† These authors contributed equally.
Academic Editor: Peter A. McCullough
We evaluated the lipidomic profile of patients with very high-risk
atherosclerotic cardiovascular disease (ASCVD) by ultra-performance liquid
chromatography-quadrupole time-of-flight mass spectrometry (UPLC-MS). A total of
64 patients with a very high risk of ASCVD were recruited and randomLy divided
into the atorvastatin group (20 mg, every night, 4 weeks) or the combined group
(evolocumab, 140 mg, once every 2 weeks on top of atorvastatin (20 mg per day)).
The level of serum lipids was detected before and after treatment for 4 weeks.
The lipid classes of triacylglycerols, cholesteryl esters, and sphingomyelins
were analyzed using an ultra-performance liquid chromatography-quadrupole
time-of-flight mass spectrometry system. There were 32 patients in each group.
After 4 weeks of treatment, the levels of total cholesterol (TC) and low-density
lipoprotein cholesterol (LDL-C) in both groups and the level of lipoprotein-a
(Lp-a) in the combined group were lower. In the combined treatment group, the
levels of TC, LDL-C, and Lp-a decreased significantly (P
Atherosclerotic cardiovascular disease (ASCVD) is still the leading cause of death worldwide [1]. Low-density lipoprotein cholesterol (LDL-C) is not only the main component of atherosclerotic plaque formation but also a crucial risk factor for ASCVD. The management of cardiovascular events in very-high-risk patients with ASCVD has always attracted much attention [2, 3]. There is no doubt that intensive lipid-lowering therapy can further reduce the incidence of cardiovascular events in patients with very-high-risk ASCVD.
Statins are considered to be the cornerstone of lipid-lowering therapy. Statins
are not only widely used but also have anti-inflammatory properties [4]. However,
the TNT study of 100,001 patients with stable coronary disease and LDL-C
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been a significant breakthrough in the field of cardiovascular lipid-lowering therapy in recent years [7, 8]. The results of the FOURIER study confirmed the role of PCSK9 inhibitors in the prevention of cardiovascular events on a background of statin therapy [9]. The FOURIER study, which involved 27,564 patients, showed that the use of a PCSK9 inhibitor reduced the level of LDL-C, the risk of cardiovascular events, the risk of myocardial infarction, and the risk of stroke within 2 years [9].
To date, the attainment rate of LDL-C goals in very-high-risk ASCVD populations remains elusive in many countries and needs to be improved [10]. However, the therapeutic effect of PCSK9 inhibitors on the blood lipid profile and tolerance in very-high-risk ASCVD populations remain unclear. In this study, a randomized controlled trial was conducted to investigate the effects and adverse reactions of PCSK9 inhibitors on the blood lipid profile and adverse reactions in ASCVD patients at very high risk.
This two-center study was conducted from April 2019 to June 2020. ASCVD patients
at very high risk in the Department of Cardiology, Tianjin Chest Hospital and the
Department of Cardiology, Tianjin Hospital were recruited. Very-high-risk ASCVD
patients who met the criteria of the guidelines on the management of blood
cholesterol of the American Heart Association/American Heart Association
(AHA/ACC) [2] were enrolled. Very high risk was defined by a history of multiple
(
To explore the therapeutic effect of PCSK9 inhibitors on the lipidomic profile of those patients, we compared the alterations in blood lipid composition between patients treated with atorvastatin alone and patients treated with PCSK9 inhibitors on top of statins. Patients were randomly divided into the combined group and the atorvastatin group according to the envelope method. A random grouping scheme within a sealed opaque envelope was opened by the researchers according to the sequence of patients, and the patients were divided into groups according to the scheme within the envelope.
Atorvastatin was given 20 mg orally once a night for 4 weeks in the atorvastatin group. Evolocumab injection (Amgen Manufacturing Limited, USA) was used (140 mg, every 2 weeks) in the combined group for 4 weeks on top of atorvastatin (20 mg per day). All patients received lipid-lowering therapy for the first time. Standard secondary prevention of ASCVD, such as antiplatelet therapy, was provided equally in both groups [2, 3]. Fig. 1 shows the flowchart of this study.
