Academic Editor: Leonardo De Luca
Takotsubo syndrome (TTS) is an intriguing clinical entity, characterized by usually transient and reversible abnormalities of the left ventricular systolic function, mimicking the myocardial infarction with non-obstructive coronary arteries. TTS was initially regarded as a benign condition, however recent studies have unveiled adverse outcomes in the short- and long-term, with rates of morbidity and mortality comparable to those experienced after an acute myocardial infarction. Given the usual transient nature of TTS, this is an unexpected finding. Moreover, long-term mortality seems to be mainly driven by non-cardiovascular causes. The uncertain long-term prognosis of TTS warrants a comprehensive outpatient follow-up after the acute event, although there are currently no robust data indicating its modality and timing. The aim of the present review is to summarize recent available evidence regarding long-term prognosis in TTS. Moreover methods, timing and findings of the long-term management of TTS will be discussed.
Takotsubo syndrome (TTS) is defined as an acute and usually reversible heart failure syndrome, characterized by typical and transient regional wall motion abnormalities of left ventricle (LV) that reflect impairment of myocardial contractility without the evidence of culprit epicardial coronary artery lesions [1]. Although TTS remains an entity of relatively new definition, it is possible to find descriptions that match with this characteristic clinical picture in ancient history [2] as well as in the less recent medical literature [3]. Notwithstanding, TTS was first described in 1990 by Sato et al. [4] and derived its name from the Japanese word for the octopus trap, due to the shape of the LV at the end of the systole during the acute phase. The prevalence of TTS is currently reported to be around 2%–3% among patients presenting with suspected acute coronary syndrome (ACS) [5]. About 90% of patients with TTS are post-menopausal women. However, with the increasing knowledge of the disease, male patients are diagnosed more frequently, often in association with a physical trigger [1]. During the acute phase of TTS, hemodynamic and electrical instability may be the cause of serious adverse in-hospital complications, such as acute heart failure, arrhythmias and thromboembolic events, which occur in approximately one-fifth of TTS patients [6, 7]. While the short-term prognosis and acute management of TTS have been extensively characterized by several studies, data on long-term outcomes are less well defined. In the present review we will summarize and discuss the most recent evidences regarding long-term prognosis in TTS. Moreover methods, timing and findings of the outpatient follow-up after the in-hospital phase of TTS will be discussed.
TTS was initially perceived as a benign condition [8, 9]. This was likely due to the usual transient nature of left ventricular systolic dysfunction and electrocardiographic abnormalities, the absence of any culprit obstructive coronary artery disease (CAD) and of replacement fibrosis on cardiac magnetic resonance (CMR) imaging. The results of the first observational studies confirmed this notion [8, 9, 10]. However, these earlier studies included populations of limited size, consisting mainly of patients with “typical” TTS presentation (e.g., post-menopausal women experiencing emotional stress with LV apical ballooning). This subset of patients was indeed later recognized as that with a lower risk of adverse outcomes [11, 12], unlike patients with “atypical” TTS presentations (e.g., men with physical triggers [13]) who more frequently experience worse outcomes. Evidences collected in recent years, obtained from larger cohorts with longer follow-up, expanded our perception of the disease and clarified that TTS is a heterogeneous condition, not-entirely benign and burdened by a significant rate of in-hospital complications [6, 12, 14, 15, 16]. Moreover, despite TTS is characterized by an apparent recovery of LV systolic function, long-term adverse outcomes have been reported, with rates of morbidity and mortality comparable to those experienced after an acute myocardial infarction (AMI). Stiermaier et al. [17] described a long-term mortality rate even higher than in patients with ST-elevation AMI. In the SWEDEHEART registry, long-term (median of 25 months) risk of mortality after TTS was comparable to that of AMI [5] (adjusted hazard ratio: 1.01, 95% confidence interval, CI, 0.70–1.46, P = 0.955). In the International Takotsubo (InterTAK) registry, long-term rate of death from any cause was 5.6% per patient-year and that of major adverse cardiac and cerebrovascular events was 9.9% per patient-year, similar to that observed in a matched control-group of AMI patients [6]. Also the Takotsubo Italian Network (TIN) registry reported similar long-term mortality as compared to a propensity score matched cohort of AMI patients [18]. These findings may seem surprising when taking into account the substantial reversibility of functional abnormalities in TTS, however, the pathophysiological processes underlying these results remain to be fully elucidated.
In most cases, the acute cardiac symptoms in TTS are preceded by a triggering factor that can be of an emotional [19, 20, 21] or physical nature. Physical triggers include medical, surgical, anaesthetic, obstetric or psychiatric conditions, which are frequently the cause of a patient’s previous hospitalization [22, 23]. In these patients, TTS occurs as a complication of the primary condition or of its treatment and therefore is diagnosed as secondary TTS [22]. It has been established that secondary TTS is associated with a worse prognosis in the long-term follow-up [13], suggesting that these patients deserve more attention in the follow-up after the acute phase. Notably, the poor long-term outcome in this subgroup is mostly driven by non-cardiovascular mortality.
