Academic Editor: Peter A. McCullough
Unibody bifurcated endografts have the advantage of reducing the operative time,
avoiding migration and iliac limb dislocation in patient with abdominal aortic
aneurysm (AAA). We report our long-term experience in patients who underwent
endovascular aortic repair (EVAR) due to infrarenal AAA with Endologix
AFX
Endovascular aneurysm repair (EVAR) has gained wide acceptance as the preferred method of treating aortic aneurysms with suitable anatomy. The technique is associated with lower early mortality, morbidity, faster discharge and patient turnover by offering small incisions with minimal invasive nature [1, 2, 3, 4]. The adverse events in the endovascular procedures may be due to endograft, challenging aortic anatomy or iatrogenic. Endografts are dynamically in evolution according to the early and late results of the randomized studies to reduce the adverse events related to structural impairments.
Unibody bifurcated endografts have the advantage of reducing the operative time
and avoiding iliac limb dislocation in the long term. The Endologix
AFX
United States Food and Drug Administration (FDA) approval was originally granted
for Endologix Powerlink system in 2004 [6], a modification to this device was
introduced in 2011 as the AFX endograft, which used a lower profile 17F delivery
system. A proximal and distal landing zone length of
Endoleaks are defined by persistent blood flow within the aneurysm sac intraoperatively or following EVAR. During the follow-up, in a meta-analysis, type II, III or IV endoleak could be seen in 13.7% of patients [10]. Late type III endoleak is an uncommon but serious complication caused by fabric tears, disruptions or junctional separation of the endograft components.
We report our long-term experience of patients who underwent endovascular
infrarenal abdominal aortic aneurysm electively with Endologix
AFX
In our Cardiovascular Surgery Department, during January 2013–December 2018, 68
patients with elective infrarenal abdominal aortic aneurysm had EVAR procedure
with Endologix AFX
The patients were predominantly American Society of Anesthesiologists (ASA) class II–IV, with a high prevalence of cardiopulmonary comorbidities. 30 patients (44.1%) were symptomatic because of the aneurysm, mostly umbilical pain. All patients had undergone EVAR procedure in elective manners.
Preoperatively each patient had a customized plan upon multislice contrast enhanced computerized tomography (CT) for aneurysm anatomy, dimensions, length and endograft sizes. Graft diameter was determined by oversizing the graft 10–20% with respect to measured neck diameter and anatomy. Graft length was chosen to preserve at least one hypogastric artery. Measurement of the AAA centerline or greater curvature length is currently preferred, not the traditional straight-line method, for EVAR planning purposes.
The reporting standards according to the guidelines from the Society for Vascular Surgery were used as indications [1]. Almost all procedures were performed with unilateral femoral artery exposure for main body and percutaneously for the aortic extension for access. EVAR was performed under general, local, or regional anesthesia in the hybrid room equipped with “Siemens Artees Zee” fluoroscopy. Access was carried out through an ipsilateral open exposure and contralateral percutaneous access for all patients. When performing aortic extension, at least 3 cm of overlap was performed in all patients. A completion angiogram was performed to document the status after endograft implantation. Technical success was defined a successful deployment of the endograft and completion of the procedure with no type I or III endoleaks and without the need for a secondary intervention or open repair within the first 24 hours.
Postoperative surveillance protocol included physical examination, blood samples
and imaging control with abdominal aortic Doppler and/or multislice contrast CT.
