Academic Editor: Brian Tomlinson
The role of lipocalin 2 (LCN2) in pulmonary hypertension (PH) in pediatric
patients with congenital heart disease (CHD) remains unclear. We sought to
investigate whether LCN2 could be a potential biomarker for PH in pediatric
patients who underwent surgery for CHD. From December 2018 to February 2020,
patients undergoing surgical repair for congenital defects with and without PH
were identified. Healthy children without CHD and PH served as controls. A mean
pulmonary artery pressure (mPAP)
Pulmonary hypertension (PH) is associated with poor prognosis in children with congenital heart disease (CHD) [1, 2]. Neurohormonal activation and endothelial dysfunction have been identified as important mechanisms in PH associated with CHD [3, 4]. Prior studies have observed that N-Terminal pro Brain Natriuretic Peptide (NT-proBNP) and high-sensitive troponin T (hsTnT) can be used to assess risk in patients with PH [5, 6, 7, 8]. However, there is limited data regarding early prediction of PH in children with CHD [9].
Lipocalin 2 (LCN2), also known as neutrophil gelatinase-associated lipocalin (NGAL, 25kDa), forms part of the lipocalin superfamily. It is involved in several patho-physiological processes, such as inflammation, epithelial mesenchymal transition and angiogenesis [10, 11, 12]. The level of LCN2 has been used to assess the extent of kidney tissue damage following pediatric cardiac surgery [13, 14]. Recent work using a rat model suggests that LCN2 may be linked to PH [15, 16]. Therefore, LCN2 may be a useful biomarker for early detection of PH in pediatric cases with CHD [9, 17]. In the present study, our aim was to assess the expression levels of LCN2, NT-proBNP and hsTnT in children with PH associated with CHD, and thus determine their ability to predict PH in pediatric patients undergoing surgery for CHD.
From December 2018 to February 2020, patients admitted for surgical repair of
CHD were prospectively identified at Beijing Children’s Hospital. Their ages
ranged from 6 to 36 months. Patients with acute infection, sepsis, or renal
failure were excluded, as well as those taking cardiotoxic medication [10, 16].
For standardization of the study, patients with more than moderate post-tricuspid
shunts such as ventricular septal defect (VSD) and patent ductus arteriosus (PDA)
were included. The threshold value for the definition of PH was a mean pulmonary
artery pressure (mPAP) of
Echocardiography was conducted using a Philips iE33 ultrasound scanner (Philips Medical Systems, Amsterdam, Netherlands). Two-dimensional (2D) and Doppler echocardiography data were collected using 8 MHz or 3.5 MHz sector transducer and stored digitally on a QLAB work station for further analysis. The left ventricular ejection fraction (LVEF) was determined using the biplane method of disks (modified Simpson’s rule). Data were measured over 5 heart-beats by two blinded external reviewers according to current guidelines [19]. We calculated z-scores for right ventricular (RV) wall dimension and adjusted for patient age and sex according to standard reference values [20].
Cardiac catheterization prior to surgery was performed only in cases with CHD, and not in healthy controls. Hemodynamic measurements included pulmonary artery pressures, pulmonary capillary wedge pressure and right atrial pressure (RAP). The Fick equation was used to estimate systemic and pulmonary blood flows, while pulmonary vascular resistance was estimated using the standard equation of mean atrial pressure divided by flow. Cardiac catheterization was performed via a percutaneous femoral approach and low-dose midazolam and propofol were used for conscious sedation. Blood flow and vascular resistance were corrected for body surface area.
Blood samples for biomarkers were stored at –80
Continuous variables with normal distribution were expressed as the mean
(standard deviation [SD]). Non-normal variables were expressed as the median
(interquartile range [IQR]). Categorical variables were expressed as numbers and
percentages. Differences between groups were evaluated using one-way analysis of
variance (ANOVA) for continuous variables and Kruskal-Wallis test for categorical
variables. Pearson’s correlation test was used to evaluate correlations between
variables. Possible predictors of PH were identified by univariate logistic
regression. Statistically significant (P
A total of 102 patients (median age 10 [IQR 7.0–13.0] months) comprised the study cohort, of which 37.5% were female. Fifty-four patients had PH because of a left to right shunt, while 25 patients had a left to right shunt without PH. Twenty-three healthy children served as controls.