Left ventricular systolic and diastolic dysfunction are common features of acute TTS [7]. Interestingly, despite substantial recovery of left ventricular ejection fraction (LVEF) in the chronic phase, worse prognosis in the long-term has been detected in those patients having a higher degree of cardiac dysfunction during the acute event [24, 25]. Accordingly, signs of a more severe heart failure during the acute phase, including increased release of natriuretic peptide [26], higher Killip class [17], higher heart rate and a lower systolic blood pressure [27] are all associated with a worse long-term prognosis. Cardiogenic shock (CS) is not an uncommon clinical complication in TTS patients. The reported CS incidence in TTS ranges from 6% to 15% and is similar to that of patients with AMI [6, 15, 28, 29]. Clinical factors described to be significantly related to CS are male sex, lower LVEF at presentation, longer QT corrected (QTc) interval, the presence of left ventricular outflow tract (LVOT) obstruction, or a physical trigger [15, 28]. CS during TTS hospitalization is one of the stronger predictors of short and long-term outcome. The short-term (1 month) all-cause mortality rate is significantly high in patients with TTS complicated by CS (incidence ranges from 13% to 29%) [15, 28]. In-hospital mortality in TTS patients with CS is significantly lower than in patients with AMI complicated by CS [30], possibly implying different mechanisms underlying hemodynamic instability in these patients. Of interest, CS is associated with a 5-fold increased risk of long-term mortality, even after full recovery of LVEF [15]. Results from the multicenter Registry on Takotsubo Syndrome (RETAKO, Spain) showed that CS was the most powerful predictor of long-term mortality, with 10-year mortality of approximately 30%, mostly attributed to non-cardiovascular causes [15]. In contrast, a previous study reported higher long-term shock-associated mortality (67%) at 3.6 years of follow-up, with almost all deaths attributed to cardiovascular events [28]. Overall, higher cardiac involvement during the acute phase should be considered as a marker of risk even for long-term events, though it is still unclear whether this is due to the TTS related consequences or to an underlying pre-existing vulnerable profile [31].
Life-threatening arrhythmias including bradyarrhythmias, ventricular
fibrillation, and sustained/non-sustained ventricular tachycardia may occur
during the acute phase in 3.4–12.2% of TTS patients, as reported by the German
Italian Spanish Takotsubo (GEIST) registry [32], but their role in long-term
prognosis is debated. Prognostic studies have provided conflicting results, with
an increased [33, 34] or unchanged [35] one-year mortality rate. In the InterTAK
Registry, cardiac arrest (CA) is associated with a six-fold increase in short-
and long-term mortality. Compared with patients without CA, those with CA had
higher 60-day mortality (40.3% vs. 4.0%, P
AF is the most common arrhythmia in TTS, with a prevalence of about 15% [29, 41]
and is associated with poorer short and long-term prognosis [41]. El-Battrawy
et al. [41] retrospectively analysed a population of 114 TTS patients
and observed a considerably higher 3-year mortality rate in the AF patient
subgroup (38% vs. 16%, P
TTS recurrence is relatively uncommon [8]. The results of a meta-analysis suggest a cumulative risk of recurrences of 5%, and a rate of 1–2% per year [43]. A multicentre registry with longer follow-up found an incidence rate of 0.9% patient/year without an evident temporal trend [44]. Both the triggering event and the ballooning pattern may differ during recurrent events [45], although reiteration of the same trigger, either emotional [46] or physical [47, 48], has been associated with TTS recurrence in sporadic case reports. Though specific risk factors related to recurrence were not initially found [14, 48, 49], other studies found that the presence of comorbidities (such as hypertension [50], chronic obstructive pulmonary disease [44] and psychiatric diseases [51]) and some clinical features during the acute phase of the first event (reduced LVEF [43], right ventricle involvement [50] and physical trigger [44]) are factors linked with the recurrence of TTS. The results of a subsequent meta-analysis proved the association between therapy with inhibitors of the renin-angiotensin system (angiotensin-converting enzyme inhibitors, ACEIs, and angiotensin receptor blockers, ARBs) and a reduction in recurring events [52]. Furthermore, combination therapy with inhibitors of the renin-angiotensin system and beta-blockers proved effective in conferring protection against recurrence [53], while beta-blocker therapy alone did not [54]. However it should be noted that the low rate of events recorded and the nature of the studies carried out do not allow a complete analysis of the causal link between certain risk factors or medications and the rate of recurrences. Indeed, more recent multicenter studies did not found a clear impact of medication on TTS recurrence [44, 55], while in one study more than half of the population experiencing a recurrence was on treatment with a beta-blocker, an ACEI/ARBs or a combination of both [56]. Therefore, the identification of recurrence risk factors and optimal preventive medical therapy remains an open question, which requires further studies. Recurrences, as well as “familial” forms [57, 58], suggest a possible influence of the genetic background in the genesis of the syndrome. However contemporary data on genetic features of TTS do not support a clear genetic etiology in TTS [59]. While environmental triggers and concomitant comorbidities are pivotal in TTS development, to some extent a potential genetic predisposition may play a role as a contributing predisposing factor by acting on the dysregulation of the adrenergic system.
