†These authors contributed equally.
Academic Editor: Federico Ronco
Background: The impact of nodular calcifications in left ventricular
outlow tract (LVOT) and aortic annulus on the procedural outcome of transcatheter
aortic valve implantation (TAVI) with new-generation devices is yet to be
elucidated. Similarly, computational simulations may provide a novel insight into
the biomechanical features of TAVI devices and their interaction with nodular
calcifications. Methods: This retrospective single-center study included
232 patients submitted to TAVI with Evolut-R (53.4%), Portico (33.6%) and Lotus
(13.0%) devices with available preoperative computed tomography (CT) angiography
and evidence of nodular calcifications in aortic annulus and/or LVOT.
Calcification severity was defined
Transcatheter aortic valve implantation (TAVI) is an established treatment option for patients with symptomatic severe aortic stenosis at high and intermediate surgical risk [1, 2], with comparable results to surgery also in low risk patients [3, 4].
Both Evolut-R (Medtronic Inc., Minneapolis, MN, USA) and Portico aortic valves (Abbott, Minneapolis, MN, USA) are self-expanding (SE) devices [5, 6]. The Lotus valve (Boston Scientific, Marlborough, MA, USA) is the only mechanically expandable (ME) device, and, as unique feature, allows full retrievability even after complete deployment [7].
Device success depends on several features, which are related either to the aortic root and valve anatomy (e.g., calcifications, aortic angulation) and technical aspects (e.g., oversizing, implantation depth) [8, 9, 10, 11]. One of the anatomical factors that may be relevant to procedural success is the size and dimension of the annular and left ventricular outflow tract (LVOT) calcifications [8]. Prosthetic valves are meant to be expanded in a circular fashion, hence the presence of nodular calcifications may lead to partial underexpansion of the strut, with consequent paravalvular leak (PVL) [12].
Thus, the strut conformability of new-generation SE and ME devices may be relevant. In this setting, computational simulations may constitute a compelling tool to predict the device performance, based on its interaction with the aortic annulus geometry altered by nodular calcifications [13].
Aim of the present study is to assess the impact of nodular calcifications in the aortic annulus and LVOT on device success and residual PVL in patients undergoing TAVI with new-generation devices and to test by computational simulations their biomechanical behaviour with respect to the severity of nodular calcification.
This retrospective, observational, single-center study included patients with symptomatic severe aortic stenosis undergoing TAVI with Evolut-R, Portico and Lotus devices at the IRCCS Policlinico San Donato from January 2016 to May 2021 with available computed tomography (CT) angiography aortic annulus measurements.
Inclusion criterion was the presence of discrete nodular annular and/or LVOT calcifications.
Exclusion criteria were pure aortic regurgitation as indication for TAVI, and valve-in-valve TAVI. Patients treated with Evolut Pro were excluded from the analysis; merging these patients with those treated with Evolut-R could have biased the results due to the external sealing skirt in the formers. Additionally, computational simulations could only consider the prosthetic valve stent.
Among 687 patients treated with Evolut-R, Portico or Lotus valve, 232 (33.8%) were included in the study population.
Our institutional TAVI database collected prospectively the data about baseline features, procedural aspects, echocardiography measures, CT scan and 30-days outcomes.
All patients proceeded to TAVI after Heart Team discussion and provided written informed consent before the procedure.
CT-angiography scans were performed on 64- or 128-row multidetector scanner
(Somatom Definition; Siemens healthcare, Forchheim, Germany). Image acquisition
was electrocardiography (ECG) gated. The 3-Mensio valves software (version 8.2,
Pie Medical Imaging, Maastricht, The Netherlands) permitted the multiplanar
reconstruction analysis of the aortic root, evaluating both the diastolic and
systolic phase [14]. Calcification severity was defined mild in presence of one
spherical, calcific nodule with a major diameter
 Fig. 1.
Fig. 1.Evaluation of calcification severity at CT angiography. Mild
calcification severity: a calcified nodule (max diameter 2.25 mm) in the annulus
(A); LVOT is free from calcifications (C);
It was also taken into account the index of eccentricity (IE), calculated as Eqn. 1:
IE
Areas of calcium were detected in the region of interest (from the virtual basal ring up to 4 mm in the LVOT) using a validated threshold of 800 Hounsfield Units (HU) [17]. A dedicated core laboratory of radiology technicians made all measurements. They were blinded to the implanted prosthesis before TAVI.
Transfemoral TAVI was performed under local anesthesia with or without conscious sedation according to patient’s tolerance to the procedure; trans-subclavian and transaortic TAVI were performed under general anesthesia [18].
Technical details of the Evolut-R, Portico and Lotus device have been previously reported [5, 6, 7].
