Academic Editor: Kazuhiro Izawa
Cardiovascular diseases are the leading cause of morbidity and mortality worldwide. Increased rates of morbidity and mortality have led to the increased need for the implementation of secondary prevention interventions. Exercise-based cardiac rehabilitation (CR) represents a multifactorial intervention, including elements of physical exercise and activity, education regarding healthy lifestyle habits (smoking cessation, nutritional habits), to improve the physical capacity and psychological status of cardiac patients. However, participation rates in CR programs remain low due to socioeconomic, geographical and personal barriers. Recently the COVID-19 pandemic restrictions have added another barrier to CR programs. Therefore there is an emerging need to further improve the types and methods of implementing CR. Cardiac telerehabilitation, integrating advanced technology for both monitoring and communicating with the cardiac population, appears to be an innovative CR alternative that can overcome some of the barriers preventing CR participation. This review paper aims to describe the background and core components of center-based CR and cardiac telerehabilitation, and discuss their implications for present day clinical practice and their future perspectives.
Cardiac rehabilitation (CR) is an interdisciplinary, cost-effective and integral component of the continuous care for patients with cardiovascular diseases (CVD) [1], designed to improve functional capacity, psychological health and the quality of life (QoL) [2]. It is widely acknowledged and documented that CR programs reduce morbidity, mortality rates and re-admissions of cardiac patients [3]. These favorable benefits are most likely to be obtained by tightening the control of the cardiovascular risk factors (CVRFs) and enhancing commitment to appropriate treatment for the avoidance of atherosclerosis progression thereby reducing the complicating effects of atherosclerotic lesions [4, 5, 6, 7, 8]. CR target aims are to enhance tolerance and optimize CVRFs, including cholesterol and lipoprotein profiles, body weight, levels of blood glucose, blood pressure levels, and smoking habits. A further focus is on stress, anxiety, and depression reduction [2, 9, 10] (Fig. 1, Ref. [11]).
 Fig. 1.
Fig. 1.CR key preventative components. Secondary prevention cardiac rehabilitation programs assist patients in setting goals of increasing physical activity, healthy nutrition, optimal medication adherence, bodyweight management, smoking cessation, and optimal psychosocial well-being, thereby lowering their risk of a future cardiovascular disease event, adapted from [11].
CR generally consists of three stages: Phase I, Phase II, and Phase III. Phase I lasts as long as the patient is an inpatient within a hospital and often involves: early mobilization, patient health education, medication treatment, lifestyle changes, the transfer from care to an ambulatory setting [1, 2]. Following the initial stationary restoration and referral (Phase I), Phase II consists of a supervised outpatient ambulatory program lasting up to twelve weeks with supervised exercise training (ET), medical evaluation, lifestyle education, continuous medical monitoring, and cardiac risk factor management [12]. The purpose is to control related disorders, such as diabetes mellitus, hypertension, hyperlipidemia, and more recently stress management, using an educational and consultations team approach, has been included [8]. These multi-disciplinary interventions aim to produce long-term behavior modifications during Phase III CR, to expand life expectancy, maintain behavioral changes accomplished during Phases I and II, and to improve the patients’ cardiorespiratory fitness and thus QoL [13, 14].
Exercise-based CR programs have been recognized as safe [15] and are as cost-effective as medical treatment. Exercise for the heart and coronary vasculature has been proven to have immediate positive results, including myocardial oxygen demand, endothelial function, self-reliance tone, coagulation and coagulation factors, inflammatory indicators, and the creation of collateral coronary arteries [16, 17, 18]. The findings from the first exercise-based CR analysis of CHD that Cochrane did, however, support the belief that indirect exercise benefits might potentially mediate mortality reductions through improved risk factors (e.g., lipids, smoking, and blood pressure) for atherosclerotic disease [19]. Despite breakthroughs in CVD medical and interventional therapies, regular participation in a CR program is only undertaken by a minority of patients. As a result, it is expected that an increased number of patients will survive an acute cardiovascular event, but congestive heart failure (CHF) will increase worldwide as a chronic consequence.