In the follow up period, all patients had a CT between the first three months and
after that all patients were evaluated with abdominal aortic Doppler ultrasound
every six-month period and CT annually. For patients with a shrinking aneurysm
sac and/or
The Endologix AFX
The variables were investigated using visual (histograms, probability plots) and
analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk test) to determine the
normality of their distribution. Normally distributed continuous variables were
expresses as mean
Baseline characteristics of patients are listed in Table 1. In our series, mean
age was 68.5
| Features | n (%) or mean | |
| Male gender | 63 (92.6) | |
| Age, years | 68.5 | |
| ASA grade | ||
| 2 | 25 (36.8) | |
| 3 | 29 (42.6) | |
| 4 | 14 (20.6) | |
| Comorbidities | ||
| Diabetes mellitus | 15 (22.1) | |
| Hypertension | 44 (64.7) | |
| Hyperlipidemia | 18 (26.5) | |
| Chronic obstructive pulmonary disease | 25 (36.8) | |
| Renal disease | 12 (17.6) | |
| Peripheral artery disease | 6 (8.8) | |
| Coronary artery disease | 37 (54.4) | |
| Heart failure | 6 (8.8) | |
| Smoking habit | 26 (38.2) | |
| History of cerebrovascular accident | 3 (4.4) | |
| Malignancy | 9 (13.2) | |
| Aneurysm diameter, mm | 60.4 | |
| Left ventricle ejection fraction, % | 52.0 | |
| SD, Standard deviation. | ||
| Features | n (%) or mean | |
| Endovascular aneurysm repair | 68 (100) | |
| Thoracic endovascular aneurysm repair | 2 (3) | |
| Iliac extension | 13 (19.1) | |
| Anesthesia | ||
| Local | 36 (52.9) | |
| Spinal | 4 (5.9) | |
| General | 28 (41.2) | |
| Embolectomy | 4 (5.9) | |
| Graft interposition | 4 (5.9) | |
| Mean intervention duration, minute | 132.4 | |
| Total fluoroscopy duration, minute | 17.2 | |
