†These authors contributed equally.
Academic Editors: Manuel Martínez-Sellés, Grigorios Tsigkas, Athanasios Moulias and Anastasios Apostolos
Backgroud: The “FFR or OCT Guidance to Revascularize Intermediate
Coronary Stenosis Using Angioplasty” (FORZA) trial showed that in patients with
angiographically intermediate coronary lesions (AICLs), optical coherence
tomography (OCT) guidance of percutaneous coronary intervention (PCI) reduced the
occurrence of the composite endpoint of major adverse cardiac events (MACE) or
significant angina at 13 months, while fractional flow reserve (FFR) guidance
was associated with a higher rate of medical management and with lower costs.
Safety of PCI deferral when FFR
Functional assessment of intermediate coronary stenoses by means of fractional flow reserve (FFR) has proven to be better than angiography alone in selecting lesions to be treated and in guiding percutaneous coronary intervention (PCI) [1, 2, 3]. In contrast, intracoronary imaging techniques, such as optical coherence tomography (OCT), despite useful in optimizing PCI, does not still play a clear role when it comes to choosing the lesions to treat [4, 5, 6]. The open-label, single-centre, prospective, randomized “FFR or OCT Guidance to Revascularize Intermediate Coronary Stenosis Using Angioplasty” (FORZA) trial [7] (NCT01824030) was therefore conducted in order to compare the clinical and economic implications of PCI-deferral of angiographically intermediate coronary lesions (AICLs) based on OCT evaluation or on FFR assessment. In the present report, the 24-months follow-up results of the subgroup of patients in which a strategy of FFR or OCT guided strategy of PCI deferral of the FORZA trial are presented.
FORZA trial [7] enrolled three hundred and fifty consecutive patients with stable ischemic heart disease or stabilized (culprit lesion treated previously) acute coronary syndrome and evidence of at least one AICL, for a total of 446 AICL. AICL was defined as a coronary lesion with an angiographically estimated percentage diameter stenosis ranging from 30% and 80%.
Patients were randomized in a 1:1 fashion to the use of OCT guidance or FFR
guidance for deferring or performing PCI. Specific inclusion and exclusion
criteria were previously reported [7]. The study was approved by the ethics
committee of our institution (internal code 6261/13), and all patients signed a
dedicated informed consent form. The data that support the findings of this study
are available from the corresponding author upon reasonable request. We have
already published results at 1 and 13 months. We selected patients in whom PCI,
based on FFR and OCT criteria, was initially deferred. In details, PCI was
deferred when FFR was
 Fig. 1.
Fig. 1.Study flow chart. AS, area stenosis; FFR, fractional flow reserve; MLA, minimal luminal area; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; OMT, optical medical therapy.