Table 1 shows the clinical characteristics for this study cohort. No significant
differences in age, gender, body surface area, history of AKI, creatinine and
urea nitrogen were observed between the three groups. The PH group had higher
levels of LCN2 (9.2 [5.9, 12.3] vs. 6.2 [5.3, 8.9] vs. 5.3 [4.6, 6.6], P
| Controls (n = 23) | Non-PH group (n = 25) | PH group (n = 54) | P value | |
| Age, months, median (IQR) | 11 (7.5, 12) | 10 (6, 12) | 11 (7, 14) | 0.31 |
| Female, sex, n (%) | 7 (30.4%) | 7 (28.0%) | 17 (31.5%) | 0.17 |
| Body surface area, m |
0.43 |
0.42 |
0.41 |
0.23 |
| Symptomatic, n (%) | - | 4 (16.1%) | 19 (35.2%) | 0.01 |
| History of AKI, n (%) | - | 1 (4%) | 2 (3.7%) | 0.16 |
| Creatinine, mg/dL | 29.9 |
29.6 |
31.4 |
0.16 |
| Urea nitrogen, mmol/L | 3.5 |
3.7 |
4.0 |
0.27 |
| NT-proBNP, ng/L, median (IQR) | 213 (151, 231) | 445 (369, 508) | 569 (431, 842) | 0.006 |
| HsTnT, ng/L, median (IQR) | 0.012 (0.008, 0.014) | 0.017 (0.012, 0.022) | 0.020 (0.014, 0.038) | 0.007 |
| LCN2, pg/mL, median (IQR) | 5.3 (4.6, 6.6) | 6.2 (5.3, 8.9) | 9.2 (5.9, 12.3) | |
| PH, pulmonary hypertension; IQR, interquartile range; AKI, acute kidney injury; NT-proBNP, N-Terminal pro Brain Natriuretic Peptide; hsTnT, high-sensitive cardiac troponin T; LCN2, lipocanlin 2. | ||||
The echocardiographic and hemodynamic data are shown in Table 2. No significant
differences in LVEF and RV wall dimension were seen between the three groups
(P
| Controls (n = 23) | Non-PH group (n = 25) | PH group (n = 54) | P value | ||
| Echocardiographic data | |||||
| LVEF, % | 70 |
71 |
69 |
0.21 | |
| RV wall dimension, mm | 2.8 |
3.0 |
3.2 |
0.09 | |
| RV wall dimension z-score | −0.66 |
−0.31 |
0.65 |
0.01 | |
| PH associated with PDA, n (%) | - | 7 (28.0%) | 16 (29.6%) | 0.13 | |
| PH associated with VSD, n (%) | - | 18 (72.0%) | 32 (70.4%) | 0.16 | |
| Haemodynamic data | |||||
| Mean RAP, mmHg | - | 4.1 |
6.1 |
0.001 | |
| PASP, mmHg | - | 26.8 |
46.2 |
0.001 | |
| Mean PAP, mmHg | - | 16.4 |
31.7 |
0.001 | |
| PVR index, WU × m |
- | 2.3 |
4.9 |
0.001 | |
| Qp/Qs | - | 1.36 |
1.86 |
0.02 | |
| PH, pulmonary hypertension; LVEF, Left ventricular ejection fraction; RV, right ventricular; PDA, patent ductus arteriosus; VSD, ventricular septal defect; RAP, right atrial pressure; PASP, pulmonary artery systolic pressure; PAP, pulmonary artery pressure; PVR, pulmonary vascular resistance; WU, Wood units; Qp, pulmonary flow; Qs, systemic flow. | |||||
The correlation of echocardiographic and hemodynamic data with biomarker levels
are summarized in Table 3. LCN2 was significantly correlated with mean RAP (r =
0.572, P
| LCN2 | hsTnT | NT-proBNP | ||||
| r value | P value | r value | P value | r value | P value | |
| LVEF | −0.126 | 0.15 | −0.134 | 0.13 | −0.107 | 0.23 |
| RV wall dimension z-score | 0.247 | 0.01 | 0.126 | 0.12 | 0.146 | 0.10 |
| Mean RAP, mmHg | 0.572 | 0.001 | 0.156 | 0.09 | 0.278 | 0.01 |
| PASP, mmHg | 0.651 | 0.001 | 0.298 | 0.03 | 0.304 | 0.01 |