While secondary prevention is usually advocated after an acute occurrence, CR programs were implemented in fewer than half of the eligible patients following an acute myocardial infarction (MI) or stroke. According to the resources available from health systems, particularly those in low-income countries, the coverage is less than intended for primary and/or secondary prevention [20, 21]. The majority of studies indicated decreased hospital readmissions, improvements both in clinical and life-quality status, and also revealed lower death rates in the time frame of CR. The under-use of CR is a significant problem when immediate and effective return to working conditions, for active working individuals, is required following an acute CV incident [22, 23].
Exercise training (ET) is considered as a significant non-pharmacological
component of a CR program [18, 21, 23, 24], which has been associated with a
reduction of patients’ mortality and morbidity rates and an increase in their
functional capacity and QoL [25]. The first guidelines for resistance exercise in
CR were issued by Pollock et al. [26]. Resistance training is a type of
exercise that helps to increase muscle strength and endurance. Stretching or
flexibility activities can commence as soon as 24 hours after a bypass procedure
or two days following an acute myocardial infarction. Current guidelines propose
that dynamic resistance exercise are implemented in II Phase CR with caution,
beginning with low-intensity training (
Exercise-based CR programs, combining elements of aerobic endurance and dynamic resistance training have now been applied in CVD patient populations [27]. Notably, the implementation of exercise-based CR programs has led to an increase in the percentage of middle-aged and older persons who believe that “more activity is better for the preservation of their cardiac health status” [29].
Exercise risk stratification is essential for safety and is accomplished with clinical, blood, ergometric, echocardiographic, and other medical examination findings [30]. The cardiovascular risk management of exercise may also be evaluated differently [31]. For example several different protocols are available for cardiac patient risk stratification such as the Protocol of the American College of Sports Medicine, the Protocol of the Brazilian Society of Cardiology, and the Protocol of the American Heart Association [31]. These differing protocols enable CR practitioners to choose the most suitable one for their CR program.
Initially, a profound evaluation of the patient status is performed, both clinically and functionally. The patients’ risk of stratification is linked to the probability of severe events caused by exercise whilst performing the CR program, this must be estimated, which results in a specific plan pertaining to the form and intensity of work to be assigned. Factors to be considered for the stratification include the risk of morbidity and mortality during the exercise. According to this evaluation, the usual classifications for patients are low, moderate, or high risk Stratification is also useful for planning the CR process, and enables the professional to determine the proper level of monitoring in agreement with the risk level [31].
High risk patients should not engage in CR until all causes of instability have been addressed. Before opting for a CR program, the patients must be categorized into the moderate-risk category. Typically, these patients must complete the whole rehabilitation pathway, including an intense CR program in a specialized medical center. Patients with a higher clinical risk require safer training methods, lower levels of exercise training and extended supervision by CR specialists [17, 28, 32]. Low-risk, stable patients do not require special care and can be assigned to center-based or home-based CR. Both approaches are beneficial to one’s cardiovascular status and well-being. It is also critical for patients to have easy access to hospital and local healthcare facilities, preferably the same one from which they were released. For the most significant clinical outcomes, regular contact between outpatient hospital services and each patient is required [33, 34, 35].
Over the last few years, several national and international entities have developed and validated protocols for cardiac risk stratification relating to participation in exercise programs. These may include multivariate analyses, which have provided clinicians and researchers with a wide array of data. This has resulted in more sufficient and safer configurations of CR interventions or exercise protocols, leading to minimalized probabilities of acute cardiovascular events [34].
The indications for participation in a CR program vary from country to country. CR is not just indicated for low-moderate cardiac risk patients. All patients with a diagnosis of acute myocardial infarction, coronary revascularization (coronary artery bypass graft (CABG), percutaneous coronary interventions) or other cardiac surgery (for instance, valvular, transplantation, correction of congenital heart diseases), chronic stable angina, heart failure (HF), peripheral arterial disease and high-risk groups for CVD, such as diabetes and metabolic syndrome, can also partake in CR programs [36].
