Infective endocarditis (IE) represents one of the most challenging clinical entities, requiring a multidisciplinary approach. The increasing number of surgical and transcatheter heart valves replacements performed annually lead to a higher incidence of prosthetic valve endocarditis. Transcatheter aortic valve implantation (TAVI) brought a new alternative for the treatment of aortic stenosis and a new subgroup of IE with its features. We aimed to compare the incidence of IE in TAVI and surgical valve replacement (SAVR) to identify risk factors for TAVI-IE, evaluate the possible impact on mortality, and clarify the best treatment strategies. A digital scan in PubMed and SCOPUS databases was performed. 68 publications were selected to perform a meta-analysis and systematic review on epidemiology, risk factors, and mortality predictors in TAVI-IE. No significant difference in IE rate was noted between patients with TAVI and those with SAVR for in-hospital, early, mid-term and late IE. Male gender, intubation, new pacemaker implantation IE and CKD were correlated with TAVI-IE. Surgical treatment was performed in 22.3% of cases. Overall mortality for the pooled cohort was 38.3%. In a multivariate logistic regression model, surgical treatment and self-expandable device were linked to lower mortality in TAVI-IE. Even if the invasive procedure can trigger bacteremia, exposing the TAVI valve to future infection, no significant difference in IE rate was noted in our analysis between patients with TAVI and those with SAVR for in-hospital, early, mid-term and late IE. Surgical treatment of TAVI-IE can be a viable option in patients with a prohibitive risk score.
Infective endocarditis (IE) represents one of the most challenging clinical entities, which requires a multidisciplinary approach and a high level of expertise from practitioners (Habib et al., 2015). Despite being a rare complication of heart valve replacement, it can occur in 1-6% of patients (Vongpatanasin et al., 1996). An increasing number of surgical and transcatheter heart valves replacements performed annually lead to a higher incidence of prosthetic valve endocarditis (PVE) worldwide (Conen et al., 2020).
Even with all the advances in antimicrobial and surgical treatment, a proper management strategy is still not clarified, and mortality after PVE remains high (24-46%) (Akowuah et al., 2003; Cresti et al., 2017). Transcatheter aortic valve implantation (TAVI) brought not only a new alternative for the treatment of aortic stenosis but also a new subgroup of IE with its own morphological, microbiological, and clinical features. A recent meta-analysis of randomized control trials (RCT) comparing PVE after TAVI and SAVR were recently published (Ando et al., 2019), however, due to rigorous selection of patients enrolled in this type of studies, the results observed in daily medical practice could differ.
The purpose of this study was to compare the incidence of IE in TAVI and surgical valve replacement (SAVR), to identify risk factors for TAVI-IE, evaluate the possible impact on the mortality and clarify best treatment strategies. Our analysis included RCT, observational, and propensity match score studies to minimize the differences.
All relevant papers were systematically searched in the electronic databases of PubMed and SCOPUS between May 2002 and December 2019, without any restriction for publication language. Two authors (A.T. and M.E.) independently screened titles and abstracts for eligibility of the studies using the following query terms: TAVI, TAVR, transcatheter aortic valve replacement, transcatheter aortic valve implantation, infective endocarditis. The systematic search of the literature was performed in accordance with PRISMA (the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement) see Fig. 1.