| Mean PAP, mmHg | 0.689 | 0.001 | 0.265 | 0.02 | 0.292 | 0.01 |
| PVR index, WU × m |
0.256 | 0.03 | 0.287 | 0.01 | 0.239 | 0.03 |
| Qp/Qs | 0.297 | 0.01 | 0.129 | 0.15 | 0.273 | 0.02 |
| LCN2, Lipocalin 2; hsTnT, high-sensitive cardiac troponin T; NT-proBNP, N-Terminal pro Brain Natriuretic Peptide; LVEF, left ventricular ejection fraction; RV, right ventricular; RAP, right atrial pressure; PASP, pulmonary artery systolic pressure; PAP, pulmonary artery pressure; PVR, pulmonary vascular resistance; WU, Wood units; Qp, pulmonary flow; Qs, systemic flow. | ||||||
In univariate logistic regression, LCN2 (OR = 2.69 [1.06–5.31], P
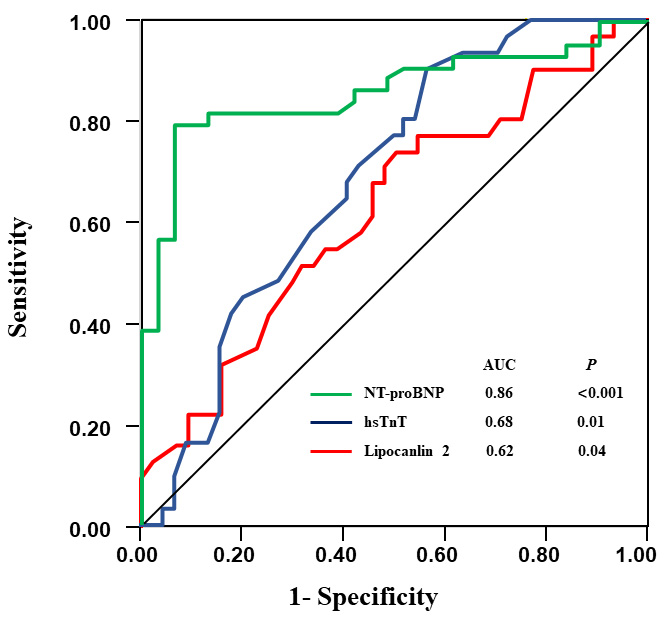 Fig. 1.
Fig. 1.Receiver operating curve (ROC) for biochemical markers in prediction of PH. PH, pulmonary hypertension; NT-proBNP, N-Terminal pro Brain Natriuretic Peptide; hsTnT, high-sensitive troponin T.
| Variables | Odds ratio (95% CI) | P value |
| Gender (Female) | 3.65 (0.89–16.3) | 0.68 |
| Age, months | 1.26 (0.91–2.38) | 0.12 |
| NT-proBNP | 1.91 (1.21–7.56) | 0.03 |
| Lipocanlin 2 | 2.69 (1.06–5.31) | |
| hsTnT | 1.36 (1.01–3.57) | 0.01 |
| RV wall dimension z-score | 1.19 (0.85–2.76) | 0.06 |
| NT-proBNP, N-terminal pro B-type natriuretic peptide; hsTnT, high-sensitive cardiac troponin T; RV, right ventricular. | ||
The three important findings from this study are: (1) pediatric patients with PH and associated CHD had higher levels of LCN2; (2) the LCN2 level was significantly correlated with invasive indices of PH; and (3) LCN2, hsTnT and NT-proBNP may all be predictors of PH, but only LCN2 was independently associated with PH in multivariate regression analysis.
Previous studies showed that LCN2 may play a role in the pathogenesis of PH in children with CHD. However, most of these studies focused on gene expression at the molecular level. The predictive value of LCN2 for PH in the clinical setting remains unknown [15, 21]. In this study, we found the concentration of LCN2 was greater in CHD patients with PH compared to those without PH. Takahashi and coworkers [16] found increased LCN2 expression in dermal microvascular endothelial cells and speculated this may contribute to PH progression in systemic sclerosis. Li et al. [22] found LCN2 to be the only gene significantly expressed in females with PH. In our study, increased expression of LCN2 was associated with PH and hence we postulate this may explain the increased incidence of pulmonary vascular disease associated with CHD. Another major result from our study was the positive correlation of LCN2 with invasive indices of PH such as mean PAP, PASP and PVR index. This contrasts with the findings of Takahashi and coworkers in systemic sclerosis patients with PH, who reported an inverse relation between LCN2 levels and RV systolic pressure in patients with normal renal function [16]. These discordant results could be due to the different etiologies and mechanisms in the development of PH.