Cardiovascular events occur in the long-term follow-up of TTS patients with an incidence rate similar to that of patients with previous AMI [17]. Moreover, several studies demonstrated a long-term mortality mainly driven by non-cardiovascular causes [15, 17, 18, 60, 61, 62]. The cardiovascular and non-cardiovascular morbidity is likely as high in TTS patients due to the common presence of comorbidities such as psychiatric diseases [6], pulmonary diseases, dyspepsia, cerebrovascular diseases, diabetes mellitus [13, 17], liver and kidney diseases [63] and AF [42, 64]. Long term cardiac abnormalities after TTS have been observed [65], though not yet clearly linked to a worse outcome. The association between TTS and neoplastic diseases deserves a special mention. In fact, the prevalence of malignancy in patients with TTS is high and considerably exceeds that in the normal population [66, 67]. Malignant tumours are among the main determinants of long-term mortality in TTS and are associated with a worse prognosis [67]. The physio-pathological mechanisms underlying this association are not known and the reasons for the high prevalence of malignancy in TTS patients remain speculative. A recent interTAK registry paper showed higher cardiovascular mortality in patients without malignancy, but not in those with malignancy, as compared to patients who suffered an ACS [68]. Both cancer diagnosis or treatment may acutely trigger TTS [69]. Additionally, given the long-time interval often observed between cancer diagnosis and TTS occurrence [70], cancer and TTS may share similar activation mechanisms, such as activation of the sympathetic nervous system or the genetic background [66].
In summary, it is established that long-term prognosis in TTS patients is not completely benign, and several clinical prognostic variables have been identified to predict adverse events which are often of a non-cardiovascular nature. However, reliable risk scores are lacking to identify those patients who might benefit most from a more intensive and prolonged follow-up. Here we propose to define as “high-risk” patients, e.g., who would need a closer long-term monitoring, those with at least one of the following characteristics: severe comorbidities, in-hospital complications, TTS recurrence, physical trigger, persistent symptoms or incomplete recovery of LV function (Tables 1,2).
While the link between comorbidities and long-term outcome appears intuitive, it may be less clear why the extent of transient acute cardiac dysfunction is possibly associated as well with long-term adverse events [15, 28]. Based on this evidence, it has been speculated that noncardiac conditions may contribute to characterize a vulnerable phenotype prone to heart failure, which may explain the higher susceptibility to functional abnormalities of TTS both in the acute phase and in the long-term [71]. This vulnerability would be therefore the cause of the long-term negative prognosis, and not the acute cardiac dysfunction which largely regresses already during the first days [31]. In this perspective, cardiac dysfunction may be a proxy of a comorbid state, which is actually responsible for the prognosis.
| RISK STRATIFICATION | |||
| High Risk | |||
| One of the following: | |||
| MANAGEMENT | |||
| Timing outpatient follow-up | Subgroups of patients with previous TTS | Recommended exams | Optional exams |
| 1 month | All | ECG | BNP |
| TTE | GLS | ||
| CMR |
ECG-Holter | ||
| 3–6 months | All | ECG | BNP and GLS |
| TTE |
CPET | ||
| 12 months and yearly | High risk | ECG | BNP, GLS and CPET |
| TTE |
Cardiac-CT | ||
TTS, Takotsubo syndrome; ECG, electrocardiogram; TTE, Transthoracic echocardiography; CMR, Cardiovascular magnetic resonance; BNP, B-type natriuretic peptide; GLS, Global Longitudinal Strain; CPET, Cardiopulmonary exercise testing; CT, Computed Tomography; QTc, QT corrected; CAD, coronary artery disease. Adapted from L. Arcari; L. Cacciotti; L. R. Limite; M. Sclafani; I. Passaseo; G. Marazzi et al. Tako-Tsubo syndrome: long term prognosis and outpatient follow-up. Outpatient Cardiology. 2018; 4: 249–261 [72]. | |||
The uncertain long-term prognosis of TTS warrants a comprehensive outpatient follow-up after the acute event, although there are currently no robust data indicating its modality and timing. A position statement published on behalf of the European Society of Cardiology (ESC, Brussels, Belgium) recommends a follow-up evaluation 3–6 months after discharge with electrocardiogram (ECG) and transthoracic echocardiogram (TTE), to assess the typical regression of acute and subacute abnormalities and to optimize the medical therapy. CMR imaging should be performed in doubtful cases and if it had not been performed during hospitalization [22]. We herein propose a strategy of scheduled follow-up visits for all TTS patients with an early evaulation, one month after discharge, to assess the recovery of ECG and echocardiographic abnormalities, to stop unnecessary and potentially harmful drugs (e.g., anticoagulation, diuretics and dual antiplatelet therapy) and, if not performed during hospitalization, to prescribe CMR. Then, a subsequent visit 3–6 months after the acute event depending on the grade of ECG and echocardiogram recovery and the persistence of symptoms. These latter, if present, may be objectified with a cardiopulmonary exercise testing (CPET). Finally, a one-year evaluation after the acute event and subsequent annual evaluations (with ECG and TTE) should be offered only to “high-risk” patients. A multidisciplinary team may be useful to discuss complicated cases such as patients with psychiatric disorders in polytherapy with a scarce control of the underlying disease, with malignancy or with extreme frailty. Table 1 (Ref. [72]) summarizes the possible approach to long-term management of TTS.
Although a stable clinical course from discharge is generally assumed, persistence of symptoms is a relatively common occurrence in TTS. A substantial number of patients experience residual symptoms (including chest pain, fatigue and breathlessness) even years after discharge [73], with a rate comparable to that of patients with a previous AMI [74]. Long-term persistence of chest pain often leads to further hospitalizations and invasive examinations [9]. For this reason, it should be investigated whether the nature of symptoms is organic or psychosomatic. The association of persistent symptoms with echocardiographic and laboratory abnormalities could suggest that symptoms are due to persistent myocardial dysfunction and not to psychosomatic factors. Consistent with this hypothesis, elevated natriuretic peptide values [75], persisting cardiac limitation on CPET and reduction in longitudinal strain on the echocardiogram were observed in symptomatic patients with previous TTS even after more than one year from the acute event [65]. Moreover, dyspnea was reported to be an independent predictor of long-term mortality [25].