At hospital discharge, patients who had an acute cardiovascular episode can undergo a CR program. However, the optimal time for training cannot be established without at least taking into account the following considerations: myocardial ischemia, the magnitude of cardiac damage and persistent ventricular dysfunction, electrical instability, clinical deterioration and comorbidity, pulmonary role and cardiac damage magnitude and persistent ventricular dysfunction, pulmonary function. Symptomatic patients (despite intensive therapy and interventional procedures and those who have complications such as by severe cardiac dysfunction, electrical instability, and/or advanced kidney disease) may be eligible for physical training sooner than patients who are symptomatic despite intensive therapy and interventional procedures [20, 37, 38].
Contraindications to CR are only related to the exercise aspect of the program, all of the other components can be pursued. Contraindications include unstable angina, decompensated heart failure (HF), complex ventricular arrhythmias, pulmonary arterial hypertension greater than 60 mmHg, intracavitary thrombus, recent thrombophlebitis with or without pulmonary embolism, severe obstructive cardiomyopathies, severe or symptomatic aortic stenosis, uncontrolled inflammatory or infectious pathologies, and any musculoskeletal condition that prohibits physical exercise [39].
Patients with stage IV HF or hemodynamic arrhythmias are included in the group containing with contraindications. In contrast for patients with CVD or stable chronic cardiac failure (CHF), regular physical training can improve physical performance, decrease symptoms, and, consequently, improved QoL [2, 9, 10, 40]. CVD and stable CHF patients should therefore be assigned to CR from the onset of their hemodynamic stabilization, even during the in-hospital phase. For patients who are difficult to stabilize, inpatient CR is more appropriate than outpatient CR. Thorough education and information regarding CVD background and motivation techniques should be provided to patients reluctant to participate in CR programs [41].
Regular ET has several implications on physical capacity, including enhanced endothelial function and increased aerobic capacity with a greater oxidative efficiency. These alterations result in improved diastolic and contractile dysfunction, reduced blood pressure (BP) and heart rate, higher muscle mass, and even higher cognitive functioning [42]. In a systemic review, Streese et al. [43] demonstrated that involvement in physical activity (PA) influences the health of the microvascular retinal in all age groups and that exercise can prevent small vessel diseases. Physical activity is defined as any movement of the body caused by skeletal muscle contractions that results in an increase in energy expenditure beyond the basal level and, as such, is a type of lifestyle intervention [43].
Exercise training is a systematic activity that takes place over a certain length of time and is described as a sub-category of physical activity in which planned, structured, and repeated bodily motions are conducted to maintain or develop one or more qualities of physical fitness [44]. Another systematic review by Dallas et al. [45] provided a solid, evidence-based approach showing that exercise based CR programs are suitable for HF patients of both genders and of all ages. Furthermore the benefits of ET appeared to be similar, regardless of the exercise type utilized in the CR routine, as both, aerobic and resistance exercise lead to beneficial changes in functional capacity levels, in the patients’ QoL and reduced re- hospitalization rates [45]. A recent meta-analysis conducted by Taylor et al. [46] identified that CR based on training increased the consumption and QoL and lowered HF patient hospital admission incidence, compared to control groups. Different exercise modalities have distinct impacts on central hemodynamics, arterial stiffness, and cardiac function in individuals with CVD. Aortic systolic pressure was dramatically reduced by aerobic or resistance training. Cardiopulmonary fitness, central arterial stiffness, core function, and endothelial function were, in contrast, improved as a reaction to aerobic exercise and combined exercise [47]. High-intensity aerobic interval training induces multisystem integrative physiologic changes in the respiratory, cardiovascular, and musculoskeletal systems, which leads to increases in peak oxygen consumption in HF [48] and CVD patients [49]. It has been proven that ET reduces systemic vasoconstriction, salt and water retention, and aldosterone production by altering angiotensin production [50]. Decreasing aldosterone lowers the sympathetic tone, enhancing the efficacy of other ET-induced parasympathetic activity modulators [51]. Plasma adrenomedullin and atrio/brain-natriuretic-peptides, for example, are connected with aerobic capacity and inhibit endothelin-1 and noradrenaline, resulting in improved endothelial function and responsiveness [52].
Despite its well-established advantages and strong support from professional associations, CR is currently underutilized by CVD patients [35, 36, 40]. Approximately less than 20% of all eligible patients participate in CR globally, and only 34% of those who are recommended ultimately enroll [53]. CR in various nations significantly differs in structure, availability, integration, and healthcare systems funding [53, 54, 55, 56, 57].
