†These authors contributed equally.
Academic Editor: Brian Tomlinson
Background: For the Asian patients with STEMI undergoing PCI, ACEIs are known to have a better outcome than ARBs. However, there is limited evidence to suggest so. Methods: Among the STEMI registry consist of 1142 Korean patients, we compared the MACE, the composite of myocardial infarction, stoke, death, admission for heart failure, and target vessel revascularization, between the ACEI and ARB groups (Set 1). Further, we defined adequate medication as the administration of a dose equal to or higher than the initiation dose of ACEI according to the heart failure guideline recommendation with a mandatory addition of beta-blockers, and compared the outcomes between the inadequate and adequate medication groups (Set 2). Propensity score matching was used to eliminate difference. Results: In the Set 1 comparison, patients in the ACEI group had a better outcome than those in the ARB group for both whole and matched populations (whole and matched population: Cox regression hazard ratio [HR], 0.645 and 0.535; 95% confidence interval [CI], 0.440–0.944 and 0.296–0.967; p = 0.024 and p = 0.039, respectively). In the Set 2 comparison for the whole population, patients in the inadequate medication group had more MACE than those in the adequate medication group (HR, 0.673; 95% CI, 0.459–0.985; p = 0.042). However, no difference was observed after propensity score matching (HR, 1.023; 95% CI, 0.654–1.602; p = 0.919). Conclusion: ACEIs might be a better choice than ARBs after primary revascularization. However, this study’s findings suggest that early ACEI dose escalation combined with beta-blocker use may not improve prognosis.
The clinical benefit of angiotensin-converting enzyme inhibitors (ACEIs) in patients with myocardial infarction (MI) is well-known [1]. Angiotensin receptor blockers (ARBs) were introduced after ACEI, have a similar pathway of blocking the renin-angiotensin-aldosterone system (RAAS), and are permitted alternatives for use in patients with MI. Their role in MI has been studied in large-scale randomized controlled trials (RCTs) [2, 3]. A clinical study reported findings that support the comparative advantage of ARB in MI [4]. However, whether ARB can substitute ACEI post MI is still a matter of debate. ACEI theoretically increases bradykinin level by inhibiting degradation, which improves endothelial function and control of the hypercoagulable state through the acute release of tissue plasminogen activator in post-MI patients [5]. Some clinical studies on Asian populations support the superiority of ACEI over ARB in the clinical outcome patients, both with and without ST-segment elevation MI (STEMI) [6, 7].
In the present study, we compared the outcomes following administration of ACEI with those following administration of ARB in STEMI patients who underwent primary revascularization therapy (PCI). ACEI is either prescribed without a beta-blocker, or a very small dose of a beta-blocker is introduced at the time of discharge due to a concern for a drop in blood pressure or cardiac output. This may decrease the benefits of the ACEI. Therefore, we additionally compared the outcomes between the group with an inadequate discharge dose of ACEI or use of ACEI without a beta-blocker and the group with proper use of ACEI with a beta-blocker.
Data from 1485 patients enrolled in the INTERSTELAR registry cohort between 2007 and 2015 were reviewed. The INTERSTELLAR registry was designed to find out the prognosis of the patients after primary revascularization for STEMI in four regional hospitals (ClinicalTrials.gov identifier: NCT02804958) in Korea. Most of the participants in this study were Koreans, while approximately 3% were Chinese, Southeast Asian, Uzbekistani, and Mongolian. To compare the outcomes of separate strategies of RAAS blockade, we split the patients into two groups by two different standards. We excluded 58 patients with in-hospital death and 285 patients who were discharged without a RAAS blocking agent. Finally, 1142 patients were included in the first review.