Due to our internal policy, Evolut-R was the most employed prosthesis at our Institution. In light of this consideration, the device was used in about half procedures (124/232; 53.4%), followed by Portico (78/232; 33.6%) and by Lotus (30/232; 12.9%). Given this premise, in each case, prosthesis choice was left to first operator’s discretion.
Implantation depth was defined as the maximal distance between the bioprosthetic intraventricular edge and the aortic annulus at the level of the non-coronary cusp (NCC) and left coronary cusp (LCC), measured by angiography in the deployment projection [19].
Repositioning was defined as partial valve resheathing to enable movement from its initial deployment site forward or backward in the ventricle. Recapture was defined as complete valve retraction into the delivery catheter [20].
Transthoracic echocardiography (TTE) was performed with a GE Vivid 9 ultrasound unit (GE Healthcare, Horten, Norway) before and after TAVI. Postprocedural TTE was performed the same day of procedure and repeated at discharge. Post-procedural PVL was assessed in line with Valve Academic Research Consortium-3 (VARC-3) criteria and classified in four categories (absent/trivial, mild, moderate, severe) by experienced echocardiographers [21].
An independent reader blinded both to aortic annulus measurements and to
prosthesis type manually reviewed the cine-loops; discrepancies in PVL grading
were resolved by consensus. Moreover, discharge TTE data were used for the
analysis in case of discrepancies with the post-procedural ones, as the
self-expanding mechanism of steel may contribute to improve PVL severity during
periprocedural period. At last, trivial jets were grouped with no PVL, whereas
moderate and severe PVL were grouped together as
Device success was defined according to VARC-3 definition upon fulfilling the
following criteria: (1) absence of procedural mortality (within 72 h from the
procedure); (2) correct positioning of a single prosthetic transcatheter heart
valve (THV) into the proper anatomical location; (3) intended prosthetic THV
performance (no patient-prosthesis mismatch, mean aortic gradient
Evolut-R, Portico and Lotus were compared. Geometrical models were reconstructed from micro-CT scans of real device samples (Evolut-R 26 mm, Portico 25 mm, and Lotus Edge 23 mm) and Nitinol material properties were assigned [22]. Only the prosthetic valve stent was considered since the valve was not visible from CT images and the post-operative configurations of the stent and aortic root were assumed not to be influenced by prosthetic leaflets.
An idealized aortic root model composed of three regions (annulus/LVOT, valsalva
sinuses and ascending aorta) was conceived as previously reported [10]. Only the
aortic root was considered, native leaflets were not taken into account in the
simulation framework. In a previous study the impact of an elliptic vs. circular
annulus has been evaluated [10]. Moreover, as in the present study the IE was
0.21
 Fig. 2.
Fig. 2.Nodular calcification model. Example of TAVI simulation with Lotus valve in a 0.25 IE aortic root having nodular calcifications of increasing size (maximum diameter: mild 8 mm, moderate 12 mm, severe 14 mm).
Material properties to model the arterial soft tissue and calcifications were derived from a previous publication [23].
These idealized aortic root models were used as the initial geometries for finite element simulation of TAVI using the commercial software Abaqus (v. 2019, Simulia, Dàssault Systems, Providence, RI, USA). The simulation setup consisted of two steps: crimping of the stent inside its catheter and stent re-expansion within the aortic root models with a final implantation depth according to each device’s instructions by the manifacturer. More details about the simulation procedure were given in a previous publication of our research group [24]. Post-processing of simulation outcomes was then performed and three variables were measured: the stent-root interaction area, Von Mises stress distribution, and paravalvular orifice area [24].
The measure of the stent-root interaction area could represent an indication of stent anchoring and adesion to the wall. It was computed by means of an in-house Matlab script (The MathWorks, Inc., Natick, MA, USA) as the sum of the areas of the aortic wall elements with contact pressure higher than zero (Fig. 3A).
 Fig. 3.
Fig. 3.Stent-rott interaction model. (A) Stent-root interaction area:
contact area between the stent and the internal surface of the aortic root. (B)
Von Mises stress map: distribution of stress values in the inner aortic root. (C)
Paravalvular orifice area: definition of a cutting plane
Von Mises stress distribution is a measure of the stress induced by the device expansion onto the inner wall of the aortic root (Fig. 3B). Only the annulus/LVOT internal wall region was considered. Both the average stress and the maximum one were computed with a Matlab script. To make the interpretation of potential dishomogeneity in the stress distribution clearer, the ratio between the average and the maximum stress value was shown (low values meant a more dishomogeneous stress distribution against the aortic wall). Paravalvular orifice area was derived from the area of the orifices generated after stent expansion between the device and the inner aortic root wall. The area of the such orifices was quantified using the open source software Image J (1.52t (30 January 2020), JAVA, NIH, USA) [24] (Fig. 3C).
Categorical and dichotomous variables are shown as frequencies and percentages; they were compared by Pear- son chi-square or Fisher exact tests, as appropriate.