The published literature reveals that 50–70% of CR-eligible patients do not attend CR, with 30–60% not completing CR [51, 53, 55, 56]. Mortality rates appear lower in patients attending more CR sessions than in individuals attending fewer sessions (Fig. 2, Ref. [58]) [59]. Determinants concerning CR participation involve, in particular, patients’ referral, availability, and inclusion of CR in the comprehensive care management. Concerning participation and adherence to the exercise-based CR, main barriers include work and transportation-related parameters, financial costs, or lack of motivation [60, 61].
 Fig. 2.
Fig. 2.Barriers to participation in CR sessions, adapted from [58]. The figure shows a detailed view of the barriers in CR participants. It is recommended to introduce alternative CR approaches such as telerehabilitation or mobile health to influence the barriers.
Furthermore, women frequently feel uncomfortable participating in conventional CR programs, where the majority of the participants are presently male, thus forcing them to either avoid enrolling in the first place or causing them to drop out of CR programs early [62, 63]. In addition to gender, racial and ethnic discriminations play significant roles in low CR referral and adherence rates [64, 65, 66, 67, 68]. Several studies have found that non-white people had lower CR referral rates than white individuals [69, 70, 71]. A systematic review by Castellanos et al. [69] showed significantly lower CR referral and participation rates among individuals from rural communities, women, and racial and ethnic groups when compared to the general population. Similar to geographic region, socioeconomic status (SES) appears to directly impact the use of CR programs [69]. Similar results have been demonstrated in another systematic review where older participants, women, patients with comorbidities, unemployed and uncoupled persons, less educated people and those lower incomes had lower participation rates [72]. Minorities, notably Black, Hispanic, and Asian patients, were 20%, 36%, and 50% respectively less likely than white patients to obtain referral for CR following a MI, according to a major US-based registry. Communication barriers are most likely to blame for these referral and participation disparities. Patients who are unable to communicate in English as their first language may feel marginalized, excluded, and anxious, resulting in decreased enrolment and greater drop-out rates.
Patients with lower income, education, and socioeconomic levels (SES) have displayed lower CR participation rates than those with a higher socioeconomic status. These patients usually have insufficient health insurance coverage and fewer health benefits, thus making CR participation economically non affordable [72]. Patients from lower socioeconomic backgrounds also lack transportation to CR facilities, and an even larger factors is lack of appropriate childcare, making enrollment and the participation in CR programs difficult to achieve [73].
Additionally, psychological factors can influence patient adherence in CR [74, 75]. Patients with depression are less likely to pursue their personal well-being and adhere to their medication requirements, and these patients are correlated to higher healthcare utilization, emergency room visits, and hospital readmissions [76, 77]. A retrospective cohort study by Rao et al. [78] revealed that patients with moderate depression, anxiety, or stress symptoms were substantially less likely to successfully participate in a CR program than those with mild to moderate symptoms.
There are two types of CR programs available, Center-Based Cardiac Rehabilitation (CBCR) and Home-Based Cardiac Rehabilitation (HBCR). The CBCR exercises are carried out in hospitals or in specialized facilities, this is safer for people with heart diseases as they receive specialist supervision. However, the cost of CBCR programs is relatively high as these long-term programs require transportation, and additionally parking near facilities may cost extra money making them less suitable for rural patients [62]. For patients with CBCR barriers, HBCR is therefore advised. HBCR is particularly appropriate for patients who are not in favor of group exercises [79].
Within the CVD populations, myocardial infarction, or low-risk HF patients, both CBCR programs and HBCR have successfully increased clinical outcomes and enhanced health-related quality of life. Indeed the efficacy of HBCR in aerobic capacity, exercise adherence, physical level, regulated BP, and cholesterol level was equivalent to CBCR. In addition HBCR decreased heart mortality by approximately 30 percent [80, 79, 81].