Our results also showed significantly higher serum hsTnT levels in patients with PH compared to those without PH, as also noted by Kayali et al. [23]. Ventricular hypertrophy in response to higher ventricular pressure limits myocardial perfusion and increases oxygen demand, leading to a higher risk of myocardial injury and increased troponins [24]. In the present study, PH patients had a greater prevalence of RV hypertrophy compared to patients without PH. In the pediatric population, increased hsTnT levels may reflect underlying myocardial damage resulting from pressure or volume overload. Serum hsTnT may therefore be a useful predictor of myocardial injury in pediatric cases with PH due to CHD.
Earlier workers found that NT-proBNP could be used to assess risk in PH patients [3, 8]. It has previously been reported that NT-proBNP was associated with a worse outcome in PH children who underwent surgery for CHD [5, 7]. Our results agree with a previous study [23] that found significant correlations between NT-proBNP levels and hemodynamic indices of PH. Elevated NT-proBNP might be explained by the pathological stretching of cardiac myocytes as a result of volume and pressure overloading because of the left-to-right shunt. These findings suggest that serum NT-proBNP might serve as a useful biomarker to detect heightened wall tension resulting from increased pulmonary flow in pediatric patients with CHD-associated PH.
Previous animal and clinical studies showed that LCN2 has major roles in the remodeling of pulmonary arterioles and the progression of PH. However, the association between LCN2 and the development of PH has not been thoroughly investigated [16, 22]. Our results showed that LCN2 was independently associated with PH. This may be because LCN2 promotes stress of the endoplasmic reticulum by increasing the amount of intracellular iron in human pulmonary arterial smooth muscle cells [21]. Another explanation might be that LCN2 inhibits the apoptosis of pulmonary artery smooth muscle cells induced by oxidative stress via a decrease in intracellular ROS and elevation of SODs. A previous animal study found significantly elevated LCN2 levels in a rat PH model induced with monocrotaline [15]. These studies support our conclusion that pediatric CHD patients with higher levels of serum LCN2 have an increased risk of developing PH. The relationship between LCN2 and PH provides a novel and important insight into the clinical management of pediatric CHD patients with PH.
Our study has several limitations. First, the study population is small and from a single center. Secondly, heart catheterization is an invasive procedure and hence the relationship between LCN2 and hemodynamic measures could not be evaluated longitudinally. Finally, serial measurements of LCN2 levels were not performed. Further studies that focus on changes in LCN2 levels over time and on disease progression, as well as longer patient follow up periods should help to shed more light on the role of LCN2 in this population.
The current study demonstrated that children with PH have alterations in LCN2 expression and that LCN2 levels positively correlated with invasive indices of PH. LCN2 could help in predicting the presence of PH in pediatric CHD cases. Further studies that include changes in LCN2 levels over time, larger sample size and longer patient follow up are required to substantiate these findings.
HJZ: Conceptualization, methodology, writing-original draft and funding acquisition. TS: Conceptualization, data curation and formal analysis. JY: Methodology, resources and software. YS: Resources and validation and visualization. GWL: Investigation, validation and formal analysis. CK: Methodology, validation and formal analysis. EAEA: Conceptualization, writing-reviewing and editing. RGBC: Methodology and writing-editing. LYX: Investigation and visualization. ZKY: Resources, software and validation. ZQL: Resources, writing-reviewing and validation. NM: Conceptualization, methodology, writing-reviewing, editing and funding acquisition.
The research protocol was approved by the human research ethics committee of the Beijing Children’s Hospital (approval ID: IR.IEC-C-008-A08-V.03.2). Informed consent was obtained from the patient’s guardian(s). The study was performed in accordance with the Declaration of Helsinki.
The authors thank all the staff members who participated in data collection for their contribution to the current study.
This research was supported by the Natural Science Foundation of Beijing Municipality under Grant NO. 719062 and Grant NO. 7202046; the National Natural Science Foundation of China under Grant No. 81641071.
The authors declare no conflict of interest.