| Amount of contrast agent (mL) | 66.3 | |
| SD, Standard deviation. | ||
Mean follow-up was 45.5
Three patients with type III endoleak did not accept any intervention who were
ASA class IV (Fig. 1). These patients all died because of aneurysm rupture at
12–18 months after the diagnosis of type III endoleak. One patient referred to
hospital with ruptured aneurysm and died intraoperatively. Other two patients had
also symptomatic contained ruptured aneurysm and firstly endovascular
intervention was attempted, however both failed and, open surgical repair was
performed and discharged (Fig. 2). One of these patients experienced fabric tear
close to bifurcation after a blunt trauma [11]. The last patient was discharged
after successful endovascular intervention (Fig. 3). All type III endoleaks were
symptomatic and 5 patients were already diagnosed with CT. The other two patients
were referred to our outpatient clinic and type III endoleak was detected firstly
with CDUS and then confirmed by CT. The mean time interval between the primary
EVAR procedure and type III diagnosis was 24.7
 Fig. 1.
Fig. 1.Preoperative, 6th and 14th month CTA and first month overlap measurements of a type III endoleak of patient #4 (IFU compatible). Although there was an adequate overlap at first month scans, the separationand kinking of the endografts during the follow-up can be seen.
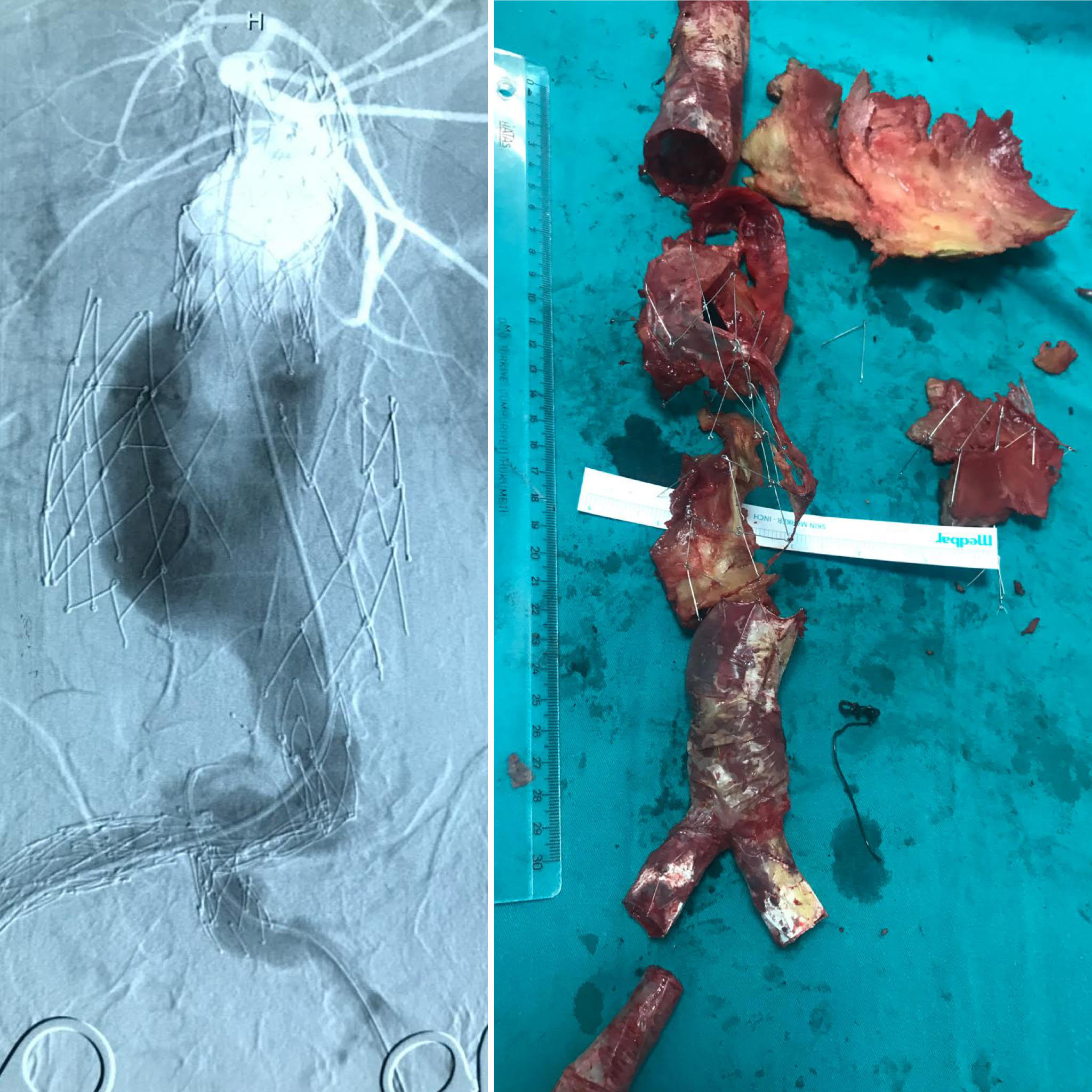 Fig. 2.
Fig. 2.The enlargement of the endoskeleton and the demonstrative main body deployment problem over the guidewire, the endovascular attempt converted to a successful open repair (patient #1). Structural deterioration and the separation of endografts can be seen on angiographic views. Endovascular rescue for this patient was unsuccessful and the extreme deterioration of the endograft is presented on the right side.
