during or within 24 hours after surgery (n = 2) were excluded from the final analysis because of the difficulty in measuring the progression of renal dysfunction. In addition, 1 patient was excluded due to incomplete data. All patients received standard of care and were transferred to the intensive care unit (ICU) after the completion of surgery. Prompt resuscitation of the circulation with fluids, vasopressors and inotropes was applied after patients were diagnosed with AKI.
 Fig. 1.
Fig. 1.The patient selection process.
Postoperative AKI was diagnosed according to the Kidney Disease Improving Global
Outcomes (KDIGO) criteria [22] by measuring the change of sCr levels and urine
output. The severity of AKI was determined according to the KDIGO guidelines as
follows: stage-1: increase of sCr by
Patient information including demographic characteristics, medical histories, physical examination results, laboratory tests, imaging findings, treatments and outcomes were collected. All enhanced CT images were independently evaluated by 2 experienced radiologists and disagreements were resolved by further consultation with a third radiologist.
2 mL of venous blood was collected from each patient before surgery and at
0-hour, 1-hour, 3-hour, 6-hour, 12-hour, and 24-hour after ICU admission
following surgery. Blood samples were centrifuged at 1500
The primary outcome was the difference in serum TLR4 levels between patients with and without postoperative AKI at different timepoints. Secondary outcomes included the correlation between changes of serum TLR4 levels at different timepoints and stage-3 AKI as well as 30-day mortality.
The operation procedure performed in this study was described in our previous study [16]. Briefly, cardiopulmonary bypass (CPB) was established by cannulation of femoral artery or right axillary artery with right atrium. Cardiac arrest was accomplished with cold blood cardioplegia (4:1 blood:crystalloid ratio) which was infused by both anterograde and retrograde infusion method. Circulation arrest was initiated when cooling reached its target rectal temperature of 22 °C, and the temperature maintained 18–22 °C during the circulation arrest period. Total arch replacement plus frozen elephant trunk method was selected when major dissection teared around aortic arch or proximal descending aorta. To prevent postoperative AKI, the mean arterial pressure was maintained between 55 and 75 mmHg and the urine output was recorded per hour during the surgery.
SPSS 26.0 software (IBM Corp, Armonk, NY, USA) was used for all statistical
analyses. Quantitative data was presented as mean
According to retrospective study in our center, the incidence of postoperative AKI in ATAAD patients was 50.4% [16]. Therefore, we assumed that the incidence of AKI during the study period was 50.0%. The sample size calculation showed that a sample of 28 from the AKI group and 28 from the non-AKI group would achieve 80% power to detect a difference of 0.20 between the area under the receiver operating characteristic (ROC) curve (AUC) under the null hypothesis of 0.80 and an AUC under the alternative hypothesis of 0.7000 using a two-sided z-test at a significance level of 0.05.
To identify independent predictors of AKI and 30-day mortality, multivariate
logistic regression analyses were performed including variables with
p-value
A total of 64 patients, including 60 with DeBakey type I and 4 with DeBakey type
II aortic dissections, were included in the analysis (Fig. 1). Among these 64
patients, 35 patients (54.7%) developed AKI, including 18 patients (51.4%)
characterized with KDIGO stage-1, 10 (28.6%) with stage-2, and 7 (20.0%) with