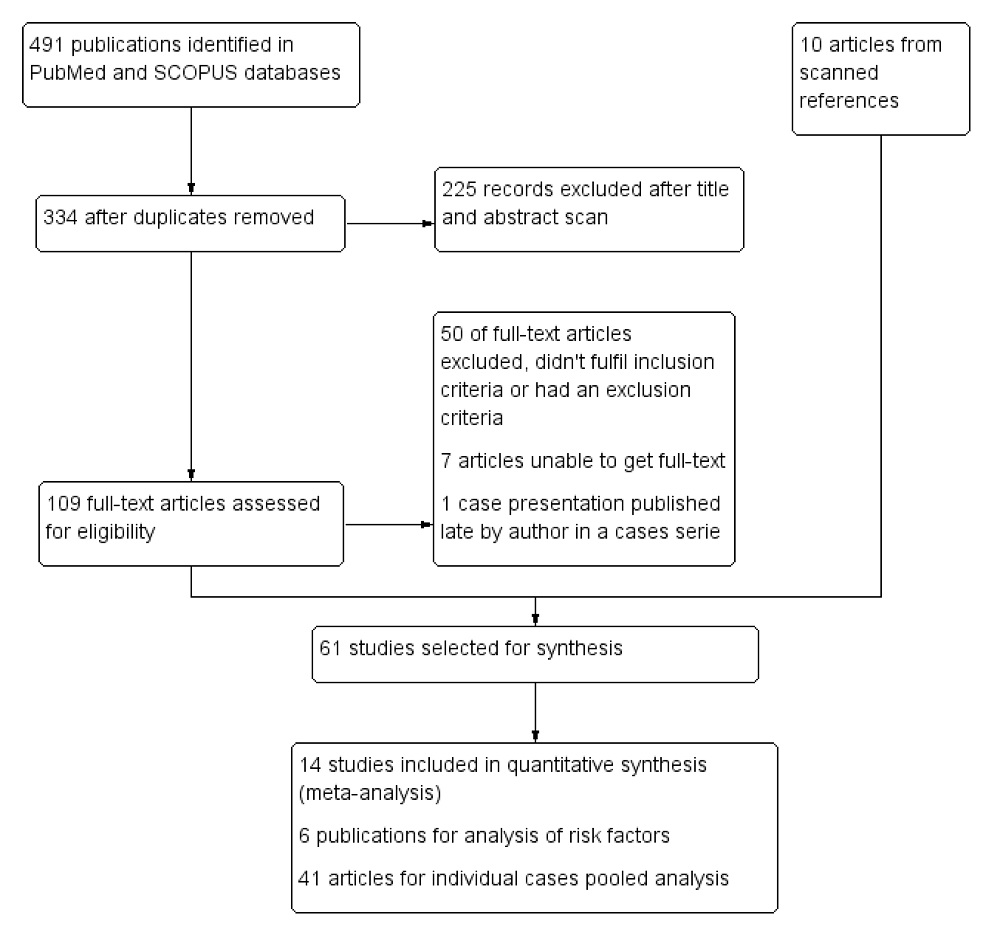 Figure 1.
Figure 1.PRISMA flow diagram of the selection process. PRISMA flow diagram representing the selection process of papers used for meta-analysis, study of the risk factors and individual cases pooled analysis in transcatheter aortic valve implantation infective endocarditis.
The International Prospective Register of Systematic Reviews (PROSPERO) registration number for this study is CRD42020165182 (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=165182).
Both authors identified separately the relevant articles which fulfill the inclusion criteria: 1. randomized clinical trials, propensity score match studies, and observational studies which compared TAVI with SAVR and reported incidence of infective endocarditis 2. observational studies which compare risk factors in TAVI-IE group and TAVI without IE 3. case reports or case series on TAVI-IE. Exclusion criteria were as follow: 1. reports on TAVI-IE for aortic insufficiency 2. systematic reviews or meta-analysis. References of selected publications were reviewed to minimize the risk of overlooking relevant studies. When there was a lack of consensus, we asked the third senior reviewer (G.T.)
In-hospital endocarditis was defined as one which emerged during indexed hospitalization, early IE — diagnosed for first-year post-TAVI, mid-term IE — between first and second year, late IE — after two years.
We used the Review Manager (RevMan) Version 5.3 (Nordic Cochrane Centre, The Cochrane Collaboration, 2012, Copenhagen, Denmark) software to calculate the pooled effect size with odds ratio (OR) and 95% confidence intervals (CI) by Mantel-Haenszel method and random effect model. I2 statistics evaluated heterogeneity of studies, and we considered 0% to 25% as low, 26% to 50% as moderate, 51% to 75% as high, and > 75% as very high. The event number was calculated from the total cohort, and the described ratio in case the ratio of events and not the number of events was mentioned. A sensitivity analysis was performed, and each study was removed once at a time, and the pooled effect size was recalculated each time. Publication bias was assessed by the construction of the Funnel plot. A P-value of less than 0.05 was considered significant.