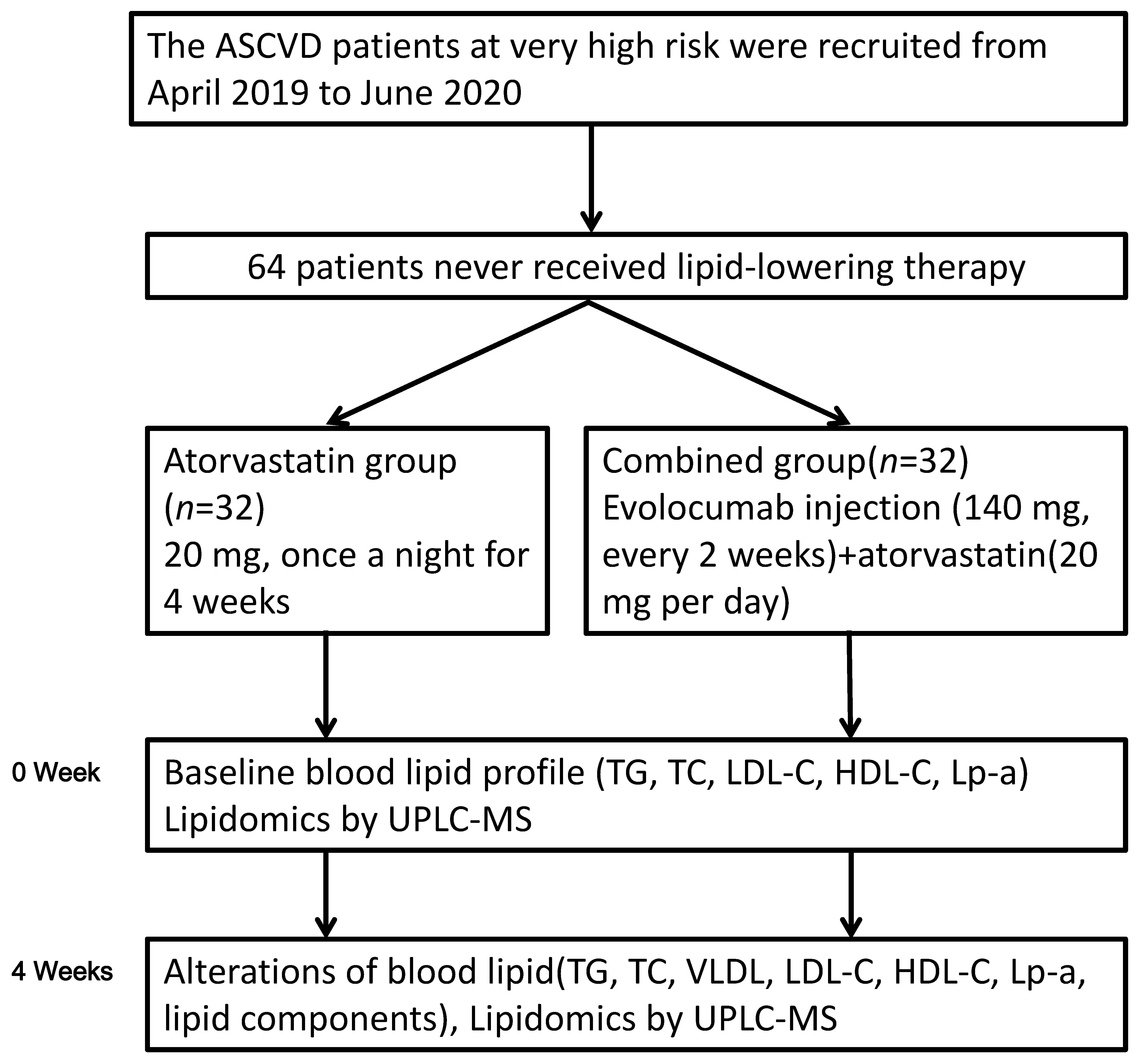 Fig. 1.