stage-3. In addition, 5 patients (7.8%) required RRT. KDIGO AKI stages and
criteria are summarized in Table 1. The median age was 57.5 years (range, 35 to
83 years) and 47 (73.4%) were males. The average body mass index was 25.9
| Patients | sCr criterion only | UO criterion only | sCr and UO criteria | RRT and sCr criteria | RRT and UO criteria | RRT, sCr, and UO criteria | All criteria AKI, n = 35 (% of AKI patients) |
| KDIGO stage 1 | 10 | 4 | 4 | 0 | 0 | 0 | 18 (51.4) |
| KDIGO stage 2 | 5 | 2 | 3 | 0 | 0 | 0 | 10 (28.6) |
| KDIGO stage 3 | 2 | 0 | 0 | 0 | 1 | 4 | 7 (20.0) |
| Total amount (% of patients with AKI) | 17 (48.6) | 6 (17.1) | 7 (20) | 0 (0) | 1 (2.9) | 4 (11.4) | |
| sCr, serum creatinine; UO, urine output; RRT, renal replacement therapy; AKI, acute kidney injury; KDIGO, Kidney Disease Improving Global Outcomes. | |||||||
| Variables | Total (n = 64) | AKI (n = 35) | Non-AKI (n = 29) | p value |
| DeBakey type I (%) | 60 (93.8) | 34 (97.1) | 26 (89.7) | 0.321 |
| Time from onset to surgery (hour) | 22.8 (12.5, 33.4) | 17.6 (8.7, 29.8) | 24.8 (14.2, 42.1) | 0.031 |
| Presenting variables | ||||
| Chest pain (%) | 58 (90.6) | 31 (88.6) | 27 (93.1) | 0.681 |
| Back pain (%) | 39 (60.9) | 20 (57.1) | 19 (65.5) | 0.494 |
| Abdominal pain (%) | 11 (17.2) | 7 (20.0) | 4 (13.8) | 0.741 |
| Vomiting (%) | 10 (15.6) | 6 (17.1) | 4 (13.8) | 1.000 |
| Demographic data | ||||
| Age (year) | 57.5 (46.0, 68.8) | 54.0 (43.0, 66.0) | 59.0 (48.0, 69.0) | 0.202 |
| Male (%) | 47 (73.4) | 28 (80.0) | 19 (65.5) | 0.192 |
| BMI (kg/m |
25.9 |
26.5 |
25.2 |
0.167 |
| Medical history | ||||
| Hypertension (%) | 53 (82.8) | 30 (85.7) | 23 (79.3) | 0.526 |
| Diabetes mellitus (%) | 2 (3.1) | 2 (5.7) | 0 (0) | 0.497 |
| Previous cardiovascular disease (%) | 14 (21.9) | 7 (20.0) | 7 (24.1) | 0.690 |
| Cerebrovascular disease (%) | 9 (14.1) | 5 (14.3) | 4 (13.8) | 1.000 |
| Smoking (%) | 16 (25.0) | 12 (34.3) | 4 (13.8) | 0.059 |
| Drinking (%) | 10 (15.6) | 5 (14.3) | 5 (17.2) | 1.000 |
| Previous cardiac operation (%) | 4 (6.3) | 3 (8.6) | 1 (3.4) | 0.620 |
| PCI (%) | 1 (1.6) | 1 (2.9) | 0 (0) | 1.000 |
| TEVAR (%) | 3 (4.7) | 2 (5.7) | 1 (3.4) | 1.000 |
| Limb ischemia (%) | 11 (17.2) | 8 (22.9) | 3 (10.3) | 0.319 |
| Cerebral ischemia (%) | 15 (23.4) | 10 (28.6) | 5 (17.2) | 0.287 |
| Coronary ischemia (%) | 4 (6.3) | 4 (11.4) | 0 (0) | 0.120 |
| Involving renal artery (%) | 35 (54.7) | 26 (74.3) | 9 (31.0) | 0.001 |
| Hypotension (%) | 6 (9.4) | 3 (8.6) | 3 (10.3) | 1.000 |
| Pericardial tamponade (%) | 29 (45.3) | 18 (51.4) | 11 (37.9) | 0.280 |
| BMI, body mass index; PCI, percutaneous coronary intervention; TEVAR, thoracic endovascular aortic repair; AKI, acute kidney injury. | ||||
| Variables | Total (n = 64) | AKI (n = 35) | Non-AKI (n = 29) | p value |
| Intro-operative variables | ||||
| TAR + FET (%) | 28 (43.8) | 15 (42.9) | 13 (44.8) | 0.874 |
| Concomitant CABG (%) | 2 (3.1) | 2 (5.7) | 0 (0) | 0.497 |
| Concomitant MVP (%) | 2 (3.1) | 1 (2.9) | 1 (3.4) | 1.000 |
| Concomitant AVP (%) | 2 (3.1) | 1 (2.9) | 1 (3.4) | 1.000 |
| Bentall (%) | 5 (7.8) | 3 (8.6) | 2 (6.9) | 1.000 |
| CPB duration (minute) | 180.0 |
196.7 |
160.3 |
0.002 |
| Aortic cross-clamp time (minute) | 131.0 |
142.5 |
117.6 |
0.009 |
| DHCA time (minute) | 26.0 |
27.2 |
24.6 |
0.253 |
| Operation time (hour) | 6.6 |
7.3 |
5.7 |
|
| Lowest nasopharyngeal temperature (°C) | 23.3 |
23.0 |
23.6 |
0.066 |
| RBC transfusion (mL) | 2125.0 (1662.5, 2645.0) | 2275.0 (1900.0, 2980.0) | 2075.0 (1525.0, 2412.5) | 0.051 |