Publications that reported a comparison of the TAVI-IE group versus the TAVI NON-IE group were analyzed to reveal predictive variables. Manuscripts and online supplements were scanned when possible to complete data. We used Hozo et al. (2005) formula to convert the median to mean when needed. The pooled sample mean and the pooled standard deviation was calculated according to the recommendation of the Cochrane Handbook for Systematic Reviews. We extracted individual data of patients reported in case series and case presentations to perform epidemiological evaluation and reveal possible risk factors that influence the treatment outcome. Continuous variables were presented as mean with standard deviation or median with interquartile range (IQR) when necessary; categorical data were presented as proportions. A Chi-square test was used to analyze dichotomous data. Means and standard deviation between studied groups were compared with the Student t-test.
Variables significant for mortality were studied in a multivariate logistic regression model. Kaplan-Meier analysis was performed for time to event data. A P-value lower than 0.05 was considered significant for each test. Statistical analysis was performed with MedCalc Statistical Software version 14.8.1 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014)
After screening the title and abstract of possible relevant publications, 68 papers were selected, retrieved as complete manuscripts and used for final synthesis as follows: 14 studies included in quantitative synthesis, six publications for analysis of risk factors, and 48 articles for individual cases pooled analysis Fig. 1.
For comparative analysis of PVE after TAVI and SAVR, we used 14 studies: 8 publications represented four randomized control trials (RCT) (Deeb et al., 2016; Kodali et al., 2012; Leon et al., 2016; Mack et al., 2015; Reardon et al., 2015; Smith et al., 2011; Søndergaard et al., 2019; Thyregod et al., 2015), 1 observational study (Shehada et al., 2018), 5 propensity score-matched studies (Johansson et al., 2016; Kolte et al., 2018; Latib et al., 2012; Thourani et al., 2016; Waksman et al., 2018) (Table 1).
| Author, year | Type of study | No. of centers | Valve type | Approach | Risk group | Type of procedure | Number of patients per arm | Age, years | Male, % | EuroScore, mean ± SD | STS score, |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Smith et al., 2011 |
RCT | 25 | EL Sapien | TF, TA | High | TAVI |
348 |
83.6 ± 6.8 |
57.8 |
29.3 ± 16.5 |
11.8 ± 3.3 |
| Reardon et al., 2015 |
RCT | 45 | CoreValve | TF, SC, TAo | High | TAVI |
390 |
83.1 ± 7.1 |
53.1 |
17.7 ± 13.1 |
7.3 ± 3.0 |
| Leon et al., 2016 |
RCT | 57 | Sapien XT | TF, TA | Intermediate | TAVI |
1,011 |
81.5 ± 6.7 |
54.2 |
- | 5.8 ± 2.1 |
| Thyregod et al., 2015 |
RCT unblinded | 3 | CoreValve | TF | Low | TAVI |
145 |
79.2 ± 4.9 |
53.8 |
8.4 ± 4.0 |
2.9 ± 1.6 |
| Shehada et al., 2018 | Prospective |
1 | - | TF, TA, TAo | High | TAVI |
100 |
81 ± 6 |
58 |
21.1 ± 13.8 |
- |
| Thourani et al., 2016 |
Prospensity score matched study | 51 | Sapien 3 | TF | Intermediate | TAVI |
1077 |
81.9 ± 6.6 |
62 |
- | 5.5 ± 0.6 |
| Waksman et al., 2018 |
Prospectiveunblinded, |
11 | Sapien 3 |
TF | Low | TAVI |
200 |
73.6 ± 6.1 |
61.5 |
- | 1.8 ± 0.5 |
| Johansson et al., 2016 | Prospensity score matched study | 1 | Sapien, Sapien XT, Lotus | TF, TA | High | TAVI |
166 |
80 ± 9 |
51 |
23 ± 15 |
- |
| Latib et al., 2012 | Prospensity score matched study | 1 | Sapien, |
TF | Moderate/ |
TAVI |
111 |
80.5 ± 6.9 |
- | 23 ± 15.1 |
4.57 ± 2.28 |
| Kolte et al., 2018 | Prospensity score matched study | National |
- | TF, TA, SC, TAo | TAVI |
15138 |
78.1 ± 13.8 |
53.4 |
- | - |
RCT: randomised clinical trial, TF - transfemoral, TA - transapical, TAo - transaortic, TAVI - transcatheter aortic valve implantation, SAVR - surgical aortic valve replaceme
No significant difference in IE rate was noted between patients with TAVI and those with SAVR for in-hospital, early, mid-term and late IE (TAVI vs SAVR: in-hospital IE: OR: 2.39, 95% CI, 0.60 to 9.44, I2 = 0%; early IE: OR: 1.21, 95% CI, 0.64 to 2.29, I2 = 0%; mid-term IE: OR: 1.11, 95% CI, 0.55 to 2.25, I2 = 4% and late IE: OR: 0.84, 95% CI, 0.44 to 1.58, I2 = 0%) (Fig. 2-5). Sensitivity analysis did not reveal any significant changes.