The Hybrid CR approach consists of one or two initial sessions at the center followed by home-based monitoring sessions conducted remotely. Initially, through in-person sessions, matters relating to cardiac patients’ safety and psychological well-being are ensured. Additionally, all of the exercise training programs are individually prescribed and tailored according to each patient’s needs and cardiac risk stratification level. Studies have demonstrated that the safety of this model is comparable with CBCR [15, 58]. A systematic review and meta-analysis conducted by Imran et al. [82] found that when compared to standard care, hybrid CR resulted in a more significant improvement in peak oxygen consumption.
Current telemonitoring approaches, such as the use of wearable sensors (heart rate chest zones, sport watches, accelerometers) and the use of web platforms to instantly upload training data, appear adequate enough to enable physical exercise and heart rate surveillance [11]. Telecommunications could replace conventional communication methods with fast-developing technology that provides speedier and more individualized opportunities [83]. The core components of HBCR should remain similar to those of CBCR for HBCR to be effective [84]. The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) emphasizes the need for specialized supervision of CR programs by a physician medical director. Additionally, it is proposed that all patients should undergo a baseline assessment and receive an individualized rehabilitation plan, consisting of nutritional education, weight counseling, mental health evaluation, risk factor control, smoking cessation, and exercise training [85].
Nearly all CR programs start with a personal evaluation of patient
functionality, medical history, and medication treatment. Physical therapists,
nurses or doctors could conduct patients’ evaluations for HBCR either in person
in the last days few of their hospitalization, or remotely via a video or phone
conference after their discharge, or during an initial visit to the CR center
when considering a hybrid center-based and home-based model CR [11]. A
personalized training and nutrition plan for each patient should be drawn up
during this initial visit. HBCR should also include assessment of lipid and blood
pressure levels, diabetes, and compliance with cardioprotective drugs (e.g.,
anti-platelet drugs,
A systematic review about CR and telemedicine has shown that both cardiovascular risk variables, such as increased cholesterol and systolic blood pressure, and barriers to CR implementation including limited adherence to training, were positively impacted following the inclusion of telemedicine. Evidence shows that cardiac telerehabilitation (TR) programs are associated with an increase in cardiac patients’ physical fitness outcomes, a promotion in their QoL and a reduction in the costs of rehabilitation programs [87, 88].
Recently the COVID-19 pandemic has imposed serious barriers on the implementation of CBCR programs. COVID-19, on the other hand, has only served to emphasize the critical role that CR plays in empowering patients to choose healthy lifestyle choices which lower the risk of atherosclerotic CVD and COVID-19-related morbidity and mortality [89]. COVID-19 also has the potential to eliminate current gaps in healthcare access. Even in the absence of the pandemic’s stress, lower socioeconomic levels have been connected to CR underutilization [90]. COVID-19 has already dramatically turned telemedicine into mainstream practice from seldom utilized solution [91]. Comprehensive patient counseling may now be carried out to integrate the Health Insurance Portability and Accountability Act (HIPAA) — enabling submission of video sessions into the electronic media record. In addition, telemedicine visits rose by 50% in the first quarter of 2020 compared to the same period in 2019 [92].
In the current pandemic era, an extra 7.3 million employees, including their families, have become unemployed [93]. Evidence from a relative study conducted in Singapore demonstrated that prior to the COVID-19 pandemic, hospitalized patients who qualified for CR could attend outpatient exercise sessions within two weeks after their hospital discharge. Nowadays, and during the COVID-19 pandemic era, CR programs are being postponed for extended periods of up to six months. Unfortunately, the delays in early CR implementation are likely to lead to poor patient outcomes [94]. As a result, cardiac populations will have no access to CR, and hence atherosclerotic CVD patients with more cardiometabolic risk factors remain at a higher risk. COVID-19 has made it difficult for patients to access CR and furthermore, COVID-19 has created dozens of problems for hospital clinics. CR during COVID-19 is not financially sustainable for many locations. Additionally, during the COVID-19 limitations, over half of all CR programs were halted completely. The increased adoption of innovative technology into standard clinical practice is promising and may lead to an improved access and participation in exercise-based CR beyond the COVID-19 era [95]. The pandemic has provided the opportunity for innovation, so patients may continue to profit safely from CR. While HBCR programs exist, they are infrequent, not very well researched, and lack clear implementation instructions. In addition to allowing CR to remain safe and effective throughout the pandemic, new technology can potentially contribute to increased utilization rates in the future.