| TAR, total arch replacement; FET, frozen elephant trunk; CABG, coronary artery bypass graft; MVP, mitral valvuloplasty; AVP, aortic valvuloplasty; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; RBC, red blood cell; AKI, acute kidney injury. | ||||
| Variables | Total (n = 64) | AKI (n = 35) | Non-AKI (n = 29) | p value |
| Postoperative complications (%) | 32 (50) | 26 (74.3) | 6 (20.7) | |
| Reintubation (%) | 5 (7.8) | 5 (14.3) | 0 (0) | 0.034 |
| Tracheotomy (%) | 1 (1.6) | 1 (2.9) | 0 (0) | 1.000 |
| Atrial fibrillation (%) | 15 (23.4) | 11 (31.4) | 4 (13.8) | 0.097 |
| ARDS (%) | 26 (40.6) | 24 (68.6) | 2 (6.9) | |
| Lung infection (%) | 19 (29.7) | 17 (48.6) | 2 (6.9) | |
| SWI (%) | 2 (3.1) | 2 (5.7) | 0 (0) | 0.497 |
| CRRT (%) | 5 (7.8) | 5 (14.3) | 0 (0) | 0.034 |
| Cerebral infarction (%) | 7 (10.9) | 6 (17.1) | 1 (3.4) | 0.116 |
| Delirium (%) | 9 (14.1) | 7 (20.0) | 2 (6.9) | 0.166 |
| Paraplegia (%) | 2 (3.1) | 2 (5.7) | 0 (0) | 0.497 |
| Osteofascial compatament syndrome (%) | 1 (1.6) | 1 (2.9) | 0 (0) | 1.000 |
| Use of diuretics (%) | 42 (65.6) | 30 (85.7) | 12 (41.4) | |
| Inotropic support (%) | 41 (64.1) | 27 (77.1) | 14 (48.3) | 0.017 |
| Inotropic support |
30 (46.9) | 22 (62.9) | 8 (27.6) | 0.005 |
| Inotropic support |
23 (35.9) | 19 (54.3) | 4 (13.8) | 0.001 |
| Drainage volume 24 hours after surgery (mL) | 475.0 (300.0, 692.5) | 540.0 (345.0, 860.0) | 410.0 (300.0, 650.0) | 0.112 |
| Ventilation time (hour) | 20.0 (12.3, 67.0) | 46.0 (17.0, 155.0) | 14.0 (7.0, 19.5) | |
| 30-day mortality (%) | 6 (9.4) | 6 (17.1) | 0 (0) | 0.028 |
| ICU stay (day) | 3.0 (2.0, 6.0) | 5.0 (2.0, 11.5) | 2.0 (1.0, 3.0) | |
| Hospital stay (day) | 14.0 (11.0,19.0) | 17.0 (13.0, 22.5) | 13.0 (10.0, 17.0) | 0.005 |
| ARDS, acute respiratory distress syndrome; SWI, sternal wound infection; CRRT, continuous renal replacement therapy; ICU, intensive care unit; AKI, acute kidney injury. | ||||
| Variables | Total (n = 64) | AKI (n = 35) | Non-AKI (n = 29) | p value |
| WBC (10 |
11.2 (8.8, 14.3) | 13.2 (9.8, 15.2) | 10.2 (7.6, 11.9) | 0.012 |
| Haemoglobin (g/L) | 126.0 |
131.8 |
121.8 |
0.808 |
| PLT (10 |
163.8 |
161.1 |
161.5 |
0.309 |
| Triglyceride (mmol/L) | 1.2 |
1.6 |
0.7 |
0.061 |
| CRP (mg/dL) | 10.1 (3.8, 31.1) | 8.6 (4.1, 13.7) | 10.1 (1.5, 31.1) | 0.626 |
| D-dimer (ng/mL) | 7.4 (3.3, 21.6) | 7.1 (5.0, 65.7) | 4.5 (2.26, 21.7) | 0.314 |
| Albumin (g/L) | 39.0 (35.5, 41.1) | 39.8 (35.7, 42.8) | 39.2 (38.6, 41.9) | 0.451 |
| TnT (ng/mL) | 0.016 (0.009, 0.065) | 0.017 (0.009, 0.199) | 0.015 (0.008, 0.127) | 0.009 |
| ALT (U/L) | 25.1 (17.0, 42.0) | 27.3 (20.9, 48.6) | 25.9 (13.1, 88.4) | 0.766 |
| Bun (mmol/L) | 6.9 |
7.4 |
6.3 |
0.028 |
| sCr ( |
88.4 |
102.1 |
74.0 |
0.003 |
| BNP (pg/mL) | 106.8 |
101.3 |
123.1 |
0.571 |
| Total bilirubin (mg/dL) | 16.1 |
14.1 |
17.0 |
0.132 |
| PT (s) | 12.1 (11.3, 13.2) | 12.2 (11.4, 13.3) | 11.9 (10.8, 12.5) | 0.134 |
| APTT (s) | 27.3 (25.7, 29.5) | 26.7 (25.7, 30.0) | 27.4 (24.9, 28.3) | 0.405 |
| Fibrinogen (g/L) | 2.3 |
2.2 |
2.6 |
0.220 |
| INR | 1.06 (1.00, 1.18) | 1.07 (1.01, 1.17) | 1.05 (0.95, 1.10) | 0.619 |
| Serum lactate (mmol/L) | 2.9 |
2.9 |
2.7 |
0.787 |
| WBC, white blood cell; PLT, platelet; CRP, c-reactive protein; TnT, troponin T; ALT, alanine aminotransferase; Bun, blood urea nitrogen; sCr, serum creatinine; BNP, brain natriuretic peptide; PT, prothrombin time; APTT, Activated Partial Thromboplastin Time; INR, international normalized ratio; AKI, acute kidney injury. | ||||
Significant differences in the time from disease onset to operation, renal
artery involvement, white blood cell, troponin T, blood urea nitrogen, and sCr
level were identified between patients with and without AKI (Tables 2,5).