Acute STEMI was diagnosed based on clinical information, including blood
samples, chest pain, and 12-lead electrocardiography. An emergency primary
revascularization call was made by
The 2016 European Society of Cardiology (ESC) Guidelines for the diagnosis and treatment of acute and chronic heart failure recommend ACEIs as class I drugs in patients with asymptomatic left ventricular (LV) systolic dysfunction and a history of MI to prevent or delay the onset of heart failure (HF) and prolong life [8]. However, in the 2017 ESC Guidelines for STEMI, the treatment indications for ACEIs have been broadened, and ACEIs should be considered in all patients with STEMI [9]. According to the guidelines, we defined adequate medication as the use of a dose equal to or higher than the recommended initiation dose of ACEIs with a concomitant use of beta-blockers. In contrast, we defined the following RAAS inhibition strategies as inadequate: (1) any use of ARB; (2) discharge with a lower-than-recommended ACEI initiation dose; and (3) use of an ACEI without a beta-blocker. Evidence-based recommended initiation doses are: captopril 6.25 mg thrice daily, enalapril (2.5 mg) twice daily, lisinopril (2.5 mg), and ramipril (2.5 mg) daily.
The primary endpoint was major adverse cerebrovascular and cardiovascular events (MACE), defined as a composite of MI, stoke, all-cause death, readmission due to symptoms of HF after PCI, and ischemia-driven target vessel revascularization. If the patients had multiple events, the first clinical event only was included in this analysis. Medical chart reviews and/or standardize phone call were included in this study. Medical status of all patients (RAAS blockade and beta-blockers) was assessed at discharge as well as at the first outpatient visit.
For set 1 comparison, we divided the patient population according to the type of RAAS blocking agent regardless of beta-blocker use: ACEI versus ARB. Among 1142 patients, 784 were prescribed an ACEI and 358 were prescribed an ARB at discharge. In set 2, we compared the adequate ACEI medication group with an inadequate RAAS blockade medication group. Among 1142 patients discharged with a RAAS blocking agent, data from 1064 patients were available for review. The study protocol was received from the institutional review board of Sejong General Hospital (SGH 2019-08-010). Written informed consent was obtained from all patients, and we complied with the Declaration of Helsinki (6th revision).
Patient characteristics were compared between the two groups using a two-sample
t-test or Mann-Whitney U test for continuous variables and Pearson’s
chi-square or Fisher’s exact tests for categorical variables. Continuous data are
expressed as the mean
For predisposition and to adjust the selection bias of RAAS inhibitor strategy,
propensity score matching was used. The nearest neighbor method was used for the
propensity matching algorithm, and 0.1 standard deviation was used for the
caliper width with a matching ratio of 1:1. Co-variates for matching were chosen
among those with p
| Variables (n) | Set 1 | Set 2 | |||||
| ARB (359) | ACEIs (784) | p-value | Inadequate (412) | Adequate (652) | p-value | ||
| Age, years | 60.7 |
59.2 |
0.065 | 61.2 |
58.7 |
0.003 | |
| Systolic BP (mmHg) | 128.5 |
125.7 |
0.121 | 126.0 |
127.1 |
0.528 | |
| Heart rate (bpm) | 77.9 |
76.7 |
0.337 | 76.5 |
77.2 |
0.542 | |
| BMI (kg/m |
24.0 |