The Kruskal-Wallis test was used to check the skewed distribution of continuous covariates.
Continuous variables following a normal distribution are reported as mean and standard deviation; they were compared using unpaired two-sided Student’s t-test. Otherwise, non-normally distributed variables were arranged as median and interquartile range; they were compared using the Mann-Whitney U-test.
Univariate logistic regression analysis was performed to investigate factors
associated with
Multivariate logistic regression analysis was performed to investigate factors
associated with device success, by using a backward stepwise method including
variables with p
All p-values were two-sided with values
Among the study population, 123 (53.0%) patients showed
Patients with mild calcifications had more frequently a history of prior coronary artery bypass grafting (CABG). There were no significant differences regarding age, other cardiovascular risk factors, Society of Thoracic Surgeons (STS) score, creatinine clearance, and echocardiographic findings.
On CT-angiography, patients with
| Variables | Overall calcium | Mild calcifications | p-value | |
| N | 232 (100%) | 109 (47.0%) | 123 (53.0%) | |
| Age (years) | 83.0 |
82.1 |
83.7 |
0.325 |
| Female sex | 131 (56.5%) | 63 (57.8%) | 68 (55.3%) | 0.700 |
| Hypertension | 169 (72.8%) | 80 (73.4%) | 89 (72.4%) | 0.859 |
| Diabetes | 55 (23.7%) | 27 (24.8%) | 28 (22.8%) | 0.720 |
| Dyslipidemia | 92 (39.7%) | 42 (38.5%) | 50 (40.7%) | 0.742 |
| COPD | 35 (15.1%) | 19 (17.4%) | 16 (13.0%) | 0.348 |
| Coronary artery disease | 76 (32.8%) | 33 (30.3%) | 43 (35.0%) | 0.448 |
| Prior CABG | 17 (7.4%) | 13 (12.0%) | 4 (3.3%) | 0.011 |
| Prior AMI | 26 (11.2%) | 13 (11.9%) | 13 (10.6%) | 0.744 |
| STS score (%) | 5.0 |
5.2 |
4.8 |
0.197 |
| Creatinine clearance (mL/min/1.73 m |
53.0 |
50.0 |
55.8 |
0.086 |
| Haemoglobin (g/dL) | 12.0 |
12.0 |
12.0 |
0.180 |
| Ejection fraction (%) | 55.5 |
54.5 |
56.6 |
0.619 |
| Mean aortic gradient (mmHg) | 46.0 |
44.82 |
47.23 |
0.649 |
| AR |
48 (20.7%) | 20 (18.3%) | 28 (22.8%) | 0.407 |
| LM height (mm) | 14.7 |
14.5 |
14.9 |
0.300 |
| RCA height (mm) | 17.8 |
17.6 |
17.9 |
0.862 |
| Annulus min diameter (mm) | 20.8 |
20.6 |
20.9 |
0.907 |
| Annulus max diameter (mm) | 26.5 |
26.4 |
26.5 |
0.965 |
| Annulus mean diameter (mm) | 23.6 |
23.5 |
23.6 |
0.863 |
| Annulus perimeter (mm) | 74.8 |
74.6 |
75.1 |
0.794 |
| Annulus area (mm |
415.2 |
397.2 |
431.2 |
0.087 |
| Sinus of Valsalva diameter (mm) | 32.4 |
31.9 |
32.7 |
0.398 |
| Calcium volume 800 HU (mm |
304.2 [175.8–549.9] | 236.5 [151.6–437.9] | 416.3 [216.4–654.8] | |
| Aortic angulation (°) | 49.9 |
49.3 |
50.4 |
0.206 |
| Index of eccentricity | 0.21 |
0.22 |
0.21 |
0.994 |
| Calcification in LVOT | 134 (57.8%) | 45 (41.3%) | 89 (72.4%) | |
| Calcification at annulus | 188 (81.0%) | 78 (71.6%) | 110 (89.4%) | |
| Calcification at annulus and LVOT | 90 (38.8%) | 14 (12.8%) | 76 (61.8%) | |
| Calcification number | 1.42 |
1.0 |
1.79 |
|
| Major calcification diameter (mm) | 4.6 |
2.9 |
6.1 |
|
| LVOT diameter (mm) | 19.3 |
19.4 |
19.1 |
0.884 |
| Ascending aorta (mm) | 34.4 |
34.0 |
34.8 |
0.980 |
| AMI, acute myocardial infarction; AR, aortic regurgitation; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; HU, Hounsfield Units; LM, left main; LVOT, left ventricular outflow tract; RCA, right coronary artery; STS, Society of Thoracic Surgeons. | ||||
Patients treated with Lotus valve were younger, had lower STS score and smaller major diameter calcifications rather than Evolut-R and Portico patients. Evolut-R patients had a higher prevalence of diabetes, meanwhile Portico patients had lower levels of haemoglobin and lower values of annulus diameter (mean and maximum).