Prolonged follow-up is recommended for the subgroup of patients with persistent symptoms [22] and possible progression of previously non-significant CAD should not be underestimated [76].
During the acute phase of TTS, there is always evidence of elevation of troponin values with peak values significantly lower compared to classical ACS [6]. The extent of LV systolic dysfunction usually exceeds that of associated myocardial necrosis biomarkers, thus reflecting a large part of stunned myocardium [77]. On the contrary, a considerable elevation of natriuretic peptides (B-type natriuretic peptide, BNP, and N-terminal pro BNP, NT-proBNP) is frequently found, with the highest level reached 24–48 h after symptom onset, mainly related to myocardial stretch. It has been described that a gradual normalization of natriuretic peptides levels occurs within a few months after the acute phase [78], however elevated values were observed in symptomatic patients after 3 months from the acute event [75]. Higher in-hospital levels of BNP [79] and troponin [6] are associated with acute complications. Moreover elevated values of BNP were reported to be independent risk factors for delayed recovery of LV function [80], so that patients with elevated BNP were suggested to deserve particular concern and surveillance [79]. In long-term follow-up, elevated natriuretic peptide values were associated with higher long-term mortality [26] and with persistent symptoms and reduced longitudinal strain on echocardiogram [65].
The ECG on admission is abnormal in the majority of patients with TTS usually demonstrating repolarization abnormalities (ST-segment elevation, T-wave inversion, or both) mimicking AMI. Electrocardiographic features of TTS on admission were reported in comparison to anterior AMI, such as absence of reciprocal changes, absence of abnormal Q waves, and predominance of ST elevation in leads V4 to V6 [81]. However, ECG changes in the acute phase of TTS are often non-specific [82] and differential diagnosis with AMI may be difficult. On the contrary, the evolution of electrocardiographic changes in the subacute phase of TTS is characteristic. Widespread T-wave inversion and QTc-interval prolongation are frequently observed in the first three days after the onset of symptoms and associated with lower in-hospital risk when present on admission [83]. Widespread T-wave inversion typically occurs in the first 24 hours, regresses in the following days, and may recur more pronounced after about one week from the acute event [84]. The distribution of T-wave inversion in the subacute phase may be an electrophysiological manifestation of myocardial stunning and the extent of the T-wave inversion has been associated with the presence of myocardial edema identified by CMR [38, 84, 85], even in the presence of recovered LVEF. The subsequent ECG evolution is more heterogeneous and ECG changes may persist for several months even after LV contractile recovery [86]. Comparison with an ECG preceding the acute event, if available, it is useful to evaluate complete regression of the alterations related to the TTS, which can also occur years after the acute event.
In conclusion, ECG is a simple but useful tool in patients with TTS that should always be performed in the follow-up evaluations, especially when CMR or more advanced echocardiographic methods (e.g., speckle tracking echocardiography) are not available. Fig. 1 shows the typical evolution of ECG changes in TTS.
 Fig. 1.
Fig. 1.Left precordial ECG leads (V4-V6) of a TTS patient. ECG recorded at the onset of symptoms (A). ECG recorded at discharge, 5 days from the acute event (B). ECG recorded after 3 months from the acute event (C). ECG recorded after 1 year from the acute event (D). During the acute phase, the ECG shows non-specific abnormalities of ventricular repolarization. Widespread inversion of the T-wave occurs during the subacute phase. The follow-up ECG recorded 3 months after discharge shows positivization of the T-wave but without complete recovery. The follow-up ECG recorded 1 year from the acute event shows complete recovery of ventricular repolarization with a higher voltage T-wave.
The incidence of life-threatening ventricular arrhythmias in the acute phase (ventricular tachycardia and ventricular fibrillation) in patients with TTS is considerably lower than in patients with AMI [87]. They occur most often in the acute and subacute phase [39, 86] and are usually associated with marked QTc-interval prolongation. However, long-term ventricular arrhythmias have been observed [35]. As previously discussed, the indication to implant an ICD in TTS patients who experienced malignant arrhythmias during the acute phase is a debated topic. In this context, 24-hours ECG monitoring may be an useful tool during outpatient follow-up to exclude any potentially life-threatening ventricular arrhythmias or conduction disorders, particularly in patients with persistent symptoms or with marked QTc-interval prolongation. Loop recorder implantation might be a valuable option in selected patients with high arrythmic risk [88], though there is still a paucity of data in the literature.