Fig. 1.Flowchart of this study. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp-a, lipoprotein-a; TC, total cholesterol; TG, triglycerides; UPLC-MS, ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry.
The serum of all patients was collected before treatment and 4 weeks after treatment in each group. Participants were required to avoid consuming high-fat, high-protein food and alcohol at the last meal before examination. After 12 hours of fasting, forearm venous blood was drawn for assessment. The laboratory results of triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C, and lipoprotein-a (Lp-a) were recorded after each assessment. Adverse reactions during drug treatment were carefully observed in all patients.
Serum was pretreated with a methyl tert butyl ether (MTBE)-methanol extraction system. To evaluate the stability of the determination process, quality control samples were inserted into the analysis sequence. The lipid classes of triacylglycerols (TAG), cholesteryl esters (CE), and sphingomyelins (SM) were analyzed using ultra-performance liquid chromatography (UPLC) (Waters Corp., Milford, MA, USA)-quadrupole time-of-flight mass spectrometry (MS) (TripleTOF 5600, AB Sciex, Framingham, MA, USA) system [11].
The prepared samples were chromatographed on an Acquity UPLC HSS C18 (2.1 mm
SIMCA-P version 13.0 (Umetrics, Umea, Sweden) was used for multivariate
statistical analysis. An orthogonal partial least squares discriminant analysis
(OPLS-DA) model was constructed, and then the data were summarized, classified
and discriminantly analyzed. The reliability of the model was tested by 7
cross-validations. According to the OPLS-DA model, the variable importance in
projection (VIP) was obtained, and the metabolites with significant changes in
serum were preliminarily screened. A combined VIP value
A total of 64 patients were included. All patients in the atorvastatin group
(n = 32) and the combined group (n = 32) completed a 4-week
treatment without loss of follow-up. The baseline patient characteristics of the
two groups are shown in Table 1. There was no significant difference in the
baseline data of age, BMI, underlying diseases, or the proportion of percutaneous
coronary intervention (PCI) between the two groups (P
| Characteristics | Atorvastatin group | Combination group | t/ |
