Academic Editor: Matina Kouvari
Central obesity is associated with increased level and activity of endothelin-1.
The waist and hip circumferences are simple indicators of central obesity. Waist
circumference correlates with visceral adiposity, whereas hip circumference
associates with gluteofemoral peripheral adiposity. Both measurements have
independent and opposite correlation with coronary artery disease (CAD) risk
factors. The relation between serum endothelin-1 in stable CAD and both
parameters of central obesityneeds to be investigated. This study aims to examine
the correlation between serum endothelin-1 level and waist and hip circumferences
as parameters of central obesity in patients with stable CAD. This was a
cross-sectional study. Consecutive subjects were enrolled among those who
underwent elective coronary angiography with significant CAD. Serum endothelin-1
was measured from peripheral blood samples taken before coronary angiography
procedure. The measurement of waist circumference, hip circumference, and ratio
derived from them, was performed. Central obesity was determined by waist
circumference cut-off for Indonesian population. The correlation analysis was
performed with Pearson test. The multivariate analysis was performed with
multiple linear regression test. The comparison of serum endothelin-1 level
between groups was performed with Student T test. We enrolled 50
subjects. The majority of subjects was male (80.0%), hypertensive (86.0%),
dyslipidemic (68%) and smoker (52%). Most subjects had history of acute
coronary syndrome (64%). Mean waist circumference was 87.6 +/– SD cm, hip
circumference was 95.3 cm +/– SD, mean waist-to-hip ratio was 0.92 +/– SD and
mean waist-to-height ratio was 0.54 +/– SD. Central obesity occurred in 32% of
subjects. Mean serum endothelin-1 level was 2.2
Cardiovascular disease (CVD) is one of the leading causes of death in many countries [1, 2]. Hypertension, diabetes mellitus, dyslipidemia, smoking behaviors and sedentary lifestyles are the established CVD risk factors for which prevalence are high and increasing in low- and middle-income countries [3]. In Indonesia, besides these risk factors, obesity, especially central obesity, continues to increase significantly and contributes meaningfully to CVD [4]. Individual with central obesity has an excess of visceral adiposity which is associated with sedentary lifestyle and metabolic diseases such as diabetes mellitus, hypertension, and dyslipidemia [5]. It adds multiple burdens of CVD risk factors. The increase in central obesity-related morbidity in Indonesia challenge CVD risk factor controls in a country which still struggles to implement CVD prevention program such as tobacco control policy, promotion of healthy diet and endorsement of healthy lifestyle [5].
Endothelin-1 is a main vasoconstrictor peptide in the circulation which operates in the peripheral, pulmonary, and coronary vascular beds through vascular smooth muscle cells contraction [6]. In a steady-state condition, it sustains the balanced vascular tones by stimulating the release of a vasodilator, nitric-oxide [7]. In vascular dysfunction, the balance between endothelin-1 and nitric-oxide is disrupted which lead to profound increased of endothelin-1, malfunctioning vasodilation, and increased vasoconstrictor tone [8]. Vascular dysfunction, which is indicated by insulin resistance and hyperinsulinemia, frequently accompanies individual with central obesity [9, 10]. Excessive visceral adipocytes express endothelin-1 which inhibit their insulin-stimulated glucose uptake, stimulate their lipolysis, release their free fatty acids and induce their production of pro-inflammatory cytokines [11]. Previous study demonstrated the enhanced endothelin-1 activity and endothelin-1-mediated adiposity-related disruption ofvasodilationin overweight and obese individuals without CVD [12, 13].
Individual with excessive fat accumulation in the visceral adipose tissue has higher rate of obesity-related CVD events [14]. Visceral adiposity is associated with increased waist circumference, which is a parameter to determine central obesity [14]. Central obesity is considered as metabolically harmful obesity [8]. Hip circumference indicates the adiposity of lower-body gluteofemoral region, which possess opposite effect to visceral adipose tissue [8]. Increasedserum endothelin-1contributes to metabolic syndrome and higher CVD events in individual with central obesity without coronary artery disease (CAD) [8, 13]. In patients with stable CAD, endothelin-1 level in blood circulation is increasing [15, 16]. Our previous study indicated that patients survived from acute coronary syndrome (ACS) and who later developed stable CAD, the majority of them were overweight and obese individuals [17]. The association between serumendothelin-1 level and central obesity in individual with stable CAD has not been investigated.
To assess central obesity, waist circumference is a superior indicator and more strongly correlated with intra-abdominal visceral adipose content, whereas hip circumference is associated with gluteofemoral peripheral adipose mass [18]. Both measurements had independent and opposite correlation with atherogenic risk factors, glucose intolerance and lipid metabolism disturbances [18]. The relation between serum endothelin-1 in patients with angiographycally-proven stable CAD and parameters of central obesity, namely waist circumference, hip circumference and ratio derived from them needs to be investigated. This study aims to examine the correlation between serum endothelin-1 level and parameters of central obesity in Indonesian patients with stable CAD.