After placement of a guiding catheter at the coronary ostium, FFR or OCT assessments have been performed according to randomization as described previously [7]. Randomization was based on a computer-generated random series of numbers and took place through the opening of an envelope in which the treatment arm was reported. Both the operator and the patient were unblinded to the technique used.
A 0.014-inch pressure-monitoring guidewire (Pressure Wire Certus or Aeris;
Abbott Vascular, Abbott Park, IL, USA) was advanced beyond the AICL under
radioscopic examination to calculate the lowest ratio of distal coronary pressure
(Pd) divided by aortic pressure (Pa) after achievement of hyperaemia using
adenosine. Lesions were deferred when the FFR values was
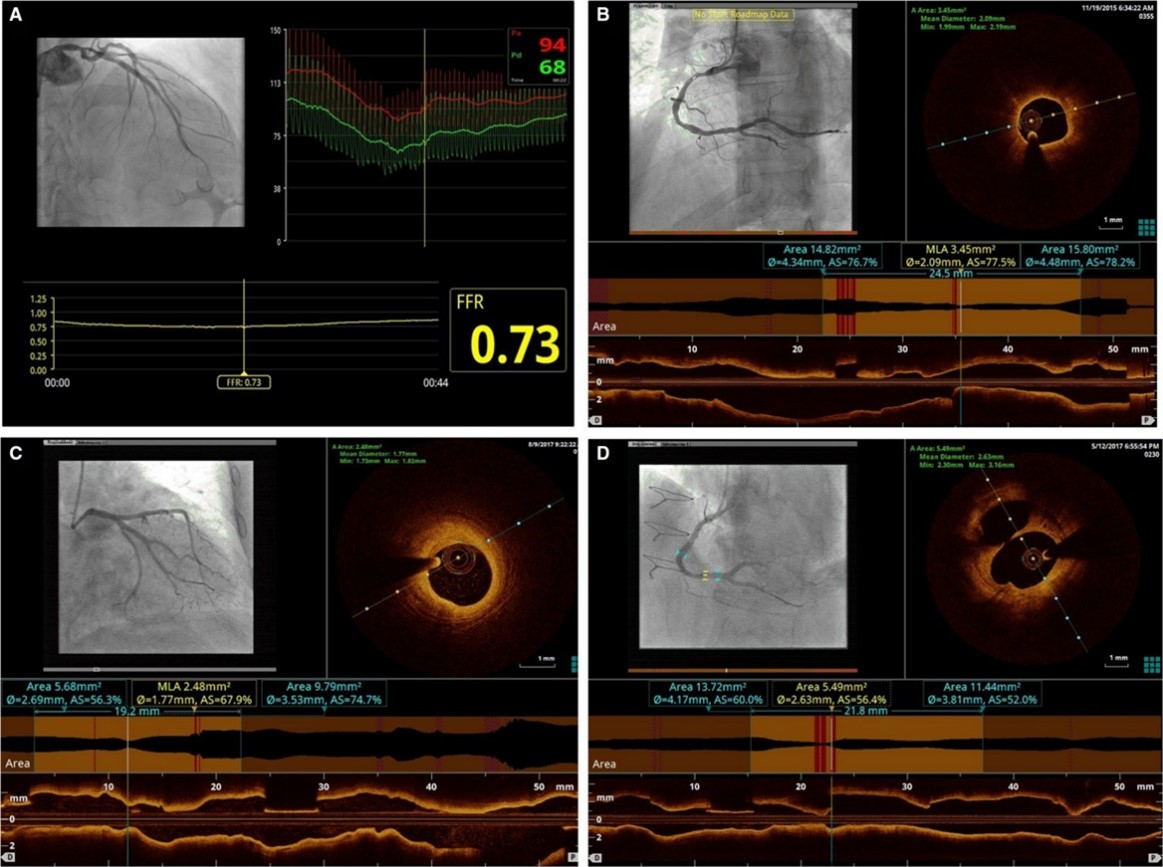 Fig. 2.
Fig. 2.The FORZA criteria for AICLs revascularization based on
FFR or OCT. (A) FFR
OCT images were acquired (after intracoronary administration of nitro-glycerine)
at the site of the AICL with commercially available systems (C7 System, LightLab
Imaging/St. Jude Medical, Westford, Massachusetts; and, after its availability,
Optis System, Abbott Vascular) after the OCT catheter (C7 Dragonfly, LightLab
Imaging/St. Jude Medical; and Dragonfly Optis, Abbott Vascular) was advanced to
the distal end of the target lesion. The entire length of the region of interest
was evaluated: minimal luminal area (MLA) (defined as cross-sectional area at the
smallest luminal area level), proximal reference luminal area (RLA) (defined as
the cross section at the frame with largest lumen within 10 mm proximal to MLA
and before any major side branch), distal RLA (defined as the cross section at
the frame with largest lumen within 10 mm distal to MLA and before any major side
branch), and mean RLA (defined as [proximal RLA + distal RLA]/2). On the basis of
these parameters, percentage of area stenosis (AS) was calculated using the
following formula: (mean RLA - MLA) / mean RLA
PCI was deferred in the absence of any of the following conditions: (1) area
stenosis
If at least 1 of the previous criteria occurred, operator proceeded to PCI. In
patients who underwent revascularization, OCT was used also to guide and optimize
PCI results. Optimization was performed in the presence of major stent
malapposition (defined as distance between strut and vessel wall
The study evaluates the clinical end point of “angina status” using the
significant residual angina (
Other outcomes of special interest were the SAQ value and the variation of angina symptoms after the 2 years of follow-up as well the overall number of medically managed patients. In addition, radioscopic time (min), amount of contrast medium (mL), post procedural length of hospitalization (days) and estimated costs associated with the two different strategies were evaluated as further secondary endpoints.
The total costs, including the cost of the first and any unplanned hospitalization after discharge was evaluated and compared between arms. Details for procedural and hospitalization cost evaluation have been previously reported [7].
This sub-study was designed to test the hypothesis that OCT imaging could be an acceptable alternative to FFR for deferral patients with AICLs after 24 months of follow-up.