P value |
| n | 32 | 32 | - | - |
| Male n (%) | 14 (43.75) | 16 (50.00) | 0.251 | 0.616 |
| Age (y) | 57.38 ± 13.63 | 58.42 ± 14.61 | 0.294 | 0.769 |
| BMI (kg/m |
28.44 ± 3.47 | 28.52 ± 3.61 | 0.088 | 0.931 |
| Current smoking n (%) | 3 (9.38) | 4 (12.50) | 0.160 | 0.689 |
| Hypertension n (%) | 16 (50.00) | 17 (53.13) | 0.063 | 0.802 |
| Myocardial infarction n (%) | 9 (28.13) | 8 (25.00) | 0.080 | 0.777 |
| PCI n (%) | 17 (53.13) | 18 (56.25) | 0.063 | 0.802 |
There was no significant difference in blood lipidomic profile between the two
groups before treatment. After 4 weeks of treatment, the levels of LDL-C in both
groups were significantly lower than before (all P
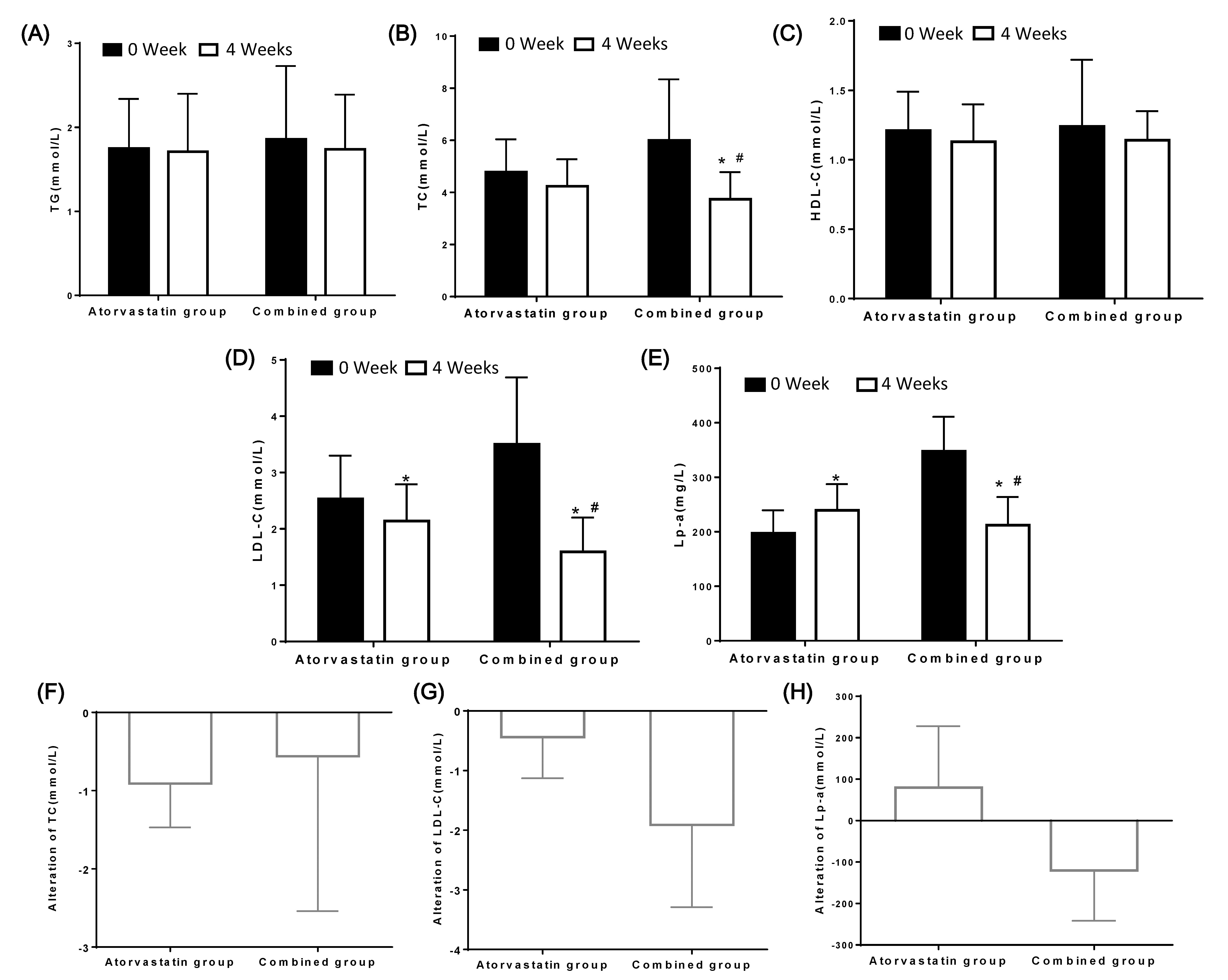 Fig. 2.
Fig. 2.The changes of lipidomic profile in ASCVD patients with a very
high-risk before and after treatment in the two groups. The levels of TG (A), TC
(B), HDL-C (C), LDL-C (D), Lp-a (E) were compared. The alteration of TC (F),
LDL-C (G), Lp-a (H) in two group. *: P
After 4 weeks of treatment, the level of LDL-C decreased to less than 1.4 mmol/L
in 16 patients in the combined group [3]. The rate of reaching the LDL-C standard
was 50.0%. However, only 7 patients treated with atorvastatin had an LDL-C level
of 1.4 mmol/L or less after 4 weeks of treatment. The rate of reaching the
standard was 21.8%, which was significantly lower than that in the combined
group (
Principal component analysis (PCA) was used to analyze the changes in all lipid classes in serum before and after treatment in each group. The principal component scores were analyzed by cluster analysis. The score chart of each sample data reflecting the dispersion degree between the two groups was obtained (Fig. 3A,B). Before treatment, the distance between the control group and the statin group was very small, and it was difficult to distinguish between the two groups (Fig. 3A). This finding means that there was no significant difference in the principal component score between the two groups before treatment. However, after 4 weeks of treatment, there was a significant difference in lipid metabolites between the combined group and the atorvastatin group. The cluster analysis of the principal component scores of the two groups was obviously separated into two parts (Fig. 3B).