This is a cross-sectional study. Subjects were patients diagnosed with stable
CAD. The subjects were enrolled consecutively during the performance of coronary
angiography (CAG) with/without stenting in Integrated Heart Center (Pusat
Jantung Terpadu) Dr. Sardjito Hospital, Yogyakarta, Indonesia. The inclusion
criteria were: (1) subjects underwent elective CAG, (2) subjects with age 30–75
years, (3) subjects with significant CAD (namely stenosis
Subjects were enrolled during hospitalization for CAG with/without PCI. The
demographic and medical histories were collected by anamnesis. Clinical data were
collected during day-1 hospitalization (before CAG procedure). The bodyweight
(kg) and body height (m) were measured by standard body scale and height ruler
respectively at day-1 hospitalization (before CAG procedure). The body mass index
(BMI) was calculated as bodyweight/(body height)
The elective CAG with/without PCI procedures and subsequent treatments for subjects were done at the discretion of attending cardiologists. The research procedure was approved by The Medical and Health Research Ethics Committee Faculty of Medicine, Public Health and Nursing Universitas Gadjah Mada and Dr. Sardjito Hospital Yogyakarta, Indonesia.
Peripheral blood samples were obtained from subjects while reclining in a supine
position from antecubital venous access on day-1 (before CAG procedure). The
blood samples were taken after at least 15 min of supine resting on vacutainer
tubes; in each subject a first sample was prepared for routine and blood
chemistry, whereas the second sample was prepared for endothelin-1
measurement. The blood samples were transferred into Vacutainer tubes (BD, USA)
and left at a room temperature to form clotting at 20–30 min. The tubes were
sent to a hospital central laboratory for routine hematology and blood chemistry
examinations. For endothelin-1 measurement, the tubes containing clotted blood
samples were centrifuged at 200 g for 20 minutes and the supernatant was stored
at –80
The normal distribution was tested with Kolmogorov-Smirnov test. The comparison
between normally distributed continuous data was performed with Student’s
t-test, while Mann–Whitney test was used for not normally distributed
continuous data. The bivariate correlation analysis was performed with Pearson
correlation test or Spearman correlation test where applicable. A multivariate
regression analysis was done to determine the strength of correlation among
different independent co-variables. Co-variables were selected from bivariate
analysis which had p value
Table 1 shows the characteristics of research subjects. Fifty-consecutive
subjects were enrolled in this study. Mean endothelin-1 level in all subjects was
2.2
| Characteristics | All subjects | |
| n = 50 | ||
| Demography | ||
| Males, n (%) | 40 (80.0) | |
| Age (year), mean |
58.8 | |
| CVD risk factors | ||
| Diabetes mellitus, n (%) | 14 (28.0) | |
| Hypertension, n (%) | 43 (86.0) | |
| Dyslipidemia, n (%) | 34 (68.0) | |
| Smoking, n (%) | 26 (52.0) | |
| History of ACS, n (%) | 32 (64.0) | |
| Clinical parameters, mean |
||
| Systolic pressure (mmHg) | 129.9 | |
| Diastolic pressure (mmHg) | 76.3 | |
| Heart rate (bpm) | 73.9 | |
| Anthropometric parameters, mean |
||
| Bodyweight (kg) | 66.3 | |
| Body height (m) | 1.62 | |
| Body mass index | 25.1 | |
| Waist circumference (cm) | 87.6 | |
| Hip circumference (cm) | 95.3 | |
| Waist-to-hip ratio | 0.92 | |
| Waist-to-height ratio | 0.54 | |
| Obesity categories | ||
| Obesity by BMI | ||
| Obese II | 5 (10.0) | |
| Obese I | 16 (32.0) | |
| Overweight | 16 (32.0) | |
| Normal | 13 (26.0) | |
| Central obesity | 16 (32.0) | |
| Laboratory, mean |
||
| Hemoglobin (g/dL) | 12.8 | |
| Leucocytes (×10 |
7.8 | |
| Platelets (×10 |
269.7 | |
| Creatinine (mg/dL) | 1.2 | |
| Glucose (mg/dL) | 140.3 | |
| Endothelin-1 level (pg/mL) | 2.2 | |
| CAD, coronary artery disease; CVD, cardiovascular disease; ACS, acute coronary syndrome; BMI, body mass index. | ||
Anthropometric parameters indicated that mean bodyweight was 66.3
 Fig. 1.