On CT-angiography, Portico treated patients had lower coronary artery take-off and annulus perimeter.
No significant differences in the rate of
| Variables | Evolut-R | Portico | Lotus | p-value |
| N | 124 (53.4%) | 78 (33.6%) | 30 (12.9%) | |
| Age (years) | 86.6 |
83.8 |
78.0 |
|
| Female sex | 68 (54.8%) | 49 (62.8%) | 14 (46.7%) | 0.274 |
| Hypertension | 85 (68.5%) | 61 (78.2%) | 23 (76.7%) | 0.285 |
| Diabetes | 38 (30.6%) | 14 (17.9%) | 3 (10.0%) | 0.020 |
| Dyslipidemia | 43 (34.7%) | 39 (50.0%) | 10 (33.3%) | 0.072 |
| COPD | 19 (15.3%) | 11 (4.7%) | 5 (15.7%) | 0.940 |
| Coronary artery disease | 42 (33.9%) | 21 (26.9%) | 13 (43.3%) | 0.247 |
| Prior CABG | 9 (7.3%) | 5 (6.4%) | 3 (10.0%) | 0.785 |
| Prior AMI | 13 (10.5%) | 9 (11.5%) | 4 (13.3%) | 0.900 |
| STS score (%) | 5.4 |
5.0 |
3.4 |
0.005 |
| Creatinine clearance (mL/min/1.73 m |
51.9 |
52.0 |
59.1 |
0.226 |
| Haemoglobin (g/dL) | 12.1 |
11.6 |
12.7 |
0.018 |
| Ejection fraction (%) | 55.2 |
55.9 |
55.7 |
0.932 |
| Mean aortic gradient (mmHg) | 46.4 |
43.5 |
50.8 |
0.109 |
| AR |
27 (21.8%) | 15 (19.2%) | 6 (20.0%) | 0.905 |
| LM height (mm) | 15.1 |
13.8 |
15.5 |
0.050 |
| RCA height (mm) | 18.3 |
16.8 |
18.1 |
0.015 |
| Annulus min diameter (mm) | 20.9 |
20.3 |
21.6 |
0.066 |
| Annulus max diameter (mm) | 26.8 |
25.6 |
27.2 |
0.004 |
| Annulus mean diameter (mm) | 23.8 |
23.0 |
24.4 |
0.014 |
| Annulus perimeter (mm) | 75.6 |
72.6 |
77.6 |
0.004 |
| Annulus area (mm |
438.5 |
396.7 |
366.0 |
0.003 |
| Sinus of Valsalva diameter (mm) | 32.7 |
31.2 |
32.7 |
0.162 |
| Calcium volume 800 HU (mm |
299. 4 [172.2–526.5] | 276.5 [169.3–479.8] | 519.2 [204.7–802.2] | 0.187 |
| Aortic angulation (°) | 50.9 |
47.9 |
50.9 |
0.164 |
| Index of eccentricity | 0.22 |
0.20 |
0.21 |
0.163 |
| Calcification at LVOT | 71 (57.3%) | 52 (66.7%) | 11 (36.7%) | 0.018 |
| Calcification at annulus | 105 (84.7%) | 56 (71.8%) | 27 (90%) | 0.031 |
| Calcification in annulus and LVOT | 52 (41.9%) | 30 (38.5%) | 8 (26.7%) | 0.305 |
| Calcification number | 1.36 |
1.51 |
1.40 |
0.378 |
| Major calcification diameter (mm) | 4.5 |
5.2 |
3.7 |
0.024 |
| LVOT diameter (mm) | 65 (52.4%) | 46 (59.0%) | 12 (40.0%) | 0.205 |
| Ascending aorta (mm) | 19.3 |
18.9 |
20.5 |
0.109 |
| AMI, acute myocardial infarction; AR, aortic regurgitation; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; HU, Hounsfield Units; LM, left main; LVOT, left ventricular outflow tract; RCA, right coronary artery; STS, Society of Thoracic Surgeons. | ||||
TAVI was performed through femoral access in 91.4% of procedures; among the