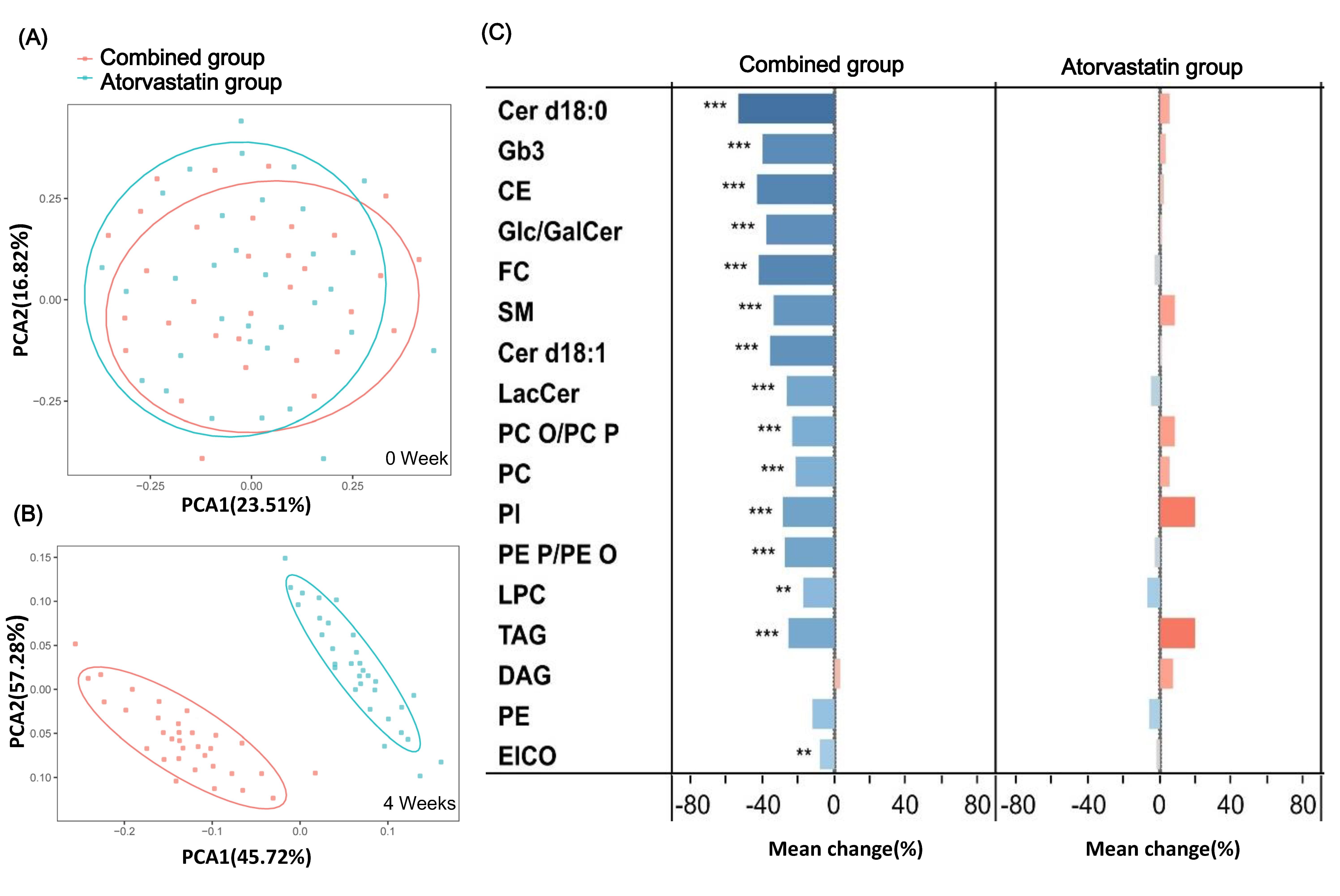 Fig. 3.