TTE is an essential tool in the follow-up evaluation of patients with TTS. Complete recovery of regional wall motion abnormalities and LVEF must be documented to confirm the diagnosis of TTS. Typically, LVEF fully recovers within a month after the acute event [89], but there are patients with a more rapid versus slower recovery [47, 90]. Nevertheless, other functional LV abnormalities may persist despite LVEF normalization. Novel, advanced echocardiographic techniques may provide an accurate and sensitive detection of these abnormalities during the recovery phase of TTS clinical course. Speckle tracking-TTE (ST-TTE) provides parametric quantification of myocardial chambers deformation and assessment of LV global longitudinal strain (GLS) by ST-TTE may have an important prognostic value. Indeed, within the first few months after TTS hospitalization, LV-GLS has been consistently described to be impaired despite normalization of LVEF [89, 91], and a reduced LV-GLS at 3 months after TTS is associated with both persistent elevation of natriuretic peptides and increased perception of impaired physical exercise capacity [92]. In addition, impairment of LV longitudinal systolic function was observed by ST-TTE even more than one year after the acute event [65]. Further research is needed to confirm whether these alterations of systolic function are a pre-existing factor, rather than a consequence of TTS, or complete recovery is possible at a later stage. In fact, long-term ST-TTE studies provided conflicting results: the complete recovery of systolic function has been described [93], as well as the persistence of disturbances of rotational strain with normalization of GLS [94]. The role of stress echocardiography in TTS follow-up is unclear since studies on this topic are small and conflicting data have been reported. A first case-control study of 22 patients with previous TTS undergoing dobutamine stress echocardiography showed no signs of diastolic or systolic dysfunction during pharmacological stress [95]. A subsequent case–control study comparing 30 patients with previous TTS and persistent symptoms who were referred for exercise echocardiography reported reversible exercise-induced LV contractile dysfunction [96]. The Authors hypothesized that exercise-induced hyperactivation of autonomic nervous system and higher levels of norepinephrine may induce endothelial dysfunction, coronary vasoconstriction, microvascular dysfunction, increased cardiac workload, and direct myocardial toxicity [96]. However the clinical implications of stress-induced LV dysfunction in TTS patients are unclear, and studies with greater cohort and longer follow-up are required to determine whether it is associated with a worse prognosis. Moreover, many case reports and a recent study reported of TTS triggered by dobutamine stress echocardiography [97, 98, 99]. Thus, the role of exercise echocardiography in TTS follow-up remains to be defined, while the use of dobutamine stress should be avoided due to the non-trivial risk of recurrence. At the present time, standard TTE appears sufficient to confirm TTS diagnosis and it is essential in the first outpatient examination both to document complete LV recovery and to evaluate the diastolic function which provides useful prognostic indications as well [90]. More advanced techniques, such as ST-TTE, could be useful in persistently symptomatic patients in order to evaluate possible abnormalities in LV systolic function [92]. Fig. 2 shows normalization of LV-GLS in a TTS patient one month after the acute event.
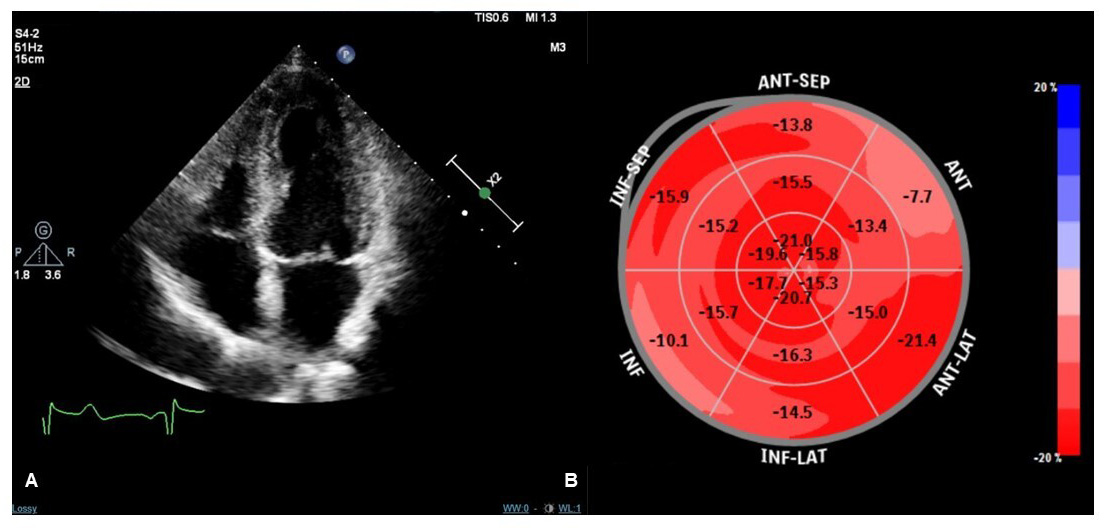 Fig. 2.
Fig. 2.Transthoracic echocardiography (TTE) imaging of a TTS patient performed one month after the acute event. Apical four-chamber view during systole showing no regional kinetic abnormalities (A). Speckle tracking-TTE (ST-TTE) showing a normal value of LV global longitudinal strain without significant regional abnormalities of myocardial chamber deformation (B).