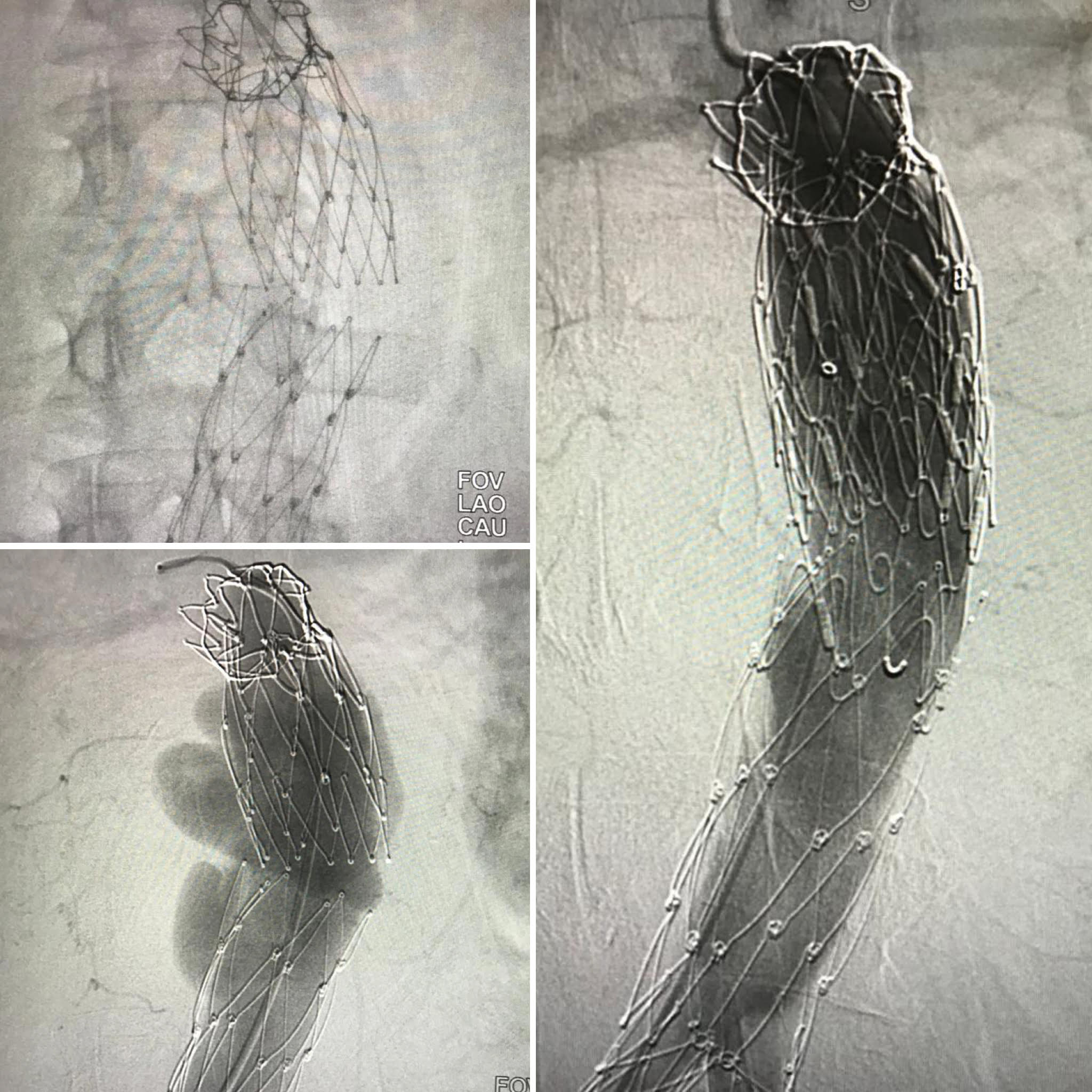 Fig. 3.
Fig. 3.Successful endovascular reintervention for type III endoleak (patient #5). The patient was admitted for ruptured aneurysm. The separation of the endografts and the endoleak between the endografts can be seen. A bridging tubular endograft was deployed between the previous endograft.
| Patient | IFU compatible | AA diameter (mm) | BSA | ASI | Outcome |
| 1 | Negative | 85 | 1.99 | 4.26 | Survived (OS) |
| 2 | Negative | 70 | 1.66 | 4.22 | Death (No intervention) |
| 3 | Positive | 60 | 1.66 | 3.6 | Death (No intervention) |
| 4 | Positive | 82 | 2.10 | 3.9 | Death (Rupture) |
| 5 | Positive | 67 | 1.94 | 3.5 | Survived (Endovascular) |
| 6 | Positive | 62 | 2.00 | 3.1 | Death (No intervention) |
| 7 | Negative | 72 | 2.00 | 3.6 | Survived (OS) |
| IFU, Instructions for use; AA, Aortic aneurysm diameter (initial); BSA, Body surface area; ASI, Aortic size index; OS, Open surgery. | |||||
There was no early mortality in the group and no conversion to open repair. Technical success was achieved in all patients. Overall survival estimated by Kaplan-Meier analysis was 94.1% at 1 year, 85.2% at 2 years, 74.1% at 3 years and 54.0% at 5 years (Fig. 4A). Freedom from second intervention and conversion was 98.4% at 1 year, 95.3% at 2 years, 93.3% at 3 years and 87.4% at 5 years (Fig. 4B). On the other hand, actual mortality was 36.8% at our patient cohort. Aneurysm related mortality occurred in six patients, and the other causes of death were cardiac problems in 12 patients, malignancies in six patients and renal failure in one patient.
No graft infection, migration and post-implantation syndrome were found during follow-up period.