Fig. 3.Lipid classes were identified in plasma samples by
ultra-performance liquid chromatography-quadrupole time-of-flight mass
spectrometry before and after the two groups. (A) Principal component analysis
of metabolites at baseline in two groups. There was no significant difference in
clustering. (B) Principal component analysis of metabolites after 4 weeks of
treatment and the clustering separation was obviously. (C) The change trend of
lipid classification in the two groups. *: P
Next, the changes in different lipid metabolism pathways were further analyzed. After 4 weeks of treatment with PCSK9 inhibitors, most of the lipid classes in plasma decreased. In the combined group, sphingolipids were the most reduced lipids, including dihydroceramide (Cer d18: 0), ceramides (Cer d18: 1), glucosyl/galactosylceramides (Glc/GalCer), globular trisaccharide ceramide (Gb3), sphingomyelins (SM), and lactosylceramides (LacCer). Significantly decreased levels of cholesterol esters (CE), free cholesterol (FC), phospholipids, triacylglyceride (TAG), and diacylglycerol (DAG) were also found, as shown in Fig. 3C and Supplementary Table 3.
During the course of 4 weeks of treatment, only one patient had an adverse drug reaction (a rash) in the combined group, which improved after anti-allergic treatment. There were no other adverse reactions, such as abnormal liver function or increased muscle enzymes, in either group.
In the present study, the lipid-lowering effect of PCSK9 inhibitors on
very-high-risk patients with ASCVD was investigated. Not only were the short-term
effects of PCSK9 inhibitors on lipidomic profile analyzed, but specific changes
in the lipidomic profile were also identified by UPLC-MS. We found that PCSK9
inhibitors on top of atorvastatin can effectively reduce the levels of TC, TG,
LDL-C, and Lp-a in patients with very-high-risk ASCVD. PCSK9 inhibitors can
further reduce residual blood lipids (ultimately be
Very-high-risk ASCVD was defined for the first time in the 2018 AHA/ACC Cholesterol Management Guide [2]. LDL-C and Lp-a are independent risk factors for ASCVD [12]. At present, intensive lipid regulation is the key treatment to improve the prognosis of ASCVD. The importance of LDL-C level control in the secondary prevention of ASCVD patients has been increasingly emphasized. The 2018 AHA/ACC Cholesterol Management Guide states that the LDL-C level of very-high-risk ASCVD patients should be below 1.8 mmol/L (70 mg/dL) [2]. Accoding to 2019 ESC/EAS Guidelines for the management of dyslipidaemias, for patients with very high risk, regardless of primary prevention or secondary prevention, the LDL-C goal should be less than 1.4 mmol/L [3].
At present, a number of studies have proven that PCSK9 inhibitors can effectively reduce the level of LDL-C [9, 13, 14, 15] and have been recommended by diverse guidelines [2, 3]. The new intensive lipid regulation regimen based on PCSK9 inhibitors is an important means and direction to improve the prognosis of very-high-risk ASCVD patients. However, in China, the application of PCSK9 inhibitors is still in the early stage of exploration. To the best of our knowledge, this is the first study on the effect of PCSK9 inhibitors on the blood lipidomic profile of patients with very-high-risk ASCVD in China.
After 4 weeks of treatment with the PCSK9 inhibitor evolocumab supplementary to
oral atorvastatin, the levels of TC, LDL-C, and Lp-a were significantly lower
than those before treatment. Although the levels of TG and HDL-C in the combined
group had a downward trend, the changes were not significant. These findings
suggest that PCSK9 inhibitors can effectively reduce the level of cholesterol,
except for HDL-C, in patients with very-high-risk ASCVD. Although the level of
LDL-C decreased in the combined group, the decrease was smaller than that
reported in other studies [16]. This difference may be related to the shorter
treatment time in our study. In the postanalysis of the FOURIER phase III study
[16], LDL-C decreased by approximately 59% at 48 weeks after treatment in the
evolocumab injection group (95% CI: 58%–60%, P
At present, there are many kinds of drugs that can reduce LDL-C, and they are widely used in the clinic, but drugs that can effectively reduce Lp-a are still in the pipeline. Although this study confirmed that PCSK9 inhibitors can reduce the level of Lp-a, the mechanism is not completely clear [17, 18, 19].