| Variables | Overall calcium | Mild calcifications | p-value | |
| N | 232 (100%) | 109 (47%) | 123 (53%) | |
| Femoral route | 212 (91.4%) | 102 (93.6%) | 110 (89.4%) | 0.261 |
| Subclavian route | 20 (8.6%) | 7 (6.4%) | 13 (10.6%) | 0.261 |
| Embolic protection system | 13 (5.6%) | 7 (6.4%) | 6 (4.9%) | 0.610 |
| Any vascular complications | 15 (6.5%) | 3 (2.8%) | 12 (9.8%) | 0.030 |
| PTA with stenting of access site | 4 (1.7%) | 1 (0.9%) | 3 (2.4%) | 0.625 |
| PCI with stenting | 12 (5.2%) | 5 (4.6%) | 7 (5.7%) | 0.705 |
| Degree of oversizing (%) | 15.9 |
15.9 |
15.8 |
0.060 |
| Predilatation | 131 (56.5%) | 58 (44.3%) | 73 (55.7%) | 0.347 |
| Implantation depth NCC (mm) | 4.4 |
4.6 |
4.2 |
0.323 |
| Implantation depth LCC (mm) | 5.9 |
6.2 |
5.7 |
0.585 |
| Implantation depth mean (mm) | 5.1 |
5.4 |
5.0 |
0.500 |
| Postdilatation | 122 (52.6%) | 58 (54.2%) | 64 (52.5%) | 0.858 |
| Repositioning | 55 (23.7) | 25 (22.9%) | 30 (24.4%) | 0.795 |
| Recapture | 47 (20.3%) | 21 (19.3%) | 26 (21.1%) | 0.723 |
| Emergent cardiac surgery | 1 (0.04%) | 0 (0.0%) | 1 (0.8%) | 1.000 |
| Need for second valve | 1 (0.04%) | 0 (0.0%) | 1 (0.8%) | 1.000 |
| Contrast volume (mL) | 155.3 |
146.1 |
164.6 |
0.101 |
| Radiation dose (Gycm |
80.1 |
72.8 |
86.5 |
0.029 |
| LCC, left coronary cusp; NCC, non-coronary cusp; PCI, percutaneous coronary intervention; PTA, percutaneous transluminal angioplasty. | ||||
Postdilatation rate was significantly lower in the Lotus group compared to Evolut-R and Portico, whereas the Evolut group had the lowest rate of predilatation. Implantation depth was greater in Portico valve patients. Degree of oversizing, as expected, was significantly different among the devices, due to the sizing chart of each valve. Similarly, Lotus valve, due to its unique feature, was the most repositioned device.
No differences were noticed regarding recapture rates, any vascular complications, radiation dose and concomitant PCI (Table 4).
| Variables | Evolut-R | Portico | Lotus | p-value |
| N | 124 (53.4%) | 78 (33.6%) | 30 (12.9%) | |
| Femoral route | 113 (91.1%) | 69 (88.5%) | 30 (100%) | 0.159 |
| Subclavian route | 11 (8.9%) | 9 (11.5%) | 0 (0.0%) | 0.159 |
| Embolic protection system | 8 (6.5%) | 2 (2.6%) | 3 (10.0%) | 0.269 |
| Any vascular complications | 8 (6.5%) | 6 (7.7%) | 1 (3.3%) | 0.711 |
| PTA with stenting of access site | 1 (0.8%) | 2 (2.6%) | 1 (3.3%) | 0.497 |
| PCI with stenting | 6 (4.8%) | 6 (7.7%) | 0 (0.0%) | 0.263 |
| Degree of oversizing (%) | 20.8 |