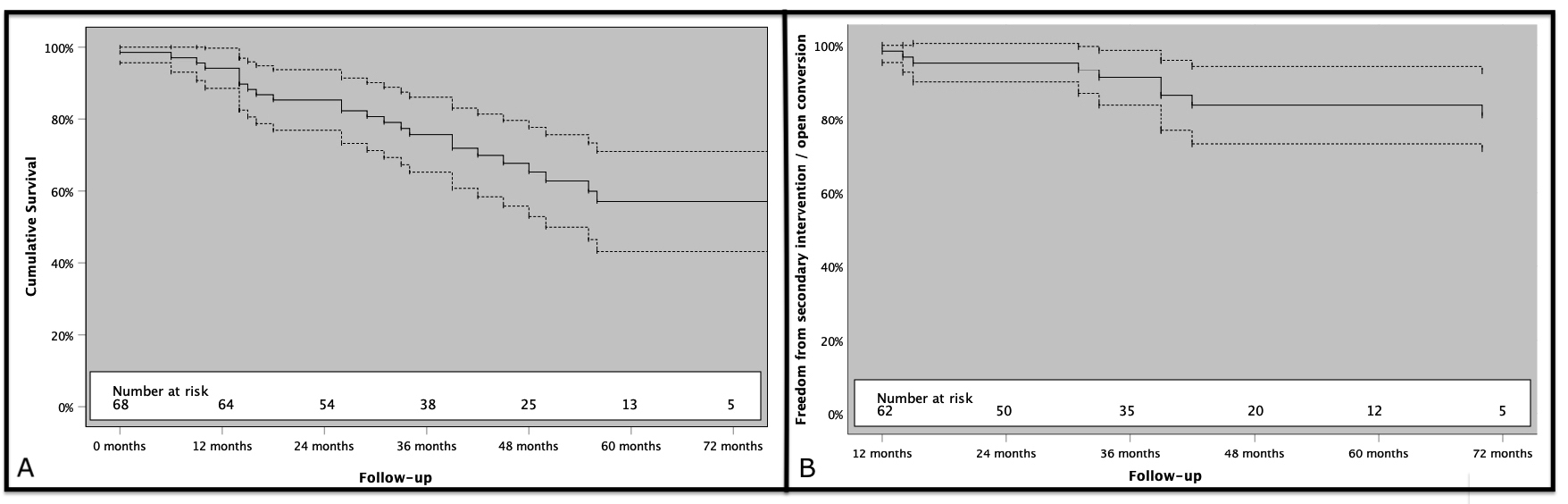 Fig. 4.
Fig. 4.Kaplan-Meier survival analysis. (A) Cumulative survival of patients. (B) Freedom from secondary interventions (dashed lines show 95.0% confidence intervals).
EVAR is widely accepted treatment of choice for AAA. The superiority of EVAR
over open surgery was seen especially in short-term outcomes [1, 2, 3, 4]. The
use of the Endologix AFX
In contrast to all other endografts, AFX stent frames are located inside to facilitate the aortic conformability. However, this endoskeleton can be challenging at reintervention times, especially for guidewire entrapment or the passage for the endograft bodies.
The texture is e-PTFE, located outside the skeleton, and only proximal and distal endings are sutured. Therefore, it provides flexibility and better adaptation to the irregular aortic neck in the proximal sitting area since the graft material can move freely in an “active seal” manner [16, 17]. This independent movement of the graft material from the stent may be a factor for uncoupling as the columnar strength may diminish when fixation is at the aortic bifurcation.