Operative parameters including CPB time, aortic cross-clamp time, and operation
time were significantly different between the 2 groups (Table 3). The incidence
of postoperative complications including reintubation (14.3% vs. 0, p =
0.034), acute respiratory distress syndrome (68.6% vs. 6.9%, p
Multivariate analysis revealed that increased level of TLR4 at 0-hour upon ICU admission was identified as a risk factor for developing postoperative AKI (OR 3.046, 95% CI 1.435–7.024; p = 0.006) and increased 30-day mortality (OR 2.604, 95% CI 1.039–6.002; p = 0.016).
The mean preoperative TLR4 of all patients was 1.8
 Fig. 2.
Fig. 2.Toll-like receptor 4 levels (mean and standard deviation) of patients in the AKI group and the non-AKI group at multiple time points.
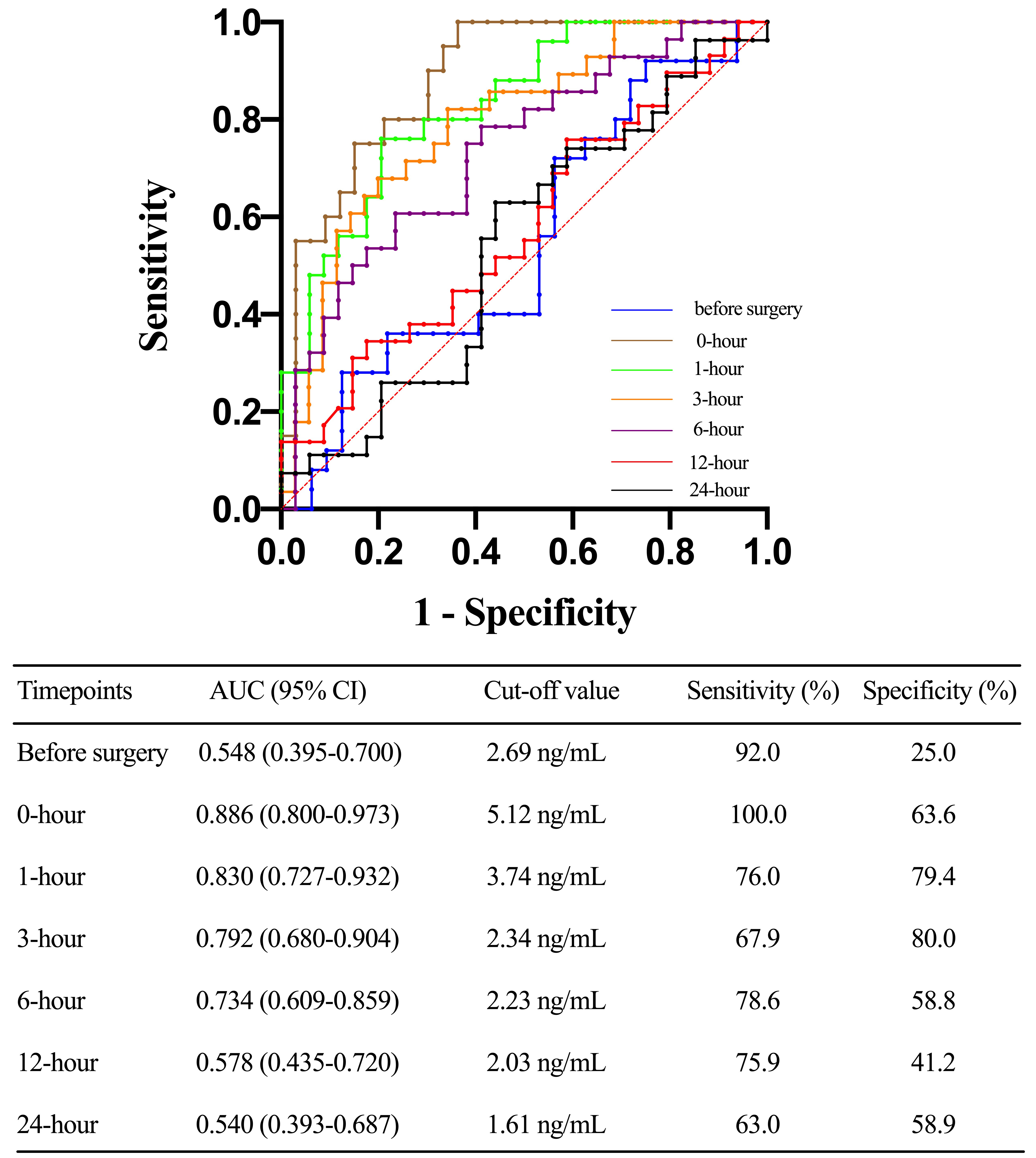 Fig. 3.
Fig. 3.Receiver operating characteristic (ROC) curves before surgery, 0-hour, 1-hour, 3-hour, 6-hour, 12-hour, and 24-hour toll-like receptor 4 levels after ICU stay in predicting acute kidney injury (AKI) after acute type A aortic dissection (ATAAD) surgery. AUC, area under the curve; CI, confidence interval.