13.8 |
1.1 |
|
| Predilatation | 52 (41.9%) | 60 (76.9%) | 19 (63.3%) | |
| Implantation depth NCC (mm) | 4.3 |
4.9 |
3.4 |
0.047 |
| Implantation depth LCC (mm) | 5.6 |
6.8 |
5.0 |
0.012 |
| Implantation depth mean (mm) | 5.0 |
5.8 |
5.1 |
0.015 |
| Postdilatation | 72 (58.1%) | 48 (61.5%) | 2 (6.7%) | |
| Repositioning | 15 (12.1%) | 26 (3.3%) | 14 (46.7%) | |
| Recapture | 27 (21.8%) | 12 (15.4%) | 8 (26.7%) | 0.352 |
| Emergent cardiac surgery | 1 (0.08%) | 0 (0.0%) | 0 (0.0%) | 0.646 |
| Need for second valve | 0 (0.0%) | 1 (1.3%) | 0 (0.0%) | 0.371 |
| Contrast volume (mL) | 151.9 |
167.2 |
137.9 |
0.132 |
| Radiation dose (Gycm |
76.1 |
81.0 |
106.8 |
0.182 |
| LCC, left coronary cusp; NCC, non-coronary cusp; PCI, percutaneous coronary intervention; PTA, percutaneous transluminal angioplasty. | ||||
There were no significant differences regarding permanent pacemaker implantation
rate, 30-day mortality and stroke among the two groups based on calcifications
severity. The
| Variables | Overall calcium | Mild calcifications | p-value | |
| N | 232 (100%) | 109 (47.0%) | 123 (53.0%) | |
| Ejection fraction (%) | 57.2 |
55.5 |
58.8 |
0.760 |
| Mean gradient (mmHg) | 9.7 |
9.6 |
9.7 |
0.353 |
| PVL absent/trivial | 82 (35.3%) | 41 (37.6%) | 41 (33.3%) | 0.496 |
| PVL mild | 132 (56.9%) | 64 (58.7%) | 68 (55.3%) | 0.598 |
| PVL |
18 (7.8%) | 4 (3.7%) | 14 (11.4%) | 0.028 |
| Device success | 212 (91.4%) | 104 (95.4%) | 108 (87.8%) | 0.039 |
| PPI | 37 (15.9%) | 14 (12.8%) | 23 (18.7%) | 0.224 |
| Stroke (including not disabling) | 5 (2.2%) | 3 (2.8%) | 2 (1.6%) | 0.555 |
| 30-day mortality | 10 (4.3%) | 4 (3.7%) | 6 (4.9%) | 0.753 |
| PPI, permanent pacemaker implantation; PVL, paravalvular leak. | ||||
When stratified by prosthesis type (Table 6), higher rates of
| Variables | Evolut-R | Portico | Lotus | p-value |
| N | 124 (53.4%) | 78 (33.6%) | 30 (12.9%) | |
| Ejection fraction (%) | 56.7 |
58.1 |
56.7 |
0.729 |
| Mean gradient (mmHg) | 8.7 |
9.2 |
12.3 |
|
| PVL absent/trivial | 36 (29.0%) | 25 (32.1%) | 21 (70.0%) | |
| PVL mild | 73 (58.9%) | 50 (64.1%) | 9 (30%) | 0.005 |
| PVL |
15 (12.1%) | 3 (3.8%) | 0 (0.0%) | 0.024 |
| Device success | 108 (87.1%) | 74 (94.9%) | 30 (100%) | 0.031 |
| PPI | 21 (16.9%) | 11 (14.1%) | 5 (16.7%) | 0.861 |
| Stroke (including not disabling) | 2 (1.6%) | 2 (2.6%) | 1 (3.3%) | 0.806 |
| 30-day mortality | 6 (4.8%) | 4 (5.1%) | 0 (0.0%) | 0.458 |
| PPI, permanent pacemaker implantation; PVL, paravalvular leak. | ||||
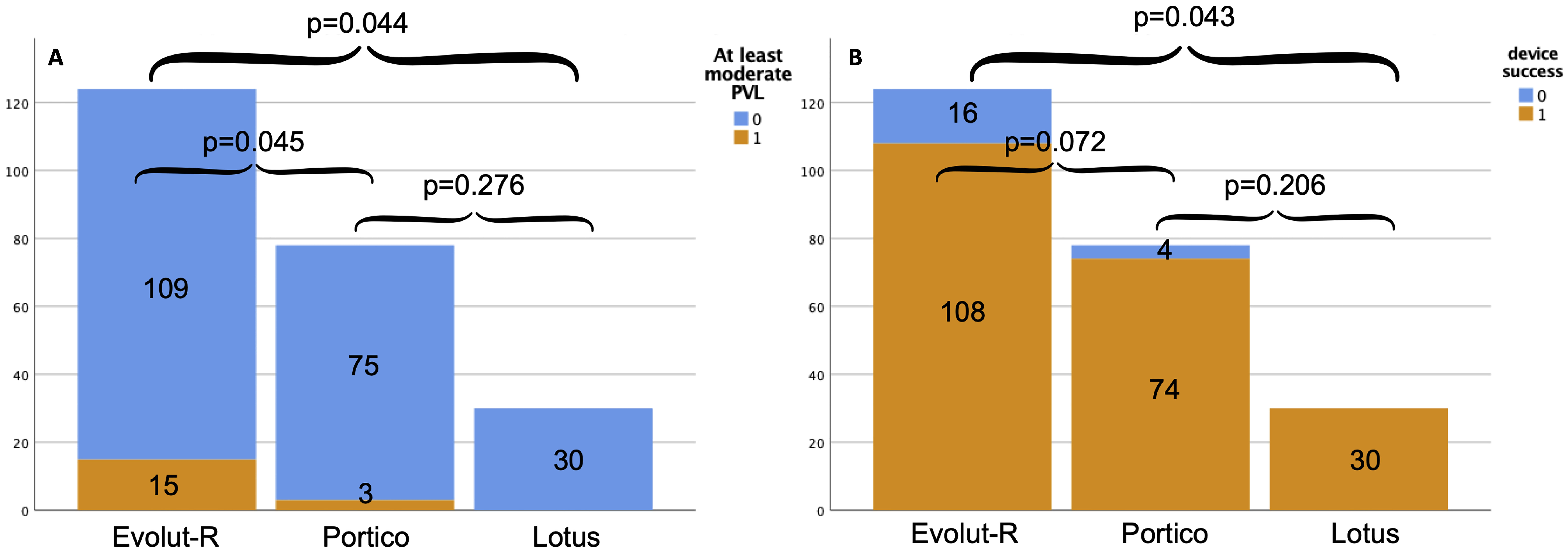 Fig. 4.