Categorical variables were expressed as percentages and analyzed by Fisher’s
exact test. Continuous variables (including clinical and economical end points)
are expressed as mean
The characteristics of the patients and lesions enrolled in the two study arms
are showed in Tables 1,2 [8]. In the FFR group, PCI was deferred in 119
patients (67.6%) vs 82 (47.1%) in the OCT group. Deferred patients according to
FFR or OCT were fairly similar (no statistically significant difference in
baseline clinical characteristic except for a significant higher statin and oral anticoagulation
use in patients evaluated by OCT). In the OCT group, higher prevalence of
multivessel disease and LCX involvement were noted. Mean FFR was 0.87
| All patients | FFR | OCT | p value | ||
| n = 201 | n = 119 | n = 82 | |||
| Age | 68 |
70 |
68 |
0.41 | |
| Male gender | 89 (74.6%) | 80 (67.2%) | 66 (55.5%) | 0.22 | |
| BMI | 27 |
27 |
27 |
0.5 | |
| Risk factors | |||||
| Diabetes | 67 (33%) | 39 (32.8%) | 28 (34.1%) | 0.88 | |
| Hypertension | 170 (84.6%) | 102 (85.7%) | 68 (82.9%) | 0.69 | |
| Dyslipidemia | 141 (70.1%) | 81 (68.1%) | 60 (73.2%) | 0.54 | |
| Smoking | 73 (36.3%) | 47 (39.5%) | 26 (31.7%) | 0.24 | |
| CKD | 40 (19.9%) | 26 (21.8%) | 14 (17.1%) | 0.47 | |
| Previous history | |||||
| Previous PCI | 83 (41.3%) | 51 (42.9%) | 32 (39%) | 0.27 | |
| Previous CABG | 4 (2.0%) | 2 (1.73%) | 2 (2.4%) | 1 | |
| Previous MI | 44 (21.9%) | 20 (16.8%) | 24 (29.3%) | 0.06 | |
| Clinical presentation | |||||
| Stable ischemic heart disease | 158 (78.6%) | 94 (79%) | 64 (78.0) | 0.86 | |
| ACS | 43 (21.4%) | 25 (21%) | 18 (21.9%) | 0.68 | |
| Unstable angina | 28 (13.9%) | 16 (13.4%) | 12 (14.6%) | 0.84 | |
| NSTEMI | 14 (7.0%) | 9 (7.6%) | 5 (6.1%) | 0.78 | |
| STEMI | 1 (0.5%) | 0 (0%) | 1 (1.2%) | 0.41 | |
| LVEF (%) | 57 |
57 |
58 |
0.55 | |
| Seattle Angina Questionnaire | 83 |
84 |
85 |
0.62 | |
| Therapy at discharge | |||||
| Aspirin | 165 (82.1%) | 97 (81.5%) | 68 (82.9%) | 0.24 | |
| P2Y12 inhibitors | 87 (43.3%) | 48 (40.3%) | 39 (47.6%) | 0.31 | |
| Beta blockers | 145 (72.1%) | 80 (67.2%) | 65 (79.3%) | 0.65 | |
| Calcium channel blockers | 51 (25.4%) | 27 (22.7%) | 24 (29.3%) | 0.63 | |
| ACE inhibitors/ARB | 145 (72.1%) | 86 (72.3%) | 59 (71.9%) | 1 | |
| Statin | 162 (80.6%) | 89 (74.8%) | 73 (89.0%) | 0.02 | |
| Nitrates | 14 (7.0%) | 7 (5.9%) | 7 (8.5%) | 0.78 | |
| Ranolazine | 16 (8.0%) | 9 (7.6%) | 7 (8.5%) | 1 | |
| Diuretics | 56 (27.9%) | 32 (26.9%) | 24 (29.3%) | 1 | |
| Oral anticoagulant | 24 (11.9%) | 9 (7.6%) | 15 (18.3%) | 0.03 | |
| FFR, fractional flow reserve; OCT, optical coherence tomography; BMI, body mass index; CKD, chronic kidney disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; MI, myocardial infarction; ACS, acute coronary syndromes; NSTEMI, non ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction; LVEF, left ventricular ejection fraction; ACE, Angiotensin-converting enzyme; ARB, angiotensin receptor blocker. | |||||
| FFR | OCT | p value | ||
| 151 lesions | 102 lesions | |||
| Multivessel disease | 27 (22.7%) | 32 (39%) | 0.018 | |
| Studied lesions | 151 | 102 | 1 | |
| Single lesion studied | 87 (57.6%) | 66 (64.7%) | 0.36 | |
| 64 (42.4%) | 36 (35.3%) | |||
| Target lesion | ||||
| LAD | 93 (%) | 64 (%) | ||
| LCX | 25 (%) | 10 (%) | 0.02 | |
| RCA | 28 (%) | 20 (%) | ||
| Visual diameter stenosis (%) | 51 |
52 |
0.19 | |
| Baseline findings according to technique of randomization | ||||
| Resting Pd/Pa | 0.95 |
N/A | ||
| FFR | 0.87 |
N/A | ||
| MLA (mm |
N/A | 3.74 |
||
| AS (%) | N/A | 53 |
||
| FFR, fractional flow reserve; OCT, optical coherence tomography; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; MLA, minimal lumen area; AS, area stenosis. | ||||
Bifurcation lesions were slightly more prevalent in the OCT arm, without significant differences in terms of prevalence and revascularization adopted strategy (single stent or double stents) (Table 3).