24.2 |
0.189 | 24.0 |
24.3 |
0.125 | |
| Males, n (%) | 285 (79.4) | 629 (80.2) | 0.751 | 327 (79.4) | 523 (80.2) | 0.737 | |
| Hypertension, n (%) | 202 (56.3) | 338 (43.1) | 205 (49.8) | 290 (44.5) | 0.093 | ||
| DM, n (%) | 110 (30.6) | 181 (23.1) | 0.007 | 131 (31.8) | 146 (22.4) | 0.001 | |
| Ever smoker, n (%) | 193 (53.8) | 365 (46.6) | 0.024 | 262 (63.6) | 289 (44.3) | ||
| Hypercholesterolemia | 112 (31.2) | 104 (13.3) | 111 (26.9) | 89 (13.7) | |||
| eGFR | 69.1 |
78.0 |
71.2 |
78.8 |
|||
| CKD |
117 (32.7) | 214 (27.3) | 0.068 | 135 (32.8) | 170 (26.1) | 0.018 | |
| Killip class | 0.005 | 0.005 | |||||
| 1–2 | 308 (86.0) | 707 (91.5) | 354 (86.6) | 591 (91.9) | |||
| 3–4 | 50 (14.0) | 66 (8.5) | 55 (13.4) | 52 (8.1) | |||
| Echocardiography | |||||||
| LVEDD | 49.3 |
49.8 |
0.135 | 49.8 |
49.7 |
0.935 | |
| E/e’ | 12.5 |
12.8 |
0.527 | 12.6 |
12.8 |
0.704 | |
| EF | 48.0 |
49.5 |
0.028 | 51.3 |
52.4 |
0.222 | |
| LV dysfunction | 66 (18.7) | 184 (23.7) | 0.063 | 93 (23.0) | 150 (23.2) | 0.934 | |
| Coronary disease | |||||||
| 1 VD | 156 (43.5) | 311 (39.7) | 0.227 | 167 (40.5) | 263 (40.3) | 0.949 | |
| 2 VD | 117 (32.6) | 247 (31.5) | 0.715 | 130 (31.6) | 206 (31.6) | 0.989 | |
| 3 VD | 85 (23.7) | 219 (27.9) | 0.131 | 113 (27.4) | 178 (27.3) | 0.964 | |
| IRA | |||||||
| LM | 2 (0.6) | 6 (0.8) | 1 (0.2) | 6 (0.9) | 0.259 | ||
| LAD | 181 (50.4) | 397 (50.6) | 0.945 | 204 (49.5) | 336 (51.5) | 0.521 | |
| LCX | 32 (8.9) | 86 (11.0) | 0.346 | 34 (8.3) | 73 (11.2) | 0.120 | |
| RCA | 143 (39.8) | 288 (36.7) | 0.316 | 171 (41.5) | 232 (35.6) | 0.052 | |
| Deployed stents | |||||||
| Zotarolimus | 96 (26.7) | 192 (24.5) | 0.416 | 124 (30.1) | 157 (24.1) | 0.030 | |
| Everlolimus | 134 (37.3) | 393 (50.1) | 191 (46.4) | 316 (48.5) | 0.503 | ||
| Biolimus | 40 (11.1) | 35 (4.5) | 38 (9.2) | 32 (4.9) | 0.006 | ||
| Others* | 38 (10.6) | 68 (8.7) | 0.323 | 12 (2.9) | 63 (9.7) | ||
| Stent diameter, mm | 3.36 |
3.36 |
0.961 | 3.27 |
3.37 |
0.001 | |
| Stent length, mm | 26.0 |
26.8 |
0.334 | 26.5 |
26.8 |
0.702 | |
| Medications | |||||||
| Beta-blockers (%) | 338/357 (94.7) | 742/782 (94.9) | 0.886 | ||||
| Potent antiplatelet |
105 (29.2) | 200 (25.5) | 0.185 | 99 (24.0) | 175 (26.8) | 0.307 | |
| Tica or prasugrel | 61 (17.0) | 33 (4.2) | 57 (13.8) | 30 (4.6) | |||
| Statin | 319 (88.9) | 671 (85.6) | 0.132 | 361 (87.6) | 565 (86.7) | 0.648 | |
| BP, blood pressure; ER, emergency room; BMI, body mass index; DM, diabetes
mellitus; eGFR, estimated Glomerular Filtration Rate was calculated by the
Cockcroft–Gault formula; CKD, chronic kidney disease; LVEDD, left ventricle
end-diastolic dimension; E/e’, the ratio of mitral peak velocity of early filling
(E) to early diastolic mitral annular velocity (e’); EF, ejection fraction; LV
dysfunction, defined as ejection fraction | |||||||
From 2007 through 2015, 1142 patients out of 1485 survived and were discharged
with an ACEI or ARB. “Mean duration of hospital stay was 5.8
In set 1, patients in the ARB group were older (60.7
A total of 1064 patients were enrolled for the set 2 comparison, with 412 and
652 patients assigned to the inadequate medication and adequate medication
groups, respectively. Patients in the inadequate group had a higher age (61.2
The stent diameters were similar in the 2 groups (3.27
In this study, we analyzed physicians’ ARB preference for patients with renal