Fig. 1.Comparison of endothelin-1 level among obese, overweight and
normal subjects. There was no significant difference in endothelin-1 level among
four groups of subjects (obese II: 2.7
The circumference measurements indicated that mean waist circumference was 87.6
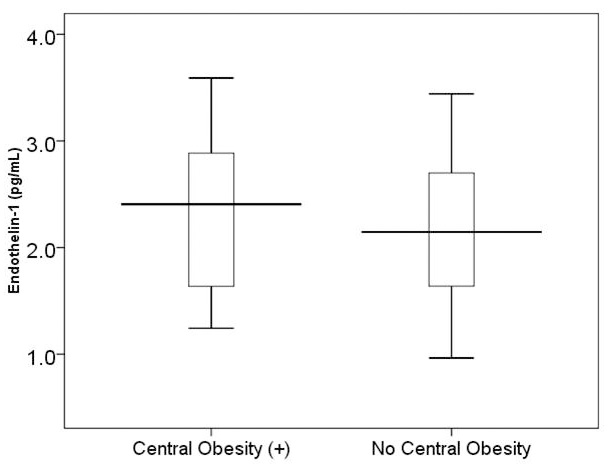 Fig. 2.
Fig. 2.Comparison of endothelin-1 level between subjects with central
obesity (n = 16) and no central obesity (n = 34). There was no signicant
difference in endothelin-1 level between two groups (mean
| Demography and CVD risk factors | Mean |
p value |
| Male (n = 40) | 2.3 |
0.203 |
| Female (n = 10) | 1.9 |
|
| Diabetes mellitus (n = 14) | 2.3 |
0.621 |
| No diabetes mellitus (n = 36) | 2.2 | |
| Hypertension (n = 43) | 2.2 |
0.661 |
| No hypertension (n = 7) | 2.3 | |
| Dyslipidemia (n = 34) | 2.3 |
0.181 |
| No dyslipidemia (n = 16) | 2.0 | |
| Smoking (n = 26) | 2.2 |
0.881 |
| No smoking (n = 23) | 2.2 | |
| History of ACS (n = 32) | 2.2 |
0.887 |
| No history of ACS (n = 18) | 2.3 | |
| ACS, acute coronary syndrome. | ||
Table 3 shows result of correlation analysis between serum endothelin-1 level and other continuous variables. Among clinical, anthropometric and laboratory variables, serum endothelin-1 level was significantly correlated with age in years (correlation coefficient of –0.376, p value = 0.007) and hemoglobin level (correlation coefficient of 0.316, p value 0.026). Serum endothelin-1 was significantly correlated with parameters of central obesity, namely waist circumference (correlation coefficient of 0.311, p value = 0.023) and hip circumference (correlation coefficient of 0.359, p value = 0.010). Other variables, i.e., age, body mass index, systolic blood pressure, diastolic blood pressure, heart rate, hemoglobin level, leucocytes count, platelet count, glucose level and creatinine level, were not significantly correlated with serum endothelin-1 level.
| Variables | Coefficient correlation | p value |
| Age (years) | –0.376 | 0.007 |
| Systolic blood pressure (mmHg) | 0.061 | 0.676 |
| Diastolic blood pressure (mmHg) | 0.251 | 0.079 |
| Heart rate (beat/min) | 0.234 | 0.102 |
| Bodyweight (kg) | 0.252 | 0.078 |
| Bodyheight (m) | 0.241 | 0.092 |
| Bodymass index | 0.172 | 0.233 |
| Waist circumference (cm) | 0.311 | 0.023 |
| Hip circumference (cm) | 0.359 | 0.010 |
| Waist-to-hip ratio | 0.017 | 0.907 |
| Waist-to-height ratio | 0.256 | 0.073 |
| Hemoglobin (g/dL) | 0.316 | 0.026 |
| Leucocytes ( |
0.035 | 0.807 |
| Platelets ( |
0.085 | 0.556 |
| Creatinine (mg/dL) | 0.058 | 0.690 |
| Glucose (mg/dL) | –0.027 | 0.853 |
Table 4 shows the result of multivariate regression analysis. Multivariable analysis were included age, hemoglobin, waist circumference and hip circumference as covariates and endothelin-1 as the dependent variable. Correlation were observed inage (coefficient of –0.353, p value = 0.007) and hip circumference (coefficient of 0.335, p value = 0.011) with endothelin-1 level. Waist circumference did not significantly correlate independently with serum endothelin-1 level.
| Co-variables | Standardized Coefficient (Beta) | p value |
| Age (years) | –0.353 | 0.007 |
| Waist circumference (cm) | –0.020 | 0.893 |
| Hip circumference (cm) | 0.335 | 0.011 |
| Hemoglobin (g/dL) | 0.115 | 0.435 |
Results of our study indicated that in patients with angiographycally-proven stable CAD, serum endothelin-1 level was positively correlated with both waist circumference and hip circumference. The independent correlation was significantly observed in age (inverse correlation) and hip circumference (positive correlation). Serum endothelin-1 tended to be higher in stable CAD patients with central obesity and patients with BMI-derived obesity compared to their counterparts. The measurement of serum endothelin-1 as a prognostic indicator in stable CAD patients with central obesity needs to be corroborated by larger and more extensive research.