| FFR | OCT | p value | |
| 225 lesions | 221 lesions | ||
| Bifurcation PCI (%) | 17 (7.5) | 24 (10.9) | 0.24 |
| Single stent treatment (%) | 13 (5.8) | 22 (9.9) | 0.31 |
| Double stent treatment (%) | 4 (1.8) | 2 (0.9) | 0.08 |
| FFR, fractional flow reserve; OCT, optical coherence tomography; PCI percutaneous coronary intervention. | |||
Radioscopic time, dose area product (DAP), consumption of contrast medium and postprocedural length of stay were numerically, but not significantly, higher in OCT than in FFR group (Table 4).
| FFR | OCT | p value | |
| 119 patients | 82 patients | ||
| 151 lesions | 102 lesions | ||
| Radioscopic time (min) | 13.1 |
14.4 |
0.58 |
| DAP (mGy*cm |
16127 |
19645 |
0.29 |
| Contrast media (mL) | 185 |
202 |
0.21 |
| Post procedural length of stay (days) | 2.5 |
3.0 |
0.21 |
| FFR, fractional flow reserve; OCT, optical coherence tomography; DAP, dose area product. | |||
The two groups had a similar value of frequency of angina at SAQ at enrolment
and both groups improved similarly at 24 months follow-up (from 82
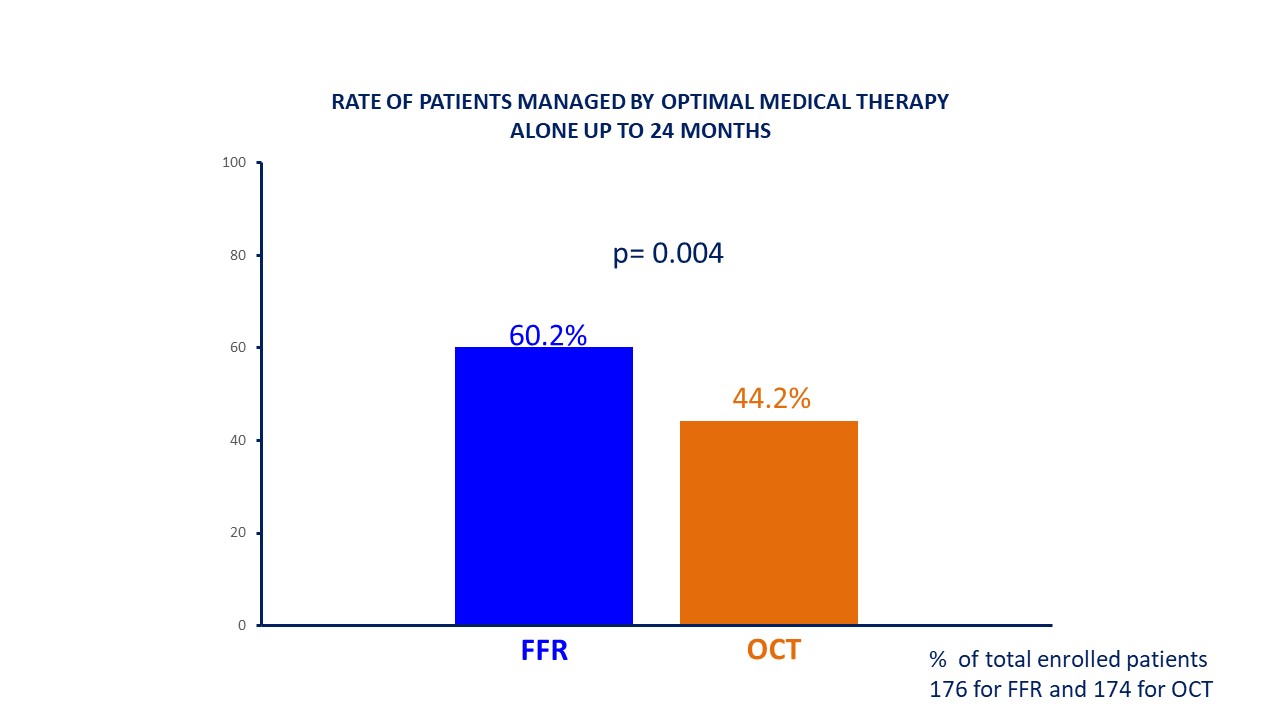 Fig. 3.