dysfunction. As shown in Table 1, eGFR was lower in the ARB group (69.1
In set 1, 278 pairs of patients were obtained after 1:1 propensity score
matching. The parameters are listed in Table 2. Table 3 shows the comparison
between the groups with adequate use of RAAS inhibitors and inadequate use of
ACEI. There were no differences in age, body mass index, ejection fraction, and
prevalence of diabetes, dyslipidemia, ever-smoking, hypertension, CKD
| Set 1 | ARB (n = 278) | ACEIs (n = 278) | p-value | |||
| Mean | SD | Mean | SD | |||
| Continuous variables | ||||||
| Age (years) | 59.8 | 12.7 | 61.1 | 13.5 | 0.250 | |
| BMI (kg/m |
24.3 | 3.4 | 24.0 | 2.8 | 0.202 | |
| Ejection fraction | 49.0 | 12.9 | 48.9 | 9.6 | 0.900 | |
| Stent length (mm) | 27.1 | 12.6 | 25.8 | 10.4 | 0.202 | |
| Categorical variables | n | Percent | n | Percent | ||
| DM | 71 | 25.5 | 73 | 26.3 | 0.846 | |
| Dyslipidemia | 62 | 22.3 | 66 | 23.7 | 0.687 | |
| Ever smoker | 130 | 46.8 | 140 | 50.4 | 0.396 | |
| Hypertension | 139 | 50.0 | 146 | 52.5 | 0.552 | |
| CKD |
90 | 32.4 | 86 | 30.9 | 0.784 | |
| MVD | 95 | 34.2 | 99 | 35.6 | 0.721 | |
| Tica or prasugrel | 25 | 9.0 | 18 | 6.5 | 0.266 | |
| Killip class 3 or 4 | 30 | 10.8 | 32 | 11.5 | 0.787 | |
| Culprit LAD | 129 | 46.4 | 137 | 49.3 | 0.497 | |
| Stent diameter (mm) | ||||||
| 2.5–3 | 184 | 66.2 | 194 | 69.8 | 0.568 | |
| 3.25–4 | 92 | 33.1 | 80 | 28.8 | ||
| 4.5–5 | 2 | 0.7 | 3 | 1.1 | ||
| 2.25 | 0 | 0 | 1 | 0.4 | ||
| BMI, body mass index; EF, ejection fraction; DM, diabetes mellitus; CKD, chronic kidney disease; MVD, multi-vessel coronary disease; Tica, ticagrelor; LAD, left anterior descending artery; stent diameter was divided four groups by diameters; SD, Standard deviation. | ||||||
| Set 2 | Inadequate use (n = 327) | Adequate use (n = 327) | p-value | |||
| Mean | SD | Mean | SD | |||
| Continuous variables | ||||||
| Age | 60.9 | 13.6 | 59.9 | 12.6 | 0.322 | |
| BMI (kg/m |
24.0 | 2.9 | 24.1 | 3.0 | 0.715 | |
| Ejection fraction (%) | 48.1 | 10.4 | 48.6 | 11.5 | 0.547 | |
| Stent length (mm) | 26.4 | 10.3 | 26.6 | 10.6 | 0.875 | |
| Categorical variables | n | Percent | n | Percent | ||
| DM | 86 | 26.3 | 86 | 26.3 | ||
| Dyslipidemia | 64 | 19.6 | 58 | 17.7 | 0.547 | |
| Ever smoker | 195 | 59.6 | 189 | 57.8 | 0.633 | |
| Hypertension | 151 | 46.2 | 144 | 44.0 | 0.582 | |
| CKD |
98 | 30.0 | 96 | 29.4 | 0.932 | |
| MVD | 108 | 33.0 | 110 | 33.6 | 0.868 | |
| Tica or prasugrel | 20 | 6.1 | 25 | 7.7 | 0.439 | |
| Killip class 3 or 4 | 34 | 10.4 | 31 | 9.5 | 0.695 | |
| Culprit LAD | 164 | 50.2 | 170 | 52.0 | 0.638 | |
| Stent diameter (mm) | ||||||
| 2.5–3 | 234 | 71.6 | 229 | 70.0 | 0.898 | |
| 3.25–4 | 91 | 27.8 | 96 | 29.4 | ||
| 4.5–5 | 2 | 0.6 | 2 | 0.6 | ||
| 2.25 | 0 | 0 | 0 | 0 | ||
| BMI, body mass index; EF, ejection fraction; DM, diabetes mellitus; MVD, multi-vessel coronary disease; Tica, ticagrelor; LAD, left anterior descending artery; stent diameter was divided four groups by diameters; SD, Standard deviation. | ||||||
In set 1, the incidence of MACE in the whole population during the follow-up was lower in the ACEI group than in the ARB group (Cox regression HR, 0.645; 95% confidence interval [CI], 0.440–0.944; p = 0.024). The outcome was similar after propensity matching (Cox regression HR, 0.535; 95% CI, 0.296–0.967; p = 0.039). The Kaplan–Meier survival curves for MACE for the whole and propensity-matched population are shown in Fig. 1.