Previous study indicated that obese individuals (BMI
An important mechanism by which obesity leads to the development of vascular diseases is the development of insulin resistance and inflammation [8]. Furthermore, in patients with stable CAD, within a spectrum of advanced atherosclerosis, insulin resistance and inflammation may have developed for long time before any stable CAD events. Evidence indicates that endothelin-1 contributes to insulin resistance and inflammation through numerous mechanisms, including impairment of insulin signaling in endothelial cells as a precursor of early atherosclerosis [22, 23]. Atherosclerosis itself is associated with excess endothelin-1, which is by several mechanisms known to enhance the atheroma formation and atherosclerotic disease progression [24, 25]. Furthermore, excess endothelin-1 inhibits insulin-stimulated glucose uptake in adipocytes and skeletal muscle cells, as well as enhancing lipolysis, release free fatty acids and pro-inflammatory cytokines in adipocytes [11]. In obese patients with stable CAD, all factors that contribute to increased serum endothelin-1 levels were found. Hypertension, which predominates in our study subjects, also contribute to elevated level of serum endothelin-1 [26].
Our study indicated positive correlation between serum endothelin-1 level with both waist and hip circumferences, but not with other obesity and central parameters. As an individual measure, waist circumference is a measure of visceral and subcutaneous adipose tissue in the abdominal region, whereas hip circumference is a measure of adipose tissue and also muscle mass in the lower body part [27]. After adjustment with covariables, unexpectedly, hip circumference independently correlated positively with serum endothelin-1. From this current observation, we speculate that increased hip circumference reflects increased subcutaneous adipose storage and muscle mass of the gluteofemoral region which had specific lipid storage and secretion of adipose tissue-related proteins [27]. Adipose tissue in the gluteofemoral region had active role in the removal of circulating nonesterified fatty acids which could limit the development of insulin resistance [28]. Similarly, thigh subcutaneous adipocytesc correlated with greater insulin sensitivity [28]. Both adipocytes and skeletal muscle cells express endothelin-1 and its receptors [11]. Whether the activity of gluteofemoral adipose tissue is mediated by endothelin-1 needed further research.
This study had several limitations. The major limitation was the small number of subjects enrolled in the analysis. Further study with larger sample size is needed to be performed to confirm our findings. Another limitation was laboratory parameters of various metabolic disturbances were not measured in this research. Further study with measurements of parameters of metabolic disturbances as additional confounding variables needs to be performed in patients with angiographycally-proven stable CAD.
In conclusion, serum endothelin-1 level was significantly positively correlated with waist circumference and hip circumference, as parameters of central obesity among Indonesian patients with angiographycally-proven stable CAD. The independent positive correlation was found between serum endothelin-1 level and hip circumference.
ABH designed the study, performed subject’s enrollment, data collection, statistical analysis and manuscript writing. JF performed data collection, laboratory analysis and manuscript writing. IP performed subjects enrollment, data collection, and statistical analysis. FSTD contributed to data collection, data analysis and manuscript writing. All authors gave final approval of the manuscript.
Ethics approval for this research had been granted by The Medical and Health Research Ethic Commitee of Faculty of Medicine, Public Health and Nursing Universitas Gadjah Mada – Dr. Sardjito Hospital, Yogyakarta, Indonesia.
Authors expressed gratitude to Ms. F. Linda Tri Pramatasari and Dr. Ahmad Musthafa from the Faculty of Medicine, Public Health and Nursing Universitas Gadjah Mada, Yogyakarta for their technical assistance on blood sample biobanking and analysis of endothelin-1. Authors expressed gratitude to Dr. Adysti Dhian Rizky Paramytha, Dr. Aras Amilla Husna and Dr. Brilliant Winona Jhundy for performing data collection of stable CAD subjects.
This research received funding from Deputi Bidang Penguatan Riset dan Pengembangan, Ministry of Research and Technology/National Research and Innovation Agency of Republic of Indonesia via Universitas Gadjah Mada with contract number: 2750/UN1.DITLIT/DIT-LIT/PT/2020 and Research Grant of Dr. Sardjito Hospital with grant number: HK.02.03/XI.2/17234/2019; both are granted to Anggoro Budi Hartopo as Principal Investigator.
The authors declare no conflict of interest.
The data used to support the findings of this study are available from the corresponding author upon request.