The TLR4 concentration at 1-hour after ICU admission could predict the occurrence of severe AKI with an AUC of 0.923 (0.852 to 0.995). The AUC of serum TLR4 levels at 1-hour, 3-hour, and 6-hour after ICU admission to predict severe AKI were 0.904 (0.811 to 0.996), 0.865 (0.736 to 0.994), and 0.844 (0.708 to 0.980), respectively (Fig. 4). TLR4 levels before surgery, 0-hour, 1-hour, and 3-hour after ICU admission were significantly different between survivors and non-survivors within 30 days after surgery (Fig. 5) and could be used to predict 30-day mortality with corresponding AUCs of 0.781 (0.602 to 0.959), 0.805 (0.648 to 0.962), 0.730 (0.515 to 0.944), and 0.731 (0.556 to 0.906), respectively (Fig. 6).
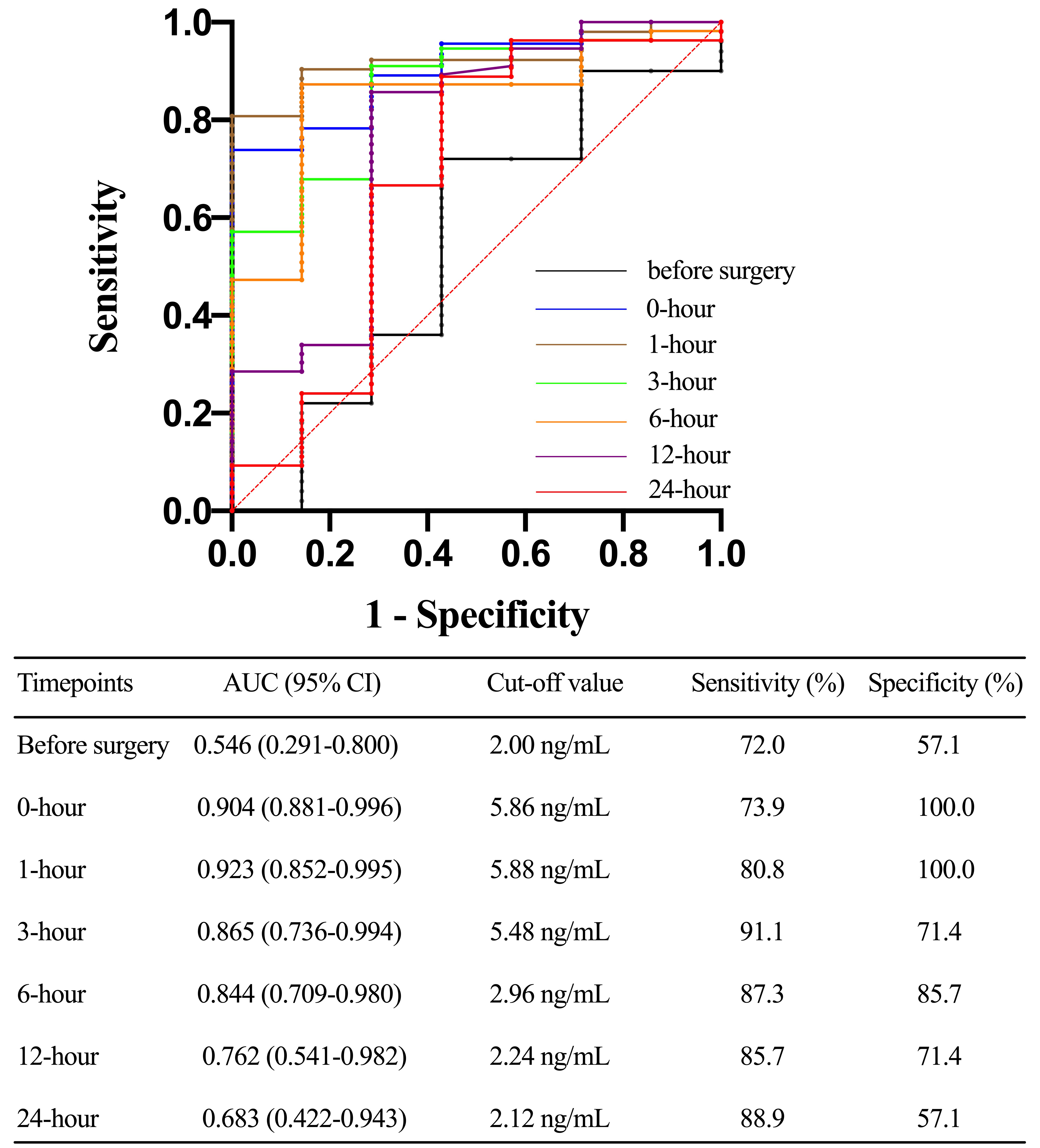 Fig. 4.
Fig. 4.Receiver operating characteristic (ROC) curve comparing prior to surgery, 0-hour, 1-hour, 3-hour, 6-hour, 12-hour, and 24-hour toll-like receptor 4 levels after ICU stay in predicting acute kidney injury (AKI) (stage 3 AKI) after acute type A aortic dissection (ATAAD) surgery. AUC, area under the curve; CI, confidence interval.