TR and mobile technology are offering appropriate options for bridging the gap over restricted CR involvement. To extend HBCR programs, TR uses information and communication technologies, telephone or video-conferencing technology, and allows for sufficient feedback, coaching, and consultation to be supplied [96]. Mobile and smartphone access also continues to grow worldwide. About 75% of the population in several high-income countries, as proposed by the World Bank classification [97], has mobile devices, and almost 80% can readily connect to the internet. In addition, the coverage of mobile internet connections and internet subscriptions is at approximately 80 percent and practically all over the world. A high prevalence of internet plus access to mobile phones indicates that advanced telemedicine and mobile medical procedures may be used, and could enhance accessibility, individuality, and the use of CR programs. Using remote technology and wearable sensors, rehabilitation program information, including exercise variables such as intensity, time, distance, and patient’s physical status indications (HR, PA and BP) and many other pertinent aspects, may be recorded, surveyed and taken into account for an optimal, individualized, safe exercise prescription. Regular feedback and counseling for patients can be provided by medical personnel. In HBCR programs, the integration of wearable and mobile technology has not yet been adequately investigated. However, several advantages are anticipated [98]. Wireless connection offers flexibility in TR use in multivariable environmental conditions and enables intervention (Fig. 3, Ref. [11, 99]) [11].
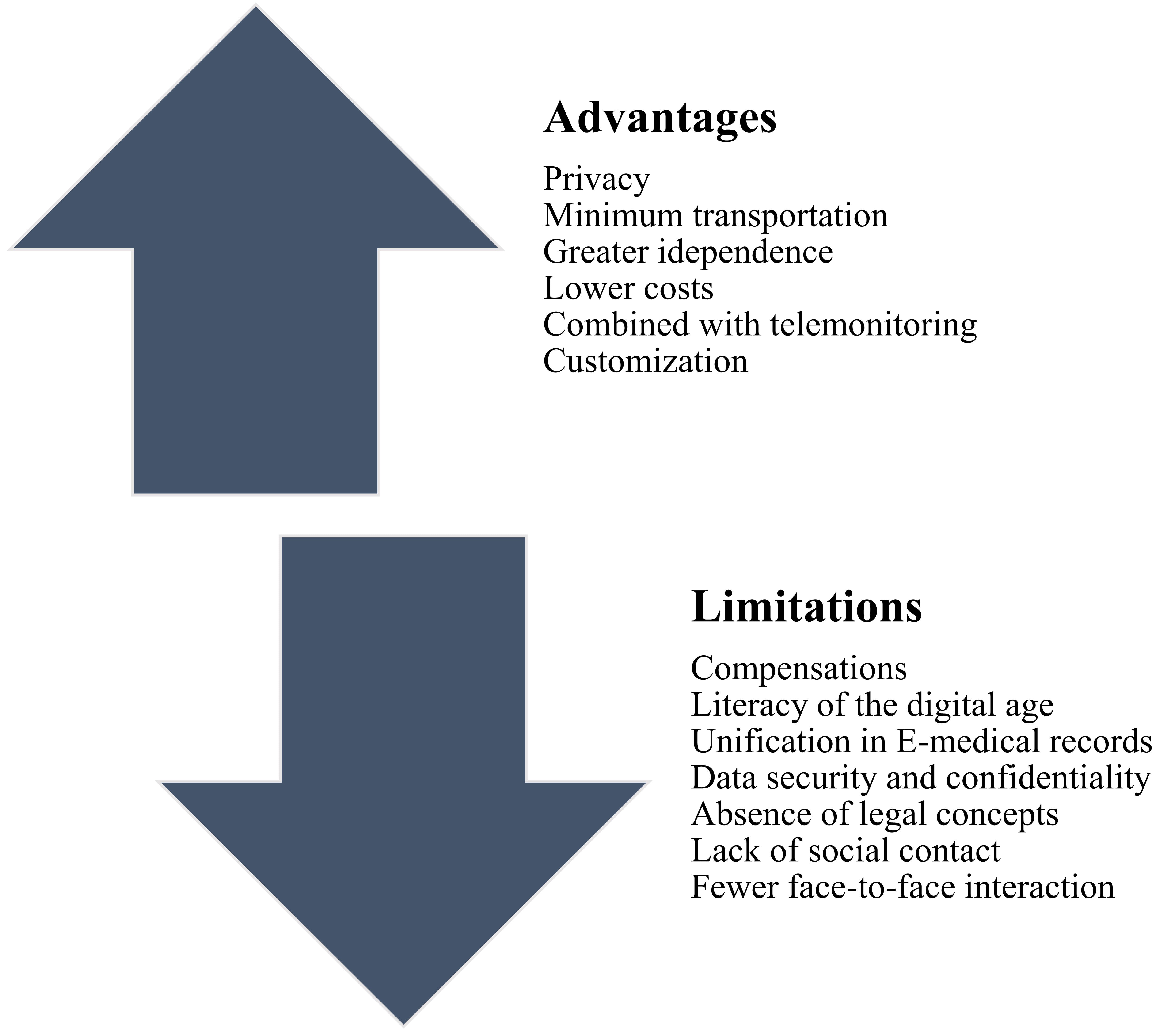 Fig. 3.