CMR is the gold standard non-invasive imaging tool in cardiovascular medicine for visualizing cardiovascular anatomy, quantifying chamber volumes and systolic function and characterize myocardial tissue [100]. Indeed it is able to identify and quantify non-vital areas, fibrosis and edema in the context of normal myocardium. Classic CMR appearance during the acute phase of TTS includes widespread myocardial edema, mostly distributed in the areas of contractile dysfunction [101], in the absence of significant replacement fibrosis at late gadolinium enhancement (LGE) imaging. However, presence of LGE areas has been reported [102], and linked to adverse outcomes [103]. Several bystander diseases associated with LGE areas might characterize patients with TTS, including previously unrelated myocarditis, AMI or known obstructive CAD [76, 104]. CMR should be performed in the acute phase, when available, in all TTS patients [22, 77] in order to differentiate TTS from other conditions with similar clinical presentation, such as myocardial infarction with non-obstructive coronary arteries (MINOCA) or myocarditis, and thus to guide the therapeutic decision-making process [105, 106, 107]. Additionally, performing early CMR would help identify typical complications of acute TTS, such as right ventricular involvement and ventricular thrombosis, which may be difficult to observe with TTE [108]. Unfortunately, CMR imaging cannot be always performed easily in the acute setting of TTS. The ESC position statement on TTS acknowledged this issue, suggesting to perform CMR preferentially for patients with dubious clinical presentation or suspected myocarditis [109]. CMR is also particularly useful in the subacute and chronic phase in order to evaluate residual edema and confirm the diagnosis, especially in case of less common presentations, such as in young, males or patients with atypical anatomical variants (midventricular [110], basal [111], and focal [112, 113] motion patterns) (Fig. 3, Ref. [72]). A low-grade myocardial edema may be still present at 3-month follow-up after the acute event, being linked to persistent symptoms as well as increased natriuretic peptide [114]. New advanced CMR techniques may provide an accurate and sensitive detection of edema and intramyocardial fibrosis and evaluation of myocardial chambers deformation (Feature-tracking CMR) similar to speckle-tracking echocardiography [115]. In particular, novel CMR mapping sequences allow a parametric quantification of interstitial expansion in the myocardium, with signal intensity mainly depending on extracellular water (T2 mapping) as well as fibrosis and infiltration (native T1 and extracellular volume mapping). Studies performed in the acute phase suggested concomitant increase of native T1 and T2 secondary to widespread edema [116, 117, 118]. High T2 values were related to lower acute LVEF [119] and delayed recovery along with native T1 [120]. At three months, persisting abnormalities can be detected [120, 121], while also in the long-term (more than one year after the acute event) native T1 has been described as persistently elevated [65] suggesting long lasting effect of the TTS event. However, native T1 is a very sensible imaging biomarker, influenced by a variety of factors including edema and, among others, previous chemotherapy [122], chronic kidney disease [123], arterial hyertension [124]. Hence, the presence of higher than normal native T1 values even before the TTS attack cannot be excluded in such a comorbid population. Fig. 4 shows CMR imaging findings of a TTS patient two weeks after the acute event.
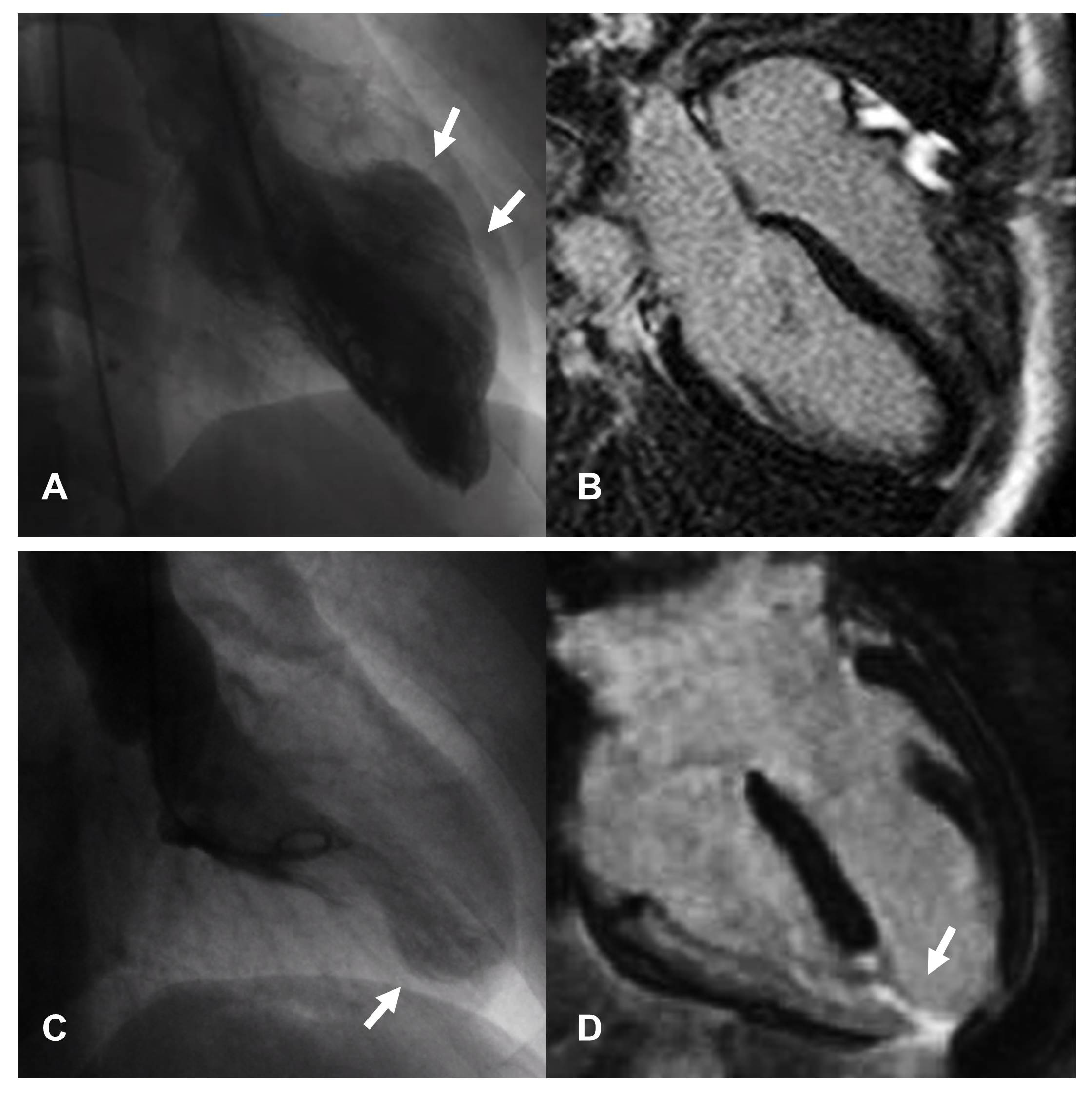 Fig. 3.