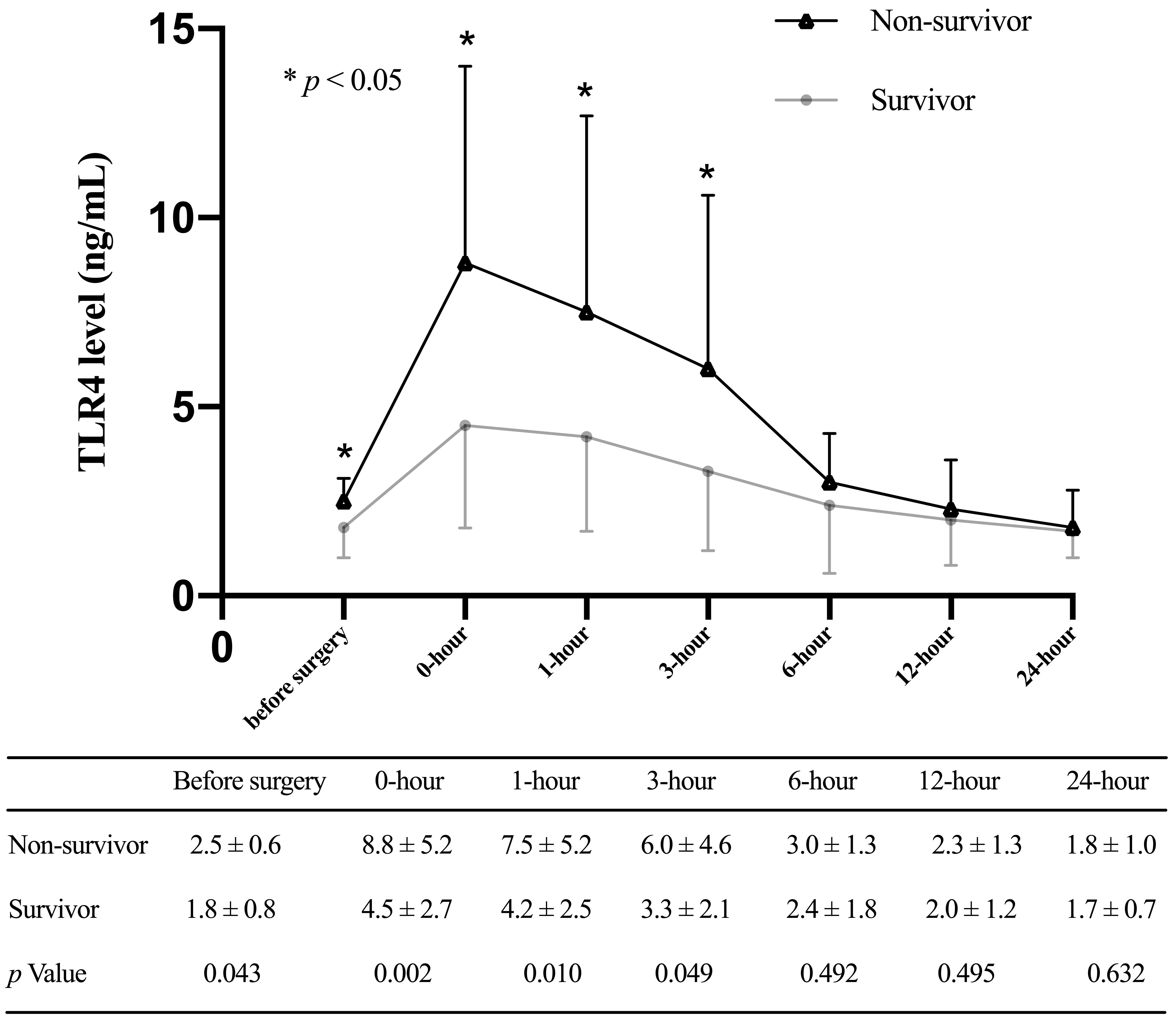 Fig. 5.
Fig. 5.Toll-like receptor 4 levels (mean and standard deviation) of patients stratified with 30-day mortality at multiple time points.