Fig. 3.Rate of patients managed with optimal medical therapy alone. FFR, fractional flow reserve; OCT, optical coherence tomography.
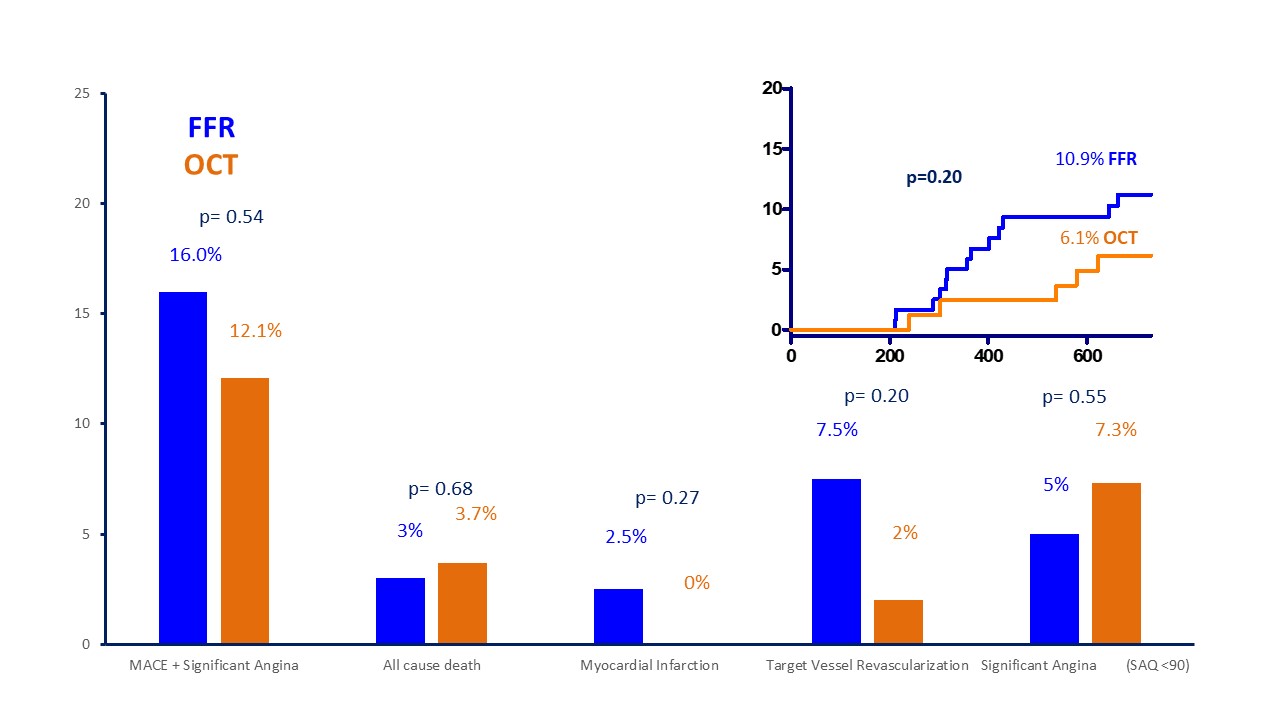 Fig. 4.
Fig. 4.Prevalence of different endpoints and Kaplan Meier curves for MACE. MACE, major cardiovascular events; SAQ, Seattle Angina Questionnaire; FFR, fractional flow reserve; OCT, optical coherence tomography.