 Fig. 1.
Fig. 1.The Kaplan–Meier survival curves for the composite of MACE (major adverse cardiac events; composite of myocardial infarction, stroke, all-cause death, readmission for heart failure, or target vessel revascularization) according to the use of angiotensin receptor blocker (ARB) or angiotensin-converting enzyme inhibitor (ACEI). (A) The left Kaplan–Meier curves shows comparison in the whole population. (B) The right Kaplan–Meier curves for the above medication groups in the matched population.
In set 2, the incidence of MACE during follow-up was higher in the inadequate medication group than in the adequate medication group (HR, 0.673; 95% CI, 0.459–0.985; p = 0.042). The outcome did not differ between the two groups after propensity score matching (HR, 1.023; 95% CI, 0.654–1.602; p = 0.919) (Fig. 2).
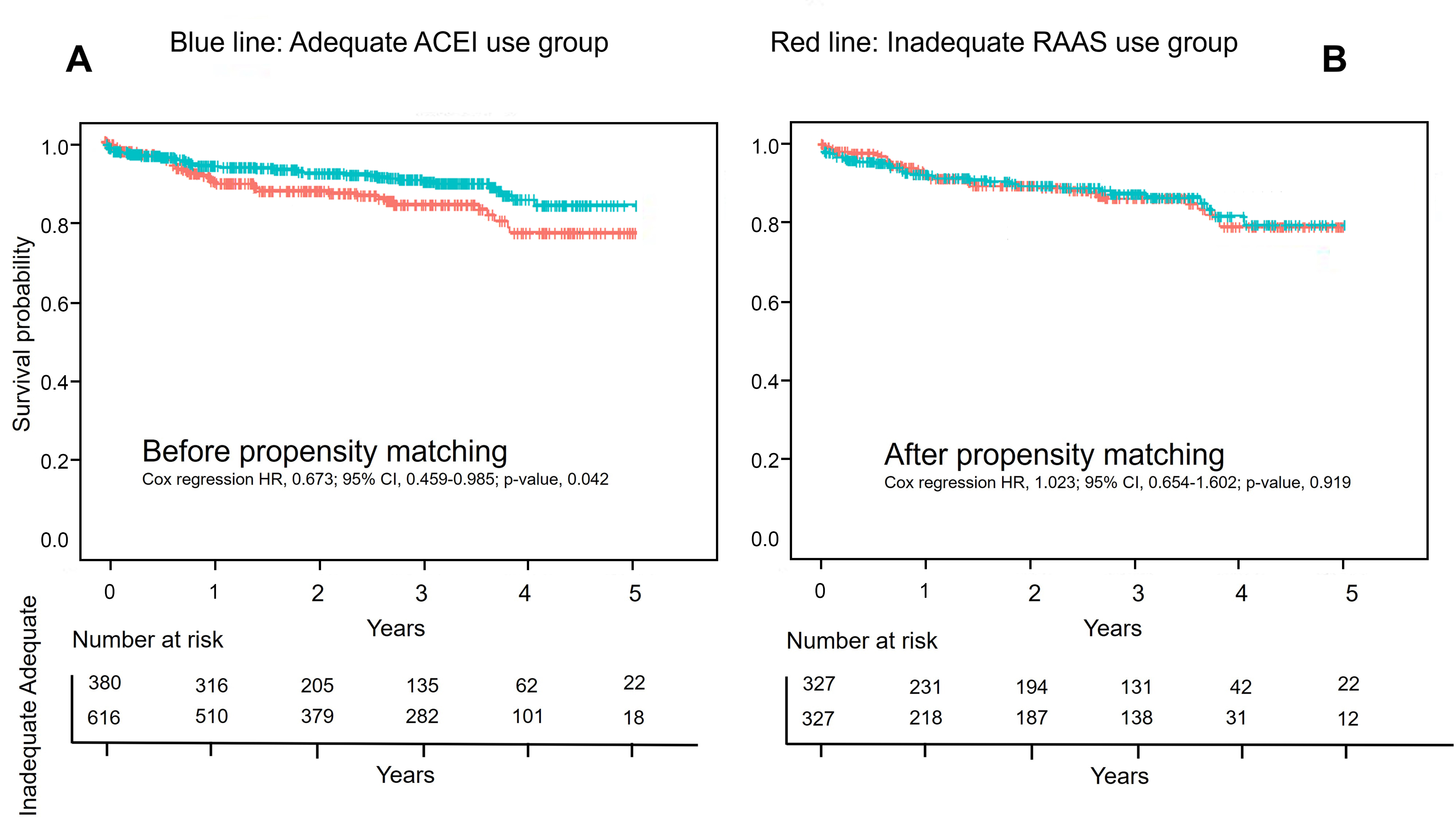 Fig. 2.