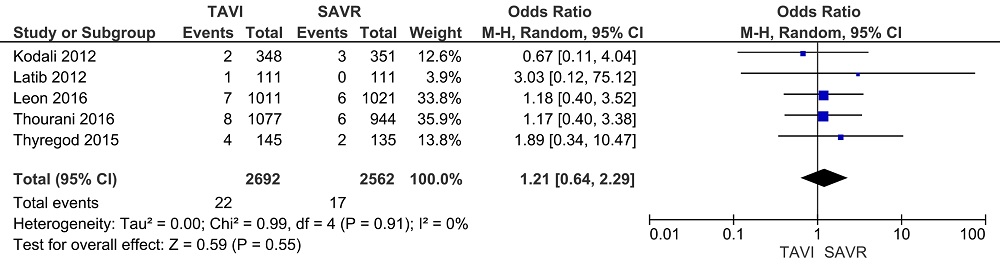 Figure 2.
Figure 2.In-hospital IE. Forest plots of the meta-analysis depicting the comparison of in-hospital infective endocarditis after transcatheter aortic valve implantation and surgical aortic valve replacement.
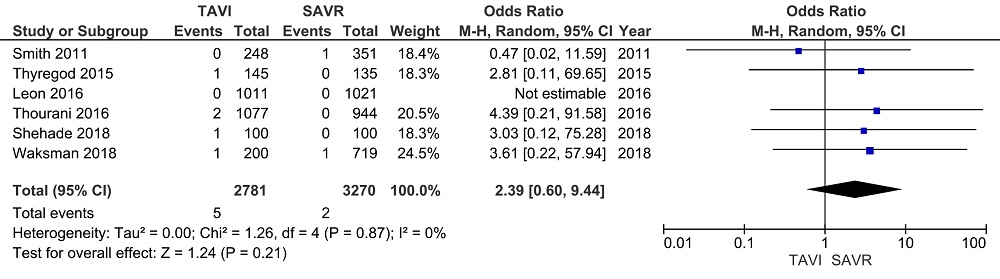 Figure 3.
Figure 3.Early IE. Forest plots of the meta-analysis depicting the comparison of the early infective endocarditis after transcatheter aortic valve implantation and surgical aortic valve replacement.
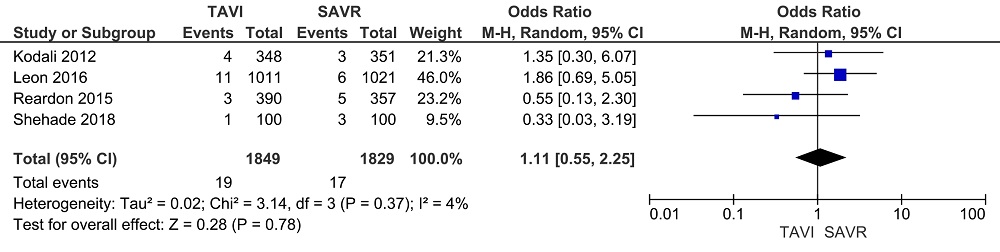 Figure 4.
Figure 4.Mid-term IE. Forest plots of the meta-analysis depicting a comparison of the mid-term infective endocarditis after transcatheter aortic valve implantation and surgical aortic valve replacement.