After 4 weeks of treatment, the level of Lp-a decreased by approximately 21.5% in the combined group, which was similar to that reported in other studies [9, 13, 15]. Stiekema et al. [20] studied the effect of intensive lipid regulation, including PCSK9 inhibitors. The authors found that although LDL-C decreased significantly, the decrease in Lp-a was small. In patients with normal Lp-a levels, the level of LDL-C decreased by approximately 60%, and the level of Lp-a was reduced by approximately 20% to 30%. Interestingly, it is suggested that the reduction in Lp-a by PCSK9 inhibitors depends on the baseline level of Lp-a. The higher the baseline level is, the smaller the decrease is [3].
The attainment of the LDL-C target is the key to affecting the prognosis of patients with very-high-risk ASCVD. In this study, after 4 weeks of PCSK9 inhibitor administration, 50% of patients met the target LDL-C level in the combined group, which was higher than that in the atorvastatin group (23.3%). This finding also indicates that PCSK9 inhibitors can not only reduce the level of LDL-C but also decrease LDL-C faster. Earlier attainment of the target means an earlier benefit for patients.
We also found that evolocumab has little effect on TG while lowering cholesterol. In addition, there was no significant change in HDL-C levels before and after the administration of the PCSK9 inhibitor. There is still a large debate on the relationship between HDL-C and cardiovascular disease, which tends to be “U-shaped”; that is, high or low levels of HDL-C may increase the risk of cardiovascular disease [21, 22]. In this study, only one patient in the combined group had an allergic reaction, which manifested as a rash. After oral anti-allergic drugs were administered, the allergic reaction was mild. Thus, the PCSK9 inhibitor was safe. As reported, the main adverse drug reactions to PCSK9 inhibitors were nasopharyngitis, upper respiratory tract infection, influenza, and back pain, and the incidence rate was less than 2% [13]. However, there was no significant difference in the occurrence of adverse drug reactions between PCSK9 inhibitors and statins, which also shows that the use of PCSK9 inhibitors is safe [23].
There are also some limitations in this study. First, this study is a double-center study with a small number of selected patients. Second, the impact of PCSK9 and atorvastatin on individual lipid species within each lipid class was not analyzed. Third, PCSK9-related genes, which may have some influence on the therapeutic effect of PCSK9 inhibitors, were not detected. Finally, we studied only the short-term changes in the blood lipidomic profile. There is a lack of long-term follow-up data for treatment effects and long-term impacts on cardiovascular events.
In conclusion, the PCSK9 inhibitor evolocumab is efficacious and safe in treating very-high-risk ASCVD patients. The universality of the efficacy needs to be further confirmed, and more large-scale, multicenter, long-term, randomized trials need to be carried out.
ASCVD, Atherosclerotic cardiovascular disease; CE, cholesteryl esters; HDL-C, high-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; Lp-a, lipoprotein-a; MS, mass spectrometry; OPLS-DA, orthogonal partial least squares discriminant analysis; PCSK9, Proprotein convertase subtilisin/kexin type 9; SM, sphingomyelins; TAG, triacylglycerols; TC, total cholesterol; TG, triglyceride; UPLC, ultra-performance liquid chromatography.
All authors contributed significantly to this study. KH, XQW, and BG designed the trial. KH, XQW, NR, LY, and BG were conducted the work and involved in data collection. KH, XQW and BG analyzed the data. NR, LY, and BG interpreted the data. KH and XQW wrote the manuscript. All authors revised the manuscript.
The study was obtained permission from the Ethics Committee of the Tianjin Chest Hospital (Ethics approval number: 2019-00161). All treatments and assessments were performed after the informed consent of patients or their families was obtained.
We thank the anonymous reviewers for excellent criticism of the article.
This work was supported by the Key R & D projects of Tianjin (ZC20078).
The authors declare no conflict of interest.