Fig. 3.Images of two female patients hospitalized for chest pain arising after emotional stress. In both cases, coronary angiography documented the absence of significant coronary artery disease and ventriculography shows patterns attributable to atypical forms of TTS, with focal involvement of the middle segments of septum and anterior wall (A) and part of the apex (C). CMR performed in the first patient, shows absence of replacement fibrosis (B) confirming the diagnosis of TTS. In the second patient, CMR documents the presence of transmural necrosis, corresponding to the area of kinetic alteration and probable consequence of a vasospasm (D); therefore excluding the diagnosis of TTS. From L. Arcari; L. Cacciotti; L. R. Limite; M. Sclafani; I. Passaseo; G. Marazzi et al. Tako-Tsubo syndrome: long term prognosis and outpatient follow-up. Outpatient Cardiology. 2018; 4: 249–261 [72].
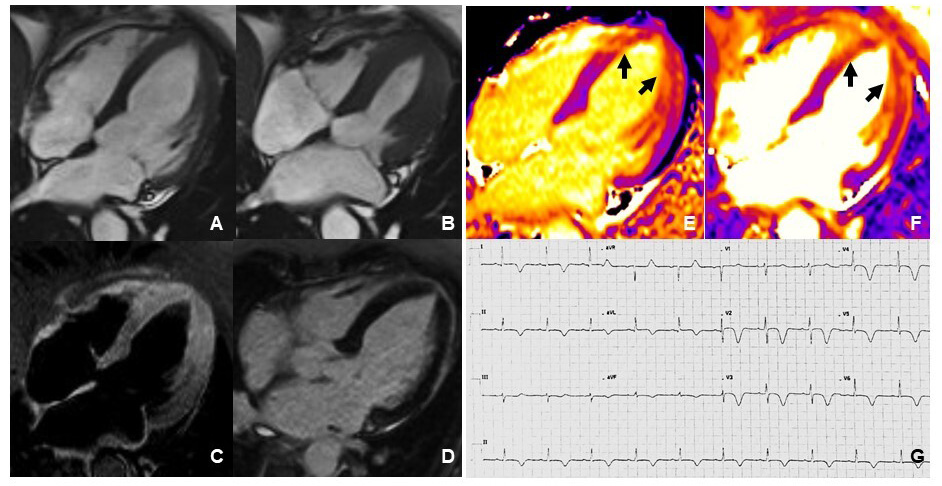 Fig. 4.
Fig. 4.Cardiovascular Magnetic Resonance imaging and ECG of a TTS patient performed 2 weeks after the acute event. Frames taken from cine sequences (balanced steady-state free-precession) showing normal kinesis of the left ventricle, end-diastole (A) and end-systole (B). T2-STIR imaging shows increased signal intensity consistent with myocardial edema at mid-apical left ventricular site (C). Late gadolinium enhancement imaging revealed absence of replacement fibrosis (D). Native T1 mapping imaging showed increased signal intensity at mid-apical left ventricular site (arrows in E) paralleling increased signal detected by T2 mapping imaging (arrows in F). ECG showed diffuse T-wave inversion (G).
CPET ensures a dynamic, non-invasive assessment of the cardiopulmonary system at rest and during exercise. It may be a useful tool in patients with previous TTS and persistent symptoms to objectify functional capacity and persisting cardiac limitation on exercise. In a population of symptomatic patients with previous TTS, CPET demonstrated a lower VO2 peak and a higher VE/VCO2 slope even more than one year after the acute event [65]. Both peak VO2 and VE/VCO2 slope are predictors of cardiovascular morbidity and mortality in heart failure of several etiologies [125]. Despite their normal LVEF, these metabolic characteristics are strongly suggestive of a heart failure phenotype [65]. An impaired autonomic function may be present in TTS patients long after the acute event. On this regard, a lower heart rate recovery during the recovery phase of the cardiopulmonary test demonstrates a blunted parasympathetic reactivation after exercise [126].
Coronary computed tomography (CT) plays an important role in the diagnostic workup of patients with acute chest pain and doubtful TTS diagnosis to rule-out other conditions such as AMI, pulmonary embolism and aortic dissection [77, 127], also allowing a more accurate detection of coronary artery anomaly, such as myocardial bridging, quite common in TTS [109, 128]. Compared with CMR, cardiac CT can be readily used in the emergency setting due to its accessibility and fast acquisition time and also provides myocardial characterization with late iodine enhancement imaging comparable to that obtained from CMR [129]. However, the role of cardiac CT in long-term follow-up of TTS patients is less well defined. Cardiac CT may be considered in some peculiar situations: such as in patients with previous TTS and new onset of stable anginal symptoms with a low clinical likelihood of new CAD [130], patients with a history of TTS and suspected recurrence, patients with low likelihood of CAD and clinical conditions usually associated with TTS where coronary angiography is highly likely to cause complications (e.g., terminal malignancy and advanced age with frailty [131]).