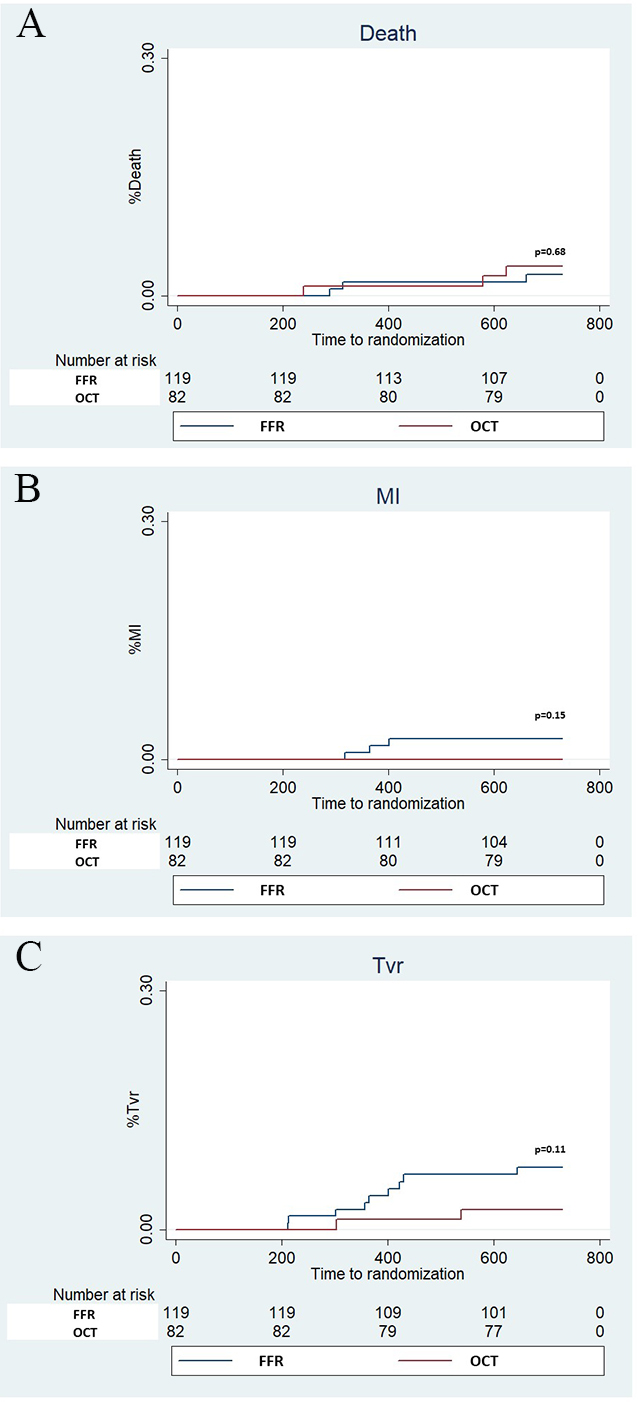 Fig. 5.
Fig. 5.Kaplan Meier curves for Death, myocardial infarction (MI) and Target vessel revascularization (TVR). Panel A (Death), Panel B (MI) and Panel C (TVR).