Fig. 2.The Kaplan–Meier survival curves for the composite of MACE (major adverse cardiac events; composite of myocardial infarction, stroke, all-cause death, readmission for heart failure, or target vessel revascularization) according to the inadequate use of renin-angiotensin-aldosterone system (RAAS) antagonist and its adequate use. An inadequate use of RAAS antagonist is defined as: any use of ARB; discharge with an initiation dose lower than the guideline recommendation; use of ACEI without a beta-blocker. (A) The left Kaplan–Meier curves shows comparison in the whole population. (B) The right Kaplan–Meier curves for the above medication groups in the matched population.
The first key finding of our study is that among discharged patients with STEMI, those on an ACEI have a better clinical outcome than those on an ARB. However, secondary findings suggest that a sufficient dose of ACEI with a mandatory combination of beta-blockers at discharge was not necessarily associated with a better prognosis.
After primary revascularization, the most important medical therapies other than administration of antiplatelets and statins are RAAS blockade and administration of beta-blockers. With the transition to the era of reperfusion, the importance of beta-blockers has diminished, while the importance of RAAS inhibition is increasing. Recently, with the development of new ARBs and reports of their better efficacy, there is a growing expectation that ARB treatment would result in a better prognosis than ACEI treatment for patients with STEMI. However, based on recently released real-world registry data, especially data from Asia, there is accumulating evidence that ACEI is associated with improved prognosis in patients with MI. Byun et al. [6], reported that ACEI use was associated with a two-year reduction in MACE and revascularization rates for NSTEMI and DM patients. Similarly, in the STEMI patient subset, ACEI plus beta-blocker use was related to a two-year decrease in MACE rate. Current research shows that ACEI treatment is associated with improvements in MACE over five years, similar to the previous two-year results [7].
Although both ACEI and ARB act by blocking a similar pathway in RAAS, their
mechanisms of action differ. Theoretically, the beneficial effects of ACEI are
strongly associated with increased levels of bradykinin. Bradykinin acts as a
potent vasodilator by activating endothelial B
ARBs work by selectively blocking angiotensin II type 1 (AT1) receptors. The compensatory increase in angiotensin II levels may mediate harmful vascular effects via other angiotensin II receptors (AT2, 3, and 4), resulting in plaque instability or rupture [14, 15]. The risk of acute MI was slightly higher with ARB (candesartan and irbesartan) than with placebo [16, 17].
Ferrari et al. [18, 19] compared the vascular and endothelial functions between ACEIs and ARBs and found that ACEIs produce a greater reduction in endothelial dysfunction, inflammation, cell adhesion, and apoptosis, and have better antithrombotic and anti-atherosclerotic effects. ARB has only partial anti-thrombotic and inflammatory benefits. The ONTARGET study compared ACEI and ARB and showed a lower tendency for the risk of MI with ramipril as compared to telmisartan (relative risk, 1.07; 95% CI, 0.94–1.22).