Fig. 3.Limitations and Advantages of Telerehabilitation, adapted from [11, 99]. Telerehabilitation interventions can overcome many barriers to CR participation and support long-term adherence to a healthy lifespan. The telerehabilitation approach enables providing motivation and guidance and allows participants to check their progress. The figure provides an overview of the possible advantages and limitations of current telerehabilitation interventions.
Essential components of a TR program include patient evaluation, physical
exercise, food counseling, treatment of the risk factor (blood pressure, weight,
lipids, diabetes mellitus), adherence to medication, and psychosocial management.
Almost every CR program begins with an in-person patient status evaluation,
history, and medication assessment. This can be done by a physical therapist,
registered nurse, or physician, either on the spot after release, or remotely via
a video or telephone conference during the initial visit at the CR center. During
the initial assessment, an individualized exercise and nutritional program are
designed. As in the case in CBCR, HBCR also includes cholesterol management,
blood pressure reduction, diabetes mellitus management, cardioprotective
medication adherence (e.g., antiplatelet drugs,
Smartphones can be used effectively for PA and dietary documentation since they allow personal data, health treatments and reminders to be retrieved. Smartphones can remotely transmit data to online portals so physicians may evaluate and offer advice. Physical activities can also be supervised indirectly via wearable appliances. These systems can include pedometers, accelerometers, and cardiac monitoring sensors that are typically included in most wearable gadgets and smartphones [83]. In addition, smartphone cameras and applications for nutritional content allow patients to record their nutritional intake and to receive instant feedback on possible adjustments at home [86].
A comprehensive TR intervention includes multi-stage evaluation, patient selection, and patient direct feedback based on counter-indications of remote training and monitoring. The parameters of the TR and the equipment used for their monitoring and recording of measurements and activities are displayed in Fig. 4 (Ref. [87]). During the COVID-19 pandemic, it has become even more challenging to implement these intervention procedures.
 Fig. 4.
Fig. 4.Telerehabilitation monitoring systems and parameters [87]. Fig. 4 summarizes the devices that can be used for telerehabilitation and remote parameters monitoring. The results can be observed and reported to the clinical center with cooperation by the patient or physician.
There are currently four European scenarios [11]:
(1) CR centers are fully operational.
(2) CR centers partially operational (settings and/or programs decreased).
(3) Staff-maintained but CR centers closed.
(4) Staff-redeployed, CR centers closed.
An initial face-to-face interaction for activity tests, health and risk factor evaluations was impossible in all temporarily closed CR centers. Now it is possible to provide exercise tests if local sanitary prerequisites are taken into account. In addition, multidisciplinary team meetings are still necessary. During the COVID-19 pandemic, these sessions can be virtual, for example by utilizing videos [101].