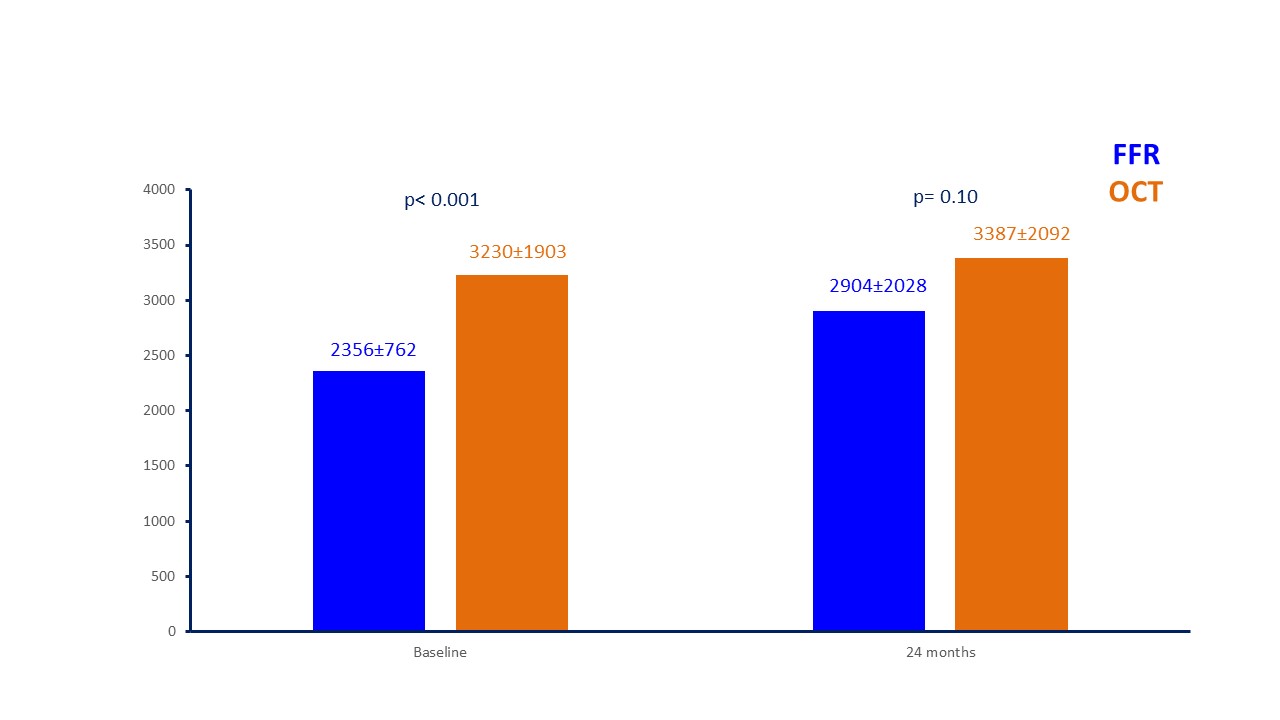 Fig. 6.
Fig. 6.Costs for patients managed with FFR and OCT at baseline and at 24-month follow-up (in euros). FFR, fractional flow reserve; OCT, optical coherence tomography.
| Event | FFR (N = 119) | OCT (n = 82) | p value | |
| Death | 3 (2.5%) | 3 (3.7%) | 0.68 | |
| Myocardial infarction | 3 (2.5%) | 0 (0%) | 0.27 | |
| Death/MI | 6 (5%) | 2 (2.4%) | 0.48 | |
| TVR | 9 (7.5) | 2 (2.4%) | 0.20 | |
| MACE | 13 (10.9%) | 5 (6.1%) | 0.32 | |
| TVF | 11 (9.2%) | 4 (4.9%) | 0.29 | |
| SAQ |
6 (5%) | 6 (7.3%) | 0.55 | |
| MACE + angina | 19 (16%) | 10 (12.1%) | 0.54 | |
| Optimal Medical Therapy (% of the starting population) | ||||
| Baseline | 119 (67.6%) | 82 (47.1%) | ||
| 13 months | 110 (62.5%) | 80 (46.0%) | ||
| 24 months | 106 (60.2%) | 77 (44.2%) | 0.0038 | |
| Cost at baseline | 2356 |
3230 |
||
| Cost at follow up | 2904 |
3387 |
0.10 | |
| FFR, fractional flow reserve; OCT, optical coherence tomography; MI, myocardial infarction; TVR, target vessel revascularization; MACE, major cardiovascular events; TVF, target vessel failure; SAQ, Seattle Angina Questionnaire. | ||||
A strategy of safe deferral of revascularization is possible only when
spontaneous cardiovascular events are lower than the predicted events due to the
procedure. A large body of evidence supports the safety of PCI deferral in case
of FFR
So far, we observed that FFR was associated with lower costs and higher medical management rate while OCT reduced the composite endpoint of major adverse cardiac events (MACE) or significant angina at 13-month [9, 10].
At 24-month of follow up FFR was still associated with a larger number of conservatively treated lesions translating into a larger and significantly higher number of medically managed patients. The high medical management rate and the overall low number of events confirmed FFR as the best and safe strategy to defer PCI.
On the other hand, the higher rate of performed PCI in the OCT arm was in line with the old previous studies, showing that visual assessment at the angiography [2], as well anatomical findings obtained by IVUS, were constantly associated with a higher rate of anatomically significant stenosis in comparison to functionally significant stenosis according to FFR.
Differently from what initially assumed in the main paper regarding the safety of “conservative” OCT criteria, a non-significant difference of MACE between the functional and imaging deferred arms was documented, with numerically less events in the OCT group.