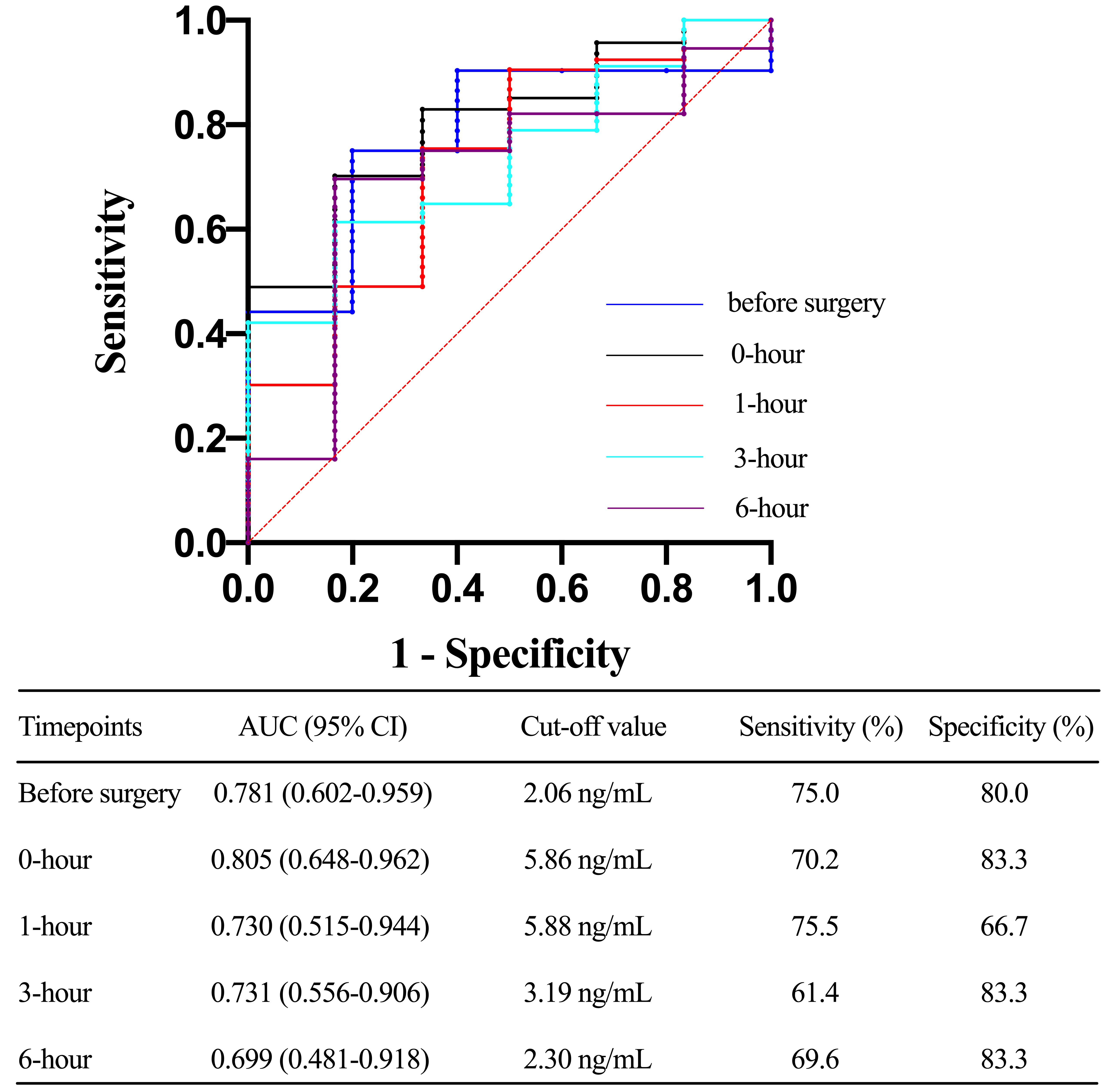 Fig. 6.
Fig. 6.Receiver operating characteristic (ROC) curve comparing the ability before surgery, 0-hour, 1-hour, 3-hour, 6-hour, 12-hour, and 24-hour toll-like receptor 4 levels after ICU stay in predicting 30-day mortality after acute type A aortic dissection (ATAAD) surgery. AUC, area under the curve; CI, confidence interval.
It has been shown in previous studies that the duration of AKI is associated with increased long-term mortality in patients who undergoing surgery for ATAAD [23]. On the other hand, early recovery of renal function after cardiac surgery is associated with improved short- and long-term survival [24]. Therefore, it is important to identify those patients with increased risk for AKI so that appropriate interventions and management can be instituted prior to, during and following surgery. To the best of our knowledge, this is the first study to demonstrate that elevated postoperative TLR4 levels are associated with a statistical increased incidence of AKI and 30-day mortality after adjusting for clinical covariates. The ROC analyses revealed that the AUCs of TLR4 levels at 0-hour after ICU admission to detect AKI, severe AKI, and 30-day mortality were 0.886, 0.904, and 0.805, respectively. Therefore, TLR4 levels after ICU admission might be a novel predictor for developing postoperative AKI in ATAAD patients undergoing surgical repair. Compared to conventional markers, the measurement of TLR4 is convenient and can be analyzed at different times during the postoperative period.
Similar to previous studies, 54.7% of our patients developed AKI following surgery for ATAAD [25]. Other studies reported a higher AKI occurrence of 67%, which might due to different criteria to diagnose AKI [26]. AKI in acute aortic dissection can be characterized into two subtypes: prerenal or intrinsic [19]. Prerenal AKI after ATAAD occurs due to decreased renal perfusion and is reversible. Prompt recognition and rapid restoration of renal perfusion may attenuate or even prevent acute tubular necrosis [27]. The use of RRT when indicated may also improve outcomes [28].