Table 2 summarizes the clinical and instrumental “red flags” in long-term follow-up of TTS patients.
| Clinical evaluation | |
| Serum biomarkers | |
| ECG | |
| 24-hour ECG monitoring | |
| Echocardiography | |
| CMR | |
| CPET | |
| BNP, B-type natriuretic peptide; ECG, electrocardiogram; QTc, QT corrected; LV, Left Ventricular; GLS, Global Longitudinal Strain; CMR, Cardiac Magnetic Resonance; CPET, Cardiopulmonary exercise testing. | |
Guidelines on the acute and chronic therapeutic management of TTS are lacking since no prospective randomized clinical trials have been performed on this patients. Therapeutic strategies are therefore based on clinical experience, expert consensus statements and on retrospective studies or meta-analyses [22, 77].
The clinical presentation of TTS is similar to that of ACS, therefore ACS guidelines should be followed until any possible culprit coronary stenosis has been ruled out [22]. This involves the administration of the dual antiplatelet therapy (DAPT), anticoagulant therapy and, when indicated, the execution of an invasive coronary angiography [106, 132]. The most recent consensus documents recommend the interruption of the DAPT once the presence of significant CAD has been excluded [22, 77]. However, in real-world practice, DAPT is used in the early period after the acute event [133], mostly relying on the pathogenetic hypotheses of a prothrombotic state induced by high levels of catecholamines [134, 135].
Anticoagulant therapy may be necessary in TTS patients for treatment or prophylaxis of ventricular thrombosis, especially in presence of risk factors such as the apical ballooning pattern and high troponin levels [136]. We recommend an early outpatient visit, within the first month after discharge, to evaluate the complete LV contractility recovery and the absence of ventricular thrombosis and to suspend unnecessary anticoagulation therapy.
Heart failure medications including beta-blockers and inhibitors of the renin-angiotensin system are indicated in TTS patients with reduced LVEF. When LVEF has recovered, weaning from the inhibitors of the renin-angiotensin system and beta-blocker might be considered in the absence of other indications [22]. Although catecholamines play an important pathophysiological role in TTS [22], beta-blocker therapy after hospital discharge does not appear to provide a survival benefit [6] or prevent recurrences [54], though were associated with lower mortality in patients with TTS complicated by CS and successfully recovered [15]. Conversely, the use of ACEIs or ARBs was associated with improved long-term survival [6, 53] and with a lower prevalence of recurrences in some [52] but not in other studies [48]. The optimal medical therapy to prevent recurrence still remains elusive.
Angiotensin receptor II blocker-neprilysin inhibitor (ARNI) has an established role in treatment of patients with heart failure with reduced ejection fraction (EF) [137]. The use of ARNI in TTS was reported in a case report and it may be reasonable to test it in the acute/subacute phase of the disease, when LVEF is impaired [138].
Diuretics are often prescribed in TTS patients at the time of discharge from hospital but, when LV function has completely recovered, they may no longer be needed and should be stopped in order to avoid electrolyte imbalances, especially in old patients or in patients with marked QTc-interval prolongation.
Considering the high prevalence of psychiatric diseases in TTS patients [6], great attention must be paid to any concomitant psychiatric drug therapy to avoid drug-induced QTc-interval prolongation during the subacute phase.
Finally, targeted therapeutic strategies for TTS may be delevoped in the next future. In this regard, a multi-centre randomised trial is underway, sequentially testing early use of intravenous N-acetylcysteine, followed by/or oral ramipril for 12 weeks [139].
The precise pathophysiological mechanisms of TTS are incompletely understood but there is considerable evidence that sympathetic stimulation is central to its pathogenesis, thus an underlying link between the brain and heart has long been proposed [1]. Specifically, stress can activate the sympathetic nervous system and lead to a complex pathophysiologic cascade including catecholamine toxicity, abnormal myocardial perfusion, myocardial stunning, and endothelial dysfunction [1]. Several neuroimaging studies demonstrated structural and functional alterations in stress-related brain networks of TTS patients during the acute phase [140], during follow-up [141, 142, 143] and even before the acute event [144]. These findings suggest long-lasting psychological stress in TTS patients which increases the risk of recurrence. Therefore non-pharmacological behavioural therapy (i.e., psychotherapy, counseling, and stress reduction approaches) are reasonable and viable options for patients that experienced TTS due to emotional triggers to reduce the risk of recurrence [145].
TTS is a clinical entity characterized by substantial long-term morbidity and mortality. In the early months after the acute event, cardiologic evaluations are essential to confirm the diagnosis, to document regression of LV systolic abnormalities and to reassess drug therapy. Long-term outpatient follow-up should aim at recognition and treatment of associated diseases, often not cardiovascular ones, which may have a greater impact on prognosis.
MS, LA, CA and MBM conceived of the presented idea. MS, LA, DR, GT and LRL performed the research. LC, MV, CA and MBM supervised the project. MS, LA, DR and GT contributed to the writing of the manuscript. All authors provided critical feedback and contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
We would like to express our gratitude to all those who helped us in writing this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