In addition to choosing between ACEI and ARB, the clinically difficult decision about hemodynamic drug prescription would be which medicine to start with, and at what dose. A combination strategy of prescribing RAAS antagonists with beta-blockers might be a clinical concern when the patient’s blood pressure is low. Some physicians start with one type of hemodynamic drug and increase the dose quickly, while others start with two drugs (ACEI and beta-blocker) and gradually increase the dose. Interestingly, previous studies or guidelines do not conclusively favor one strategy over the other. Importantly, high-risk patients with LV dysfunction might require both classes of medicine before discharge; nevertheless, they usually have a higher chance of developing hemodynamic instability during hospital stay. Thus, we attempted to analyze set 2 for these clinical concerns.
Patients undergoing primary PCI usually stabilize after pain control. Some patients may experience hypotension or compensatory tachycardia after the procedure. The treatment strategies at this stage may differ depending on the physician’s experience. We did not include patients who either died before discharge or did not receive RAAS inhibitors or beta-blockers due to instability (343/1485, 23.0%). The 2017 ESC and 2013 American College of Cardiology/American Heart Association guidelines for STEMI recommend that ACEI should be considered in all patients with STEMI [20, 21]. Unlike the recommendation that beta-blockers should be used before discharge, ACEI are recommended within the first 24 h. According to the guidelines, a RAAS inhibitor should be considered first, followed by a beta-blocker. However, due to unstable vital parameters, a lower-than-recommended starting dose may be prescribed and not titrated until discharge. Similarly, unstable patients may not receive beta-blockers until discharge. There is evidence that, in practice, a small number of patients receive suboptimal doses of ACEI [22]. Interestingly, the use of a dose equal to or higher than the recommended initiation dose of ACEI according to the HF guideline with a mandatory addition of beta-blockers was not necessarily associated with a good prognosis.
There are several reasons that may explain the observed results. First, the role
of a beta-blocker is not as strong as that of an ACEI in the primary PCI era.
Beta-blockers are known to reduce mortality in patients with acute MI; however,
most of this evidence comes from the pre-reperfusion era [23]. We must be aware
that hospitalization rates for HF may increase further with the use of
beta-blockers in the reperfusion era [23]. Second, in the absence of HF or
systolic dysfunction, an equal risk of death was observed, regardless of the use
of beta-blockers [24]. Indeed, we had less than a quarter of the population with
LV dysfunction (ejection fraction
Our study had several limitations. First, the sample size was small. Second, the analysis was conducted using non-randomized registry data. However, we used propensity score matching to minimize the selection bias. Third, the use of a RAAS inhibitor was observed at discharge as well as the first outpatient visit. Thus, the delayed titration strategies were not assessed.
ACEIs might be a better choice than ARBs after primary PCI in patients with STEMI. However, this study’s findings suggest that early ACEI dose escalation in combination with beta-blocker use may not improve prognosis. A well-designed prospective dose-related study of ACEIs versus ARBs in patients with MI is warranted to confirm our findings.
STEMI, ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; RAAS, renin-angiotensin-aldosterone system; MACE, major adverse cardiovascular and cerebrovascular event; MI, myocardial infarction; RCT, randomized controlled trial; CKD, chronic kidney disease; CI, confidence interval; HR, hazard ratio.
HJP, HJJ, and THK wrote the manuscript; JS, SWK, SDP, MGK and PCO collected and analyzed data; JM, KL, WCK, MGK and THK performed statistical analyses; THK, HJJ, and SWK conceived and designed the study.
The analysis was conducted using data obtained from the INTERSTELLAR registry of patients with STEMI who underwent primary PCI. The INTERSTELLAR registry (clinicaltrials.gov identifier NCT02804958; Am J Cardiol 2016 Jul 15;118(2):177-82; https://doi.org/10.1371/journal.pone.0159416) is a retrospective, observational, four-regional-hospital-based registry that reflects management practices, risk factors, and clinical outcomes in patients with STEMI who underwent primary PCI in the cities of Incheon and Bucheon located in the mid-western part of the Korean peninsula between 2007 and 2014. All participants provided informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki (6th revision), and the protocol was approved by the Ethics Committee of Sejong General Hospital (approval number: 1810).
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