Fig. 4.In-hospital outcomes. Differences between Evolut-R, Portico and
Lotus valves groups by
Device success was achieved by 212 patients (91.4%).
On multivariate analysis, both annulus and LVOT calcifications (OR 0.105; p = 0.023) were independent predictors of device success (Table 7).
| Odds ratio | 95% CI | p-value | ||
| Univariate predictors | ||||
| Hypertension | 1.903 | 0.739–4.900 | 0.182 | |
| Dyslipidemia | 2.839 | 0.918–8.871 | 0.070 | |
| Ejection fraction (%) | 0.956 | 0..907–1.008 | 0.099 | |
| Mean aortic gradient (mmHg) | 0.976 | 0.947–1.006 | 0.120 | |
| LVOT diameter | 1.210 | 0.976–1.500 | 0.082 | |
| Calcification number | 0.698 | 0.426–1.146 | 0.155 | |
| Major calcification diameter (mm) | 0.845 | 0.734–0.973 | 0.020 | |
| Evolut-R | 0.260 | 0.084–0.802 | 0.019 | |
| Portico | 2.145 | 0.692–6.650 | 0.186 | |
| Implantation depth LCC (mm) | 1.164 | 0.983–1.377 | 0.078 | |
| Postdilatation | 0.445 | 0.165–1.202 | 0.110 | |
| Contrast volume (mL) | 0.993 | 0.986–1.000 | 0.064 | |
| LVOT Calcification | 0.314 | 0.102–0.970 | 0.044 | |
| Both Annulus and LVOT calcification | 0.134 | 0.043–0.416 | ||
| Multivariate predictor | ||||
| Both Annulus and LVOT calcification | 0.105 | 0.015–0.736 | 0.023 | |
| LCC, left coronary cusp; LVOT, left ventricular outflow tract. | ||||
On univariate analysis, calcification location in LVOT, in both annulus and
LVOT, use of an Evolut-R prosthesis and postdilatation were predictive of
| Odds ratio | 95% CI | p-value | ||
| Univariate predictors | ||||
| Evolut-R | 4.817 | 1.355–17.121 | 0.015 | |
| Postdilatation | 3.435 | 1.095–10.774 | 0.034 | |
| Implantation depth LCC (mm) | 0.857 | 0.717–1.023 | 0.088 | |
| LVOT Calcification | 3.992 | 1.123–14.193 | 0.032 | |
| Both Annulus and LVOT calcification | 9.267 | 2.600–33.030 | ||
| LCC, left coronary cusp; LVOT, left ventricular outflow tract. | ||||
Results are reported in Fig. 5.
 Fig. 5.
Fig. 5.Simulation analysis. (A) Stent-root contact area, (B) average stress, (C) stress distribution and (D) paravalvular orifice area in relation to nodular calcific burden and valve type.
Evolut-R and Portico devices maintained similar values of stent-root interaction
area independently from calcification burden, whereas Lotus showed a worsening in
presence of severe calcifications (from 465 mm
As for PVL, Portico showed overall greater paravalvular orifice area for each
grade of calcification (respectively 35 mm
The present study showed that nodular calcifications in the aortic annulus
and/or LVOT can be found in around 34% of patients undergoing TAVI. As expected,
the presence of
On computational simulations, the Lotus valve, showed the best performance in each calcification scenario with respect to paravalvular orifice area, consistently with the clinical findings.
The Portico system exhibited an excellent stent conformability as stress distribution was substantially homogeneous by increasing calfications, but showed overall larger paravalvular orifice area apparently in contrast with the clinical data.
On the other hand, the Evolut-R showed higher values of stress on the aortic
wall than Portico, although with a more dishomogeneous distribution in each
calcification model; this finding resulted to greater paravalvular orifice areas
by increasing calcifications, and it was confirmed in the clinical setting by a
higher rate of
The extent and distribution of calcifications has been under investigation by multiple studies which showed a correlation between LVOT tract involvement and PVL [8, 9, 25]. However, evidence is still modest since data were derived from observational studies. Mauri et al. [26] compared the performance of balloon expandable (BE), SE and ME valves, and found that the SE ones were more susceptible to PVL in presence of elevated calcium at the device landing zone. It has been hypothesized that SE prostheses have lower radial force than BE valves, mending to the shape imposed by the elliptic annulus [27], and as in our case by the calcific nodules. On the other hand, probably due to the higher radial force, the BE prostheses are also associated with increased risk of aortic root injury during TAVI procedures, especially in case of calcifications in the upper LVOT below the NCC [28]. However, these results are contrastating, as BE valves were found, in another report, to be independent predictors of device failure in presence of severe LVOT calcification [29].
The distribution of the stent stress is a key factor for the interection between nodular calcification and TAVI devices deployment. In fact, simulation findings showed that calcification dimension correlated directly with PVL area. Only the Lotus valve performed well in every setting due to its optimal radial force and conformability.