Besides long-term outcomes with abdominal aortic aneurysm (AAA) treated with unibody bifurcated endograft, our study demonstrated excellent short-term results, with no intra-procedural open surgical conversion and no 30-day mortality encountered. The first finding was related to the long-term prognosis of treatment of AAA with minimally invasive procedure. Cumulative survival was 54.0% at 5th year in this patient cohort. Because of the presence of CAD in 54.4% of our patient population, one can think that all-cause mortality was high in the long term due to diseases such as CAD and other vascular system disorders. Increasing malignancy rates with advanced age may also have caused this high mortality rate. Moreover, patients who are at high risk for surgery and older patients are now treated with endovascular intervention; in the long term, death due to non-aneurysm causes is more common. In Bahia et al. [18] meta-analysis, they showed no improvement in mortality, with a mean 69.0% rate, of elderly patients over the years. There is long-term follow-up of different endografts in the literature. In a recent study, overall survival at 7 years was 50.3% to 61.4% [19]. Therefore, this high mortality in long-term; it should be kept in mind that it is more dependent on additional issues like cardiac morbidity or malignity, not aneurysm related. In order to avoid extra nephrotoxic agent, we did not perform routine coronary angiography (CAG) on patients without cardiac symptoms. We routinely used transthoracic echocardiography and electrocardiogram. In cases where surgical stress was low and no cross clamp, we did not experience cardiac morbidities in the early period. In our series with high cardiac comorbidities, long-term cardiac mortality may have caused a significant reduction in survival.
As all designs carry their own pros and cons; there seems a component uncoupling problem, as the main body and aortic extension disconnection, tried to dissolve by the manufacturer’s suggestion as to maximize the overlap and some changes over the material used. Manufacturers’ material exchange does not seem to be sufficient for eliminating the type III endoleak, so proper patient selection, sufficient overlap are the key issues.
Endoleak type III due to fabric tears or disintegration of the endograft
components has been a concern in EVAR. From study 701 EVAR patients treated with
the Endologix Powerlink and Endologix AFX
The treatment of type III endoleak often employs an endovascular approach as first line. In case of failure open late conversion and repair are mandatory. Meticulous preoperative planning and proper choice of endograft for a sufficient overlap arevery important.
To avoid type III endoleaks, maximizing component overlap or more proper patient
selection may be a choice. Proper patient selection for Endologix
AFX
It is well established that the dominant forces on modular EVAR components are directed sideways and that such force increases along the greater curvature with aortic or endograft angulation [21, 22]. The resulting sideways displacement of the endograft is more prevalent in larger aneurysms with sufficient volume in the aneurysm sac to accommodate this conformational change and is associated with an increased incidence of type I and III endoleaks [23]. Long term integrity of the central overlap zone between the two modular components thus depends on appropriate outward radial force from the proximal extension, the sealing mechanism of the expanded PTFE endograft covering, adequacy of the overlap zone between the two aortic components and the degree of angulation.
The probability of type III endoleak was increased two-fold by 0.1 unit increase at ASI. Moreover, the interpretation that AAA larger than 60 mm and ASI over 3 had the possibility of type III endoleak may be revealed as the all patients with type III endoleaks were adjusted to these criteria.
Type II endoleaks were detected in the completion angiogram in 5 patients (7.4%). They were all followed up and three of them (60.0%) disappeared in the first year while the others were closely followed for the aneurysm sac enlargement. No aneurysm sac enlargement was seen and therefore no reintervention was necessitated.
No graft infection and post-implantation syndrome were seen. There are studies suggesting e-PTFE grafts are associated with a lower probability of postoperative fever, however there is no randomized clinical support [24].
Clinical reports confirm low limb occlusion rates [14, 18]. Stent grafts each
have different variations in graft material, stent material and configuration.
These differences are made for providing the perfect adaptation of graft and
iliac artery anatomy, especially with angulated, calcified or nonuniform
atherosclerotic landing zones. Clinical studies confirm low limb occlusion rates
[9, 14, 18, 25]. Patients treated with the Endologix Powerlink and Endologix
AFX
Study limitations are the retrospective design, a single center experience with
a relatively small patient population that may prevent statistical significance.
There was no randomization as only Endologix AFX
Endologix AFX
HZİ, MK, BBA and EUÜ conceived and designed the analysis; BBA, VB, GA, EK, BA collected the data; HZİ, BBA, GA, EK and EUÜ analyzed the data; HZİ, MK, BBA, VB, GA and EUÜ wrote the paper.
No informed consent was obtained because of the retrospective nature of data collection from hospital records in the study. The institutional review board of the Turkey Yuksek Ihtisas Hospital approved, code 29620911-929-1303.
We thank three anonymous reviewers for excellent criticism of the article.
This research received no external funding.
The authors declare no conflict of interest.