In details, a similar death rate and a less rate of MI and TVR in OCT patients were detected underling the ability of imaging evaluation to characterize the natural history of coronary lesions, excluding the presence of high-risk features of untreated plaques and resulting a safe technique to defer PCI without impact on patient’s prognosis. On the other hand, the non-significant increase in TVR and hospitalizations in FFR arm affected total costs, which became not more statistically different at extended follow up of 24 months.
A similar value of frequency of angina at SAQ and, more importantly, a similar prevalence of persisting significant angina was noted between two arms. These data disclosed the ability of the “conservative” OCT criteria to be associated with hemodynamic significance of lesions and to predict stenosis-related myocardial ischemia in a way like FFR. We suggested a new role of pre-PCI OCT: not only to characterize coronary lesions and correctly select PCI materials in a “imaging-guided PCI” but also as an acceptable alternative for deferral angioplasty in intermediate lesions.
This study represents the first evidence that an anatomical guidance using OCT imaging, applying the simple FORZA criteria, could be an acceptable alternative to the physiological reference standard also for deferral of AICLs.
These are the first data supporting the use of OCT to safely defer treatment of AICL taking FFR as the gold standard. Despite the quite limited number of patients enrolled in this sub-analysis of the FORZA trial could limit the ability to draw definitive conclusions about safety of OCT to safely defer treatment of AICL, the numerically lower rate of event seen in comparison to FFR seems quite reassuring. We believe that an imaging evaluation of intermediate coronary lesions could be a comparable strategy to defer PCI like FFR, and at the same time we suppose a possible link between morphologic plaque assessment and hemodynamic significance of lesions in order to predict stenosis-related myocardial ischemia. We strongly support further investigations in this field, in particular more data are needed to assess the correlation between anatomical and functional indexes in order to predict MACE and myocardial ischemia.
Our data derived from a single centre study, with a small sample size and the result should be regarded as hypotheses generating. It was conducted locally without either a structured clinical research organization or an independent clinical events committee. The use of unconventional OCT criteria has to be acknowledged in light of the lack of clear data at the time of design of the trial [7]. However, after initiation of the study, these criteria were validated in comparison to FFR in a retrospective cohort of patients assessed with both FFR and OCT [11] and a recent randomized trial showed a safe profile of the OCT criteria to defer or perform PCI [12]. FFR assessment was made in according to the best clinical practise, however a clear definition of diffuse versus focal disease was not available because of the absence of new tools (PPG index) [13] at the start of the study. However, pullback manoeuvres were performed both in the pre and post PCI phase to assess the pressure drop distribution along the vessel. Total procedural time was not recorded but we evaluated the “procedural radioscopic time” as a surrogate, less dependent by logistic or procedural confounders.
The 24-months follow-up results of the FORZA trial, the first prospective randomized trial comparing OCT and FFR to guide PCI decision and performance in AICLs, showed that deferral of PCI based on OCT is clinically safe as compared with the (contemporary gold-standard) FFR-guided approach. Over 2 years, FFR-guidance warranted higher rate of optimal medical therapy management alone as compared with OCT-guidance.
AML and FBur—conception and design, analysis and interpretation of data, drafting, and revising critically the manuscript for important intellectual content; CA and DG—analysis of data and drafting of the manuscript; GZ, AZ, FDG, FBia and RV—collection, analysis, and interpretation of data; CT and FC—important intellectual contribution; All authors contributed to the article and approved the submitted version.
The studies involving human participants were reviewed and approved by Comitato Etico del Policlinico Gemelli di Roma (number 6261/13). The patients/participants provided their written informed consent to participate in this study.
We would like to express our gratitude to the peer reviewers for their opinions and suggestions, and to all those who helped us with writing of this manuscript.
The FORZA TRIAL was funded by internal Academic Grants (Bando Linea D. 1105536). The authors also thank the Italian Ministry of Health for the funding within the “Ricerca corrente 2021” project.
A.M.L. received speaking honoraria from Abbott, Medtronic, Abiomed and from Bracco Imaging, F.B, C.T and C.A received speaker’s fees from Abbott, Medtronic, and Abiomed. G.Z., A.Z., D.G., F.D.G., F.B., R.V. and F.C have no conflicts of interest. Antonio Maria Leone is serving as one of the Editorial Board members of this journal. We declare that Antonio Maria Leone had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Manuel Martínez-Sellés, Grigorios Tsigkas, Athanasios Moulias and Anastasios Apostolos.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