The baseline assessment should include a physical examination and an evaluation of the cardiac patients’ levels of PA (type and volume of all activities). A thorough baseline assessment is of major significance since it can result in identification of any known contraindications to exercise engagement, evaluation of each patient’s risk stratification of a possible cardiac event during exercise, and therefore help design an appropriate individual exercise prescription. An exercise based evaluation of functional capacity may be impossible in some cases, such as when patients have orthopedic restrictions. If feasible, resting blood pressure and digital pulse palpation or an electrocardiogram (ECG) should be conducted to rule out tachycardia or bradycardia, and to check for any cardiac arrhythmias or abnormal blood pressure readings that could make activity unsafe.
Exercise evaluation should also include a musculoskeletal evaluation of the major joints due to the high frequency of comorbidities, particularly musculoskeletal problems, in the population with chronic illness. All patients should be subjected to a functional capacity test if possible [80]. If neither a cycle ergometer nor a treadmill is available, a 6-minute walk test (6MWT) [102, 103, 104], a 200-metre fast-walk test [105], a step test [106], or an incremental shuttle walking test (ISWT) [107, 108, 109, 110] should be performed. Essential professional equipment for this pre-exercise evaluation includes a sphygmomanometer, stethoscope, ECG machine/monitor (or, in the absence of an ECG machine/monitor, an accurate exercise heart rate monitoring device), beacons or markers for a 6MWT, and a stopwatch [1].
Modernization of CR services with digital tools would allow for better promotion of TR programs. One challenge in the coming years will be in expediating promotion and in achieving wide implantation rates for remote HBCR for cardiac patients who are at low risk and clinically stable [111, 112, 113, 114]. These novel virtual/home-based CR programs, which can support cardiac patients in controlling their cardiac disease/medication (therapeutic education), encourage good healthy dietary habits and engage people in physical exercise, are optimized with remote monitoring sensors. Sensors may encourage patients to engage in a healthy lifestyle. Additionally, wearable sensors represent a safe way to assess a patient’s PA. Importantly, patients should always be able to contact the medical staff [99].
The interface should record, store, and remotely present data for each variable recorded by sensors (energy expenditure, body mass, glycemia, blood pressure, heart rate, ECG, and so on) to a web platform accessible to the physician, cardiologist, exercise experts, and nurses. Several nations conducted virtual HBCR studies. Some of the (non-exhaustive) experiences have demonstrated persuasive evidence relating to practicality, safety, and cardiovascular risk. However, certain obstacles remain, such as data privacy concerns and the capacity to engage elderly patients. During exercise, real-time monitoring, such as ECG and blood pressure measurements, remain an issue [115].
The COVID-19 pandemic will have a significant impact on CR worldwide. New cardiac phenotypes, such as COVID-19 myocarditis, should be addressed by CR programs. Moreover, new types CR program delivery should be adopted, involving remote CR implementation, in order to increase the capacity of CR. TR appears to be a useful, efficient, safe, and cost-effective alternative type of CR for individuals with heart disease compared to standard CBCR programs. The majority of recently published research focusing on remotely monitored TR therapies that used a holistic approach indicated substantial advancement and progress in this field [11]. The globally increased levels of internet access, the extensive use of smartphones (even among the older population), the continuous development of new wearable sensors, web applications, and platforms provide cardiac specialists with a valuable tool to expand CR implementation to a more significant proportion of cardiac patients. CR and especially TR provide promising secondary rehabilitation interventions that need further investigation regarding innovative wearable sensor technology, real-time telemonitoring, and long-term CR intervention efficacy so that most cardiac patients could benefit from their safe use.
CV, Cardiovascular; TR, Telerehabilitation; CVRFs, cardiovascular risk factors; CR, cardiac rehabilitation; PA, physical activity; CBCR, center based cardiac rehabilitation; HBCR, home based cardiac rehabilitation; ICTs, Information and communication technology; BP, blood pressure; HF, heart failure; CHF, congestive heart failure; ASCVD, atherosclerotic cardiovascular disease.
Conceptualization, MS, VA, and GP; content design, MS, LD, and GP; writing — original draft preparation, MS, LD, VA, and GP; writing — review and editing, LB, and JP; visualization, MS, GP and LB; supervision, JP; funding acquisition, LB. All authors have read and agreed to the published version of the manuscript.
Not applicable.
Not applicable.
This research was funded by the Ministry of Health, Czech Republic; conceptual development of research organization (FNBr, 65269705).
The authors declare no conflict of interest.