We tested these results in a real-world population who underwent Evolut-R,
Portico and Lotus implantation. In the Lotus group simulation findings matched
the clinical data, whereas this was not the case of Portico and Evolut-R in terms
of paravalvular area. Notably,
Thus, when using SE valves in the presence of nodular calcifications, the sole stent evaluation is too unsophisticated, as additional aspects may be relevant to achieve optimal results.
Firstly, the conformability of the valve to the altered landing zone may be an alternative feature to the high radial force, especially if it does not reach the critical crushing force. The Portico design has larger cells than Evolut-R, translating into an homogeneous stress distribution by any level of calcification and ellipticity, and ultimately to an optimal stent conformability to the shape of aortic annulus.
Secondly, the simulations were conducted without considering predilatation and
postdilatation: in our study Evolut-R group had a lower predilatation rate
compared to Portico and Lotus (41.9% vs. 76.9% vs. 63.3% respectively,
p
Thirdly, the Evolut-R could be more technically demanding because of its narrow
landing zone (3–6 mm by manufacturer) and its flared stent design. In contrast,
the Portico has a more tolerant implantation depth interval (1–9 mm by
manufacturer). In this regard, in our study, 29.8% of patients treated with
Evolut-R had a mean implantation depth
It has to be highlightened that calcifications both in the LVOT and annulus can give a higher underexpansion rather than a large calcification in a single zone, as showed by our multivariate analyses.
The clinical implications of our results are relevant as nodular calcifications
can be observed in
This study has several limitations. First, due to the retrospective design, device groups were not balanced, with Evolut-R being the predominant valve. As stated above this was due to our institutional policy.
Second, the distribution of calcifications location in the aortic annulus or LVOT were not comparable among the three devices. However, given that the device landing zone includes both annulus and LVOT, a single calcific nodule in this area of interest did not seem to be predictive of device success on multivariate analysis, whereas this was not the case if nodular calcifications in both regions were present.
Third, our study could not include patients treated with devices having an external sealing skirt such as Evolut Pro and Navitor valves which may have excellent outcomes in the setting of nodular calcifications.
As stated previously, the sealing skirt could not be integrated in the simulation model and our main focus was on the interaction between the stent struts and the aortic root.
Fourth, computational simulation could not take into account the impact of pre/postdilatation with a potential impact on simulation results. Similarly, on computational simulations, we had a simplified aortic root model with a single calcium nodule of increasing dimensions as marker of calcification severity. In a real-world setting, nodules can be multiple and of various dimensions. Finally, the results might be sensitive to the relative circumferential configuration between the calcium block and the stent mesh, which is more sparse in the case of Portico device than in the two other cases. Studies including patient-specific simulations are needed to further clarify the interaction between TAVI devices and aortic annuli with complex calcium distribution.
At least moderate nodular calcifications significantly impacted TAVI outcome,
especially in presence of nodules located both in LVOT and aortic annulus. Among
the investigated devices, Lotus and Portico seemed to perform better than
Evolut-R as for device success and
On computational simulation the three devices exhibited unique biomechanical features in terms of force and conformability of the stent frame with respect to calcifications size.
Therefore, a comprehensive assessment of both device features and aortic annulus/LVOT anatomy is pivotal in order to achieve optimal procedural outcome in these common although complex patients.
AMI, acute myocardial infarction; AR, aortic regurgitation; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CT, computed tomography; IE, index of eccentricity; HU, Hounsfield Units; LCC, left-coronary cusp; LM, left main; LVOT, left ventricular outflow tract; ME, mechanically-expandable; NCC, non-coronary cusp; PPI, permanent pacemaker implantation; PVL, paravalvular leak; RCA, right coronary artery; SE, self-expanding; STS, Society of Thoracic Surgeons; TAVI, trancatheter aortic valve implantation; THV, transcatheter heart valve; TTE, transthoracic echocardiography; VARC-3, Valve Academic Research Consortium-3.
RG designed the research study. RG and OAO performed the research, analyzed the data and wrote the manuscript. EP performed the research. AF, SM and FA performed the computational simulations, JZ, MA, MB, MS, EC, MT, NB, LT, and FB revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The study was conducted in accordance with the Declaration of Helsinki. The IRCCS Policlinico San Donato ethics committee, attached to the IRCCS Ospedale San Raffaele, approved the use of retrospective anonymized data for this study waiving the need of patients’ informed consent (code: 164/int/2020).
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
This study was partially supported by Ricerca Corrente funding from Italian Ministry of Health to IRCCS Policlinico San Donato (Ricerca corrente anno 2022).
Francesco Bedogni is consultant for Medtronic, Abbott, and Boston Scientific; Nedy Brambilla and Luca Testa are consultants for Abbott and Boston Scientific. The other authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