Toll-like receptors (TLRs) are type I membrane-associated glycoproteins that belong to the interleukin-1 receptor super-family [29]. TLRs mainly mediate the function of the innate immune system upon activation with pathogen-associated molecular patterns [30]. It has been known that the innate immune system plays a critical role in initiating the inflammatory cascade that leads to kidney damage [31]. TLRs can also recognize endogenous stress signals or damage-associated molecular patterns such as high mobility group box protein 1 (HMGB1), heat shock proteins and hyaluronan [32] and induce production of inflammatory chemokines and cytokines including interferons (IFNs). It has been demonstrated that extracellular HMGB1 released during an ischemic insult could activate the TLR4 receptor in mice, and participated in the pathogenesis of ischemia-reperfusion induced kidney injury [4]. Hyaluronan is mainly expressed in the inner medulla of the kidney but accumulates in the renal cortex under pathologic conditions such as ischemia-reperfusion injury, diabetic nephropathy, glomerulonephritis and allograft rejection [33, 34]. These studies suggest that ligand mediated TLR4 activation plays an important role in acute kidney injury.
TLR4 is the best characterized TLR in AKI. The expression of TLR4 in the kidney
is mainly located in proximal and distal tubular epithelial cells [5, 35, 36, 37].
Ischemia associated renal inflammation upregulates the expression of TLR4 mRNA
and protein in the epithelium of the distal convoluted tubule, collecting duct,
and loop of Henle [37]. The homodimer of TLR4 is formed following ligand binding
and intracellular signaling is transmitted through two major downstream pathways:
(1) the MyD88-dependent pathway, which activates early NF-
Most renal damage in AKI occurs in the tubular epithelial cells. Under pathologic conditions such as ischemia, poisoning and inflammation, renal tubular epithelial cells undergo degeneration, apoptosis, necrosis, and shedding [39]. Both innate and adaptive immune responses are involved in AKI. Except for eliminating endogenous and exogenous antigens, overly robust activation of the immune system leads to excessive production of inflammatory mediators that eventually leads to tissue damage [40]. Consequently, direct or indirect suppression of the inflammatory response has been shown to be able to ameliorate renal damage in AKI models [41].
Our data showed that the serum TLR4 levels were increased after the completion of ATAAD surgery and further increased in patients who developed post-operative AKI. The increase of TLR4 might be due to a secondary inflammatory response. Our study demonstrated an increased incidence of lung infections during the postoperative period in the AKI group. Elevated TLR4 levels might play an important role in this process. A previous study reported the therapeutic effects of TLR 4 agonistic antibodies against lung infections in mice [42]. This phenomenon might offer a new strategy for the treatment of lung infections. Additionally, we noticed that the increase of serum TLR4 levels occurred before sCr. These data indicate that extracorporeal circulation performed during surgery for ATAAD helps to promote the necroptosis in the kidney that results in an elevation of TLR 4 expression which can be used to predict the occurrence of AKI.
Previous studies confirmed that only stage 3 AKI, but not stage 1 or 2, was associated with higher postoperative mortality [43, 44, 45]. However, AKI after cardiac surgery, even in its mild form, was associated with worse short-term outcomes including 30- or 90-day mortality and morbidity, and increases medical costs [46, 47]. Our study showed that elevated TLR4 levels were associated with the occurrence of severe AKI and worse 30-day mortality. Therefore, a TLR4 targeted strategy might be a potential novel therapeutic treatment option to prevent the occurrence of AKI following ATAAD surgery.
Due to the high incidence of developing postoperative AKI after ATAAD surgical repair, patients with elevated TLR4 levels immediately after surgery should be regarded as high-risk populations and potential candidates to receive renal protective treatment. Discovering a more accurate cut-off value of TLR4 in future studies with larger sample sizes would further help to earlier diagnose postoperative AKI in ATAAD and might help to guide a more individualized treatment program.
There are several limitations in the present study. First, the sample size was relatively small and was recruited from a single center. Second, this study was not powered to examine the long-term effects of elevated TLR4 expression. Third, as TLR4 plays a vital role in inflammation and infections, infection data involving other organs systems was missing in the current dataset.
This study showed that elevated TLR4 levels immediately after ATAAD surgery could predict the occurrence of AKI with good sensitivity and specificity. These results suggest that TLR4 might be considered as a new biomarker and potential therapeutic target for postoperative AKI in ATAAD. The results are preliminary and should be verified in other studies with larger sample sizes.
AKI, acute kidney injury; ATAAD, acute type A aortic dissection; TLR4, toll-like receptor 4; ICU, intensive care unit; RRT, renal replacement therapy; SCr, serum creatinine; ROC, receiver operating characteristic; OR, odds ratios; CI, confidence interval.
HJW, CMJ, DJW, ZGW and JFX designed the research study. JFX and ZGW performed the research. JFX, ZGW, and QYZ analyzed the data and wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All subjects gave their informed consent for inclusion before participating in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Nanjing Drum Tower Hospital (No. 2022-084-01).
Not applicable.
This work was supported by grant from the Key Project of Nanjing Medical Science and Technology Development (ZKX16040).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