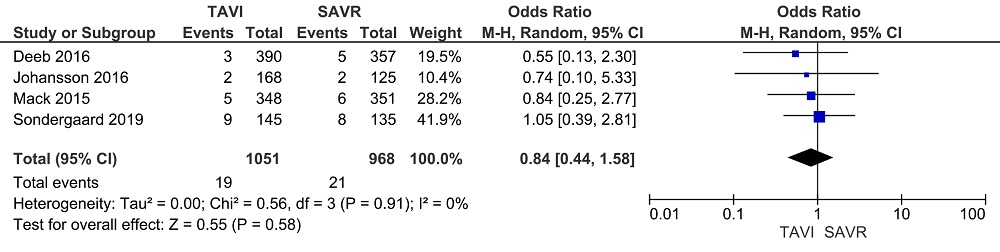 Figure 5.
Figure 5.Late IE. Forest plots of the meta-analysis depicting a comparison of the late infective endocarditis after transcatheter aortic valve implantation and surgical aortic valve replacement.
To evaluate the predictors of TAVI-IE, we pooled six cohort studies incorporating 507 patients suffering from TAVI-IE and 57531 patients free from IE (Amat-Santos et al., 2015; Mangner et al., 2016; Olsen et al., 2015; Regueiro et al., 2016; Rodriguez-Vidigal et al., 2018; Yeo et al., 2018). Patients with TAVI-IE were younger (TAVI-IE: 73.5 ± 4.2 vs. TAVI NON-IE: 79.9 ± 3.24, P < 0.001). Male gender was a risk factor for the TAVI-IE (RR: 1.24, 95% CI 1.15 to 1.33). Moreover, intubation, new pacemaker implantation, and CKD were risk factors for TAVI-IE (RR: 2.99, 95% CI 2.73 to 3.28; RR: 5.19, 95% CI 4.16 to 6.47; and RR: 1.17, 95% CI 1.05 to 1.29). Other variables showed no significant statistics and are presented in Table 2.
| Risk factor | No. of studies | No. of patents | TAVI-IE | NON-IE TAVI | RR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Age(y), mean ± SD | 6 | 57896 | 73.5 ± 4.2 | 79.9 ± 3.24 | < 0.001 | |
| Male | 5 | 55277 | 287 | 28140 | 1.24(1.15-1.33) | < 0.001 |
| COPD | 5 | 53452 | 136 | 14049 | 1.03(0.89-1.19) | NS |
| DM | 5 | 51375 | 151 | 16761 | 1.02(0.89-1.16) | NS |
| CKD | 6 | 53171 | 211 | 18783 | 1.17(1.05-1.29) | 0.003 |
| Bleeding | 3 | 6171 | 39 | 761 | 1.08(0.8-1.45) | NS |
| OTI | 3 | 53452 | 226 | 9066 | 2.99(2.73-3.28) | < 0.001 |
| New pacemaker | 5 | 51518 | 70 | 1524 | 5.19(4.16-6.47) | < 0.001 |
| Self-expandable | 3 | 10144 | 141 | 4303 | 1.03(0.9-1.16) | NS |
| Paravalvular leak ≥ 2 | 5 | 16297 | 44 | 1887 | 0.97 (0.74-1.29) | NS |
| PAD | 3 | 43339 | 36 | 10137 | 0.79(0.59-1.06) | NS |
| Vascular complications | 3 | 14013 | 32 | 5801 | 0.85(0.61-1.19) | NS |
SD, standard deviation; COPD, chronic obstructive pulmonary disease; DM, diabetes Mellitus; CKD, chronic kidney disease; OTI, oro-tracheal intubation; PAD, peripheral artery disease; RR, relative risk; NS, not significant
A total of 51 articles which reported individual data of 157 patients were identified, 3 observational studies (Amat-Santos et al., 2015; Olsen et al., 2015; Rodriguez-Vidigal et al., 2018), 5 case series (Aung et al., 2013; Gallouche et al., 2018; Martinez-Selles et al., 2016; Puls et al., 2013; Scisło et al., 2019), 43 case reports (Ahmad et al., 2016; Campana et al., 2019; Carnero-Alcazar et al., 2010; Carrel and Eberle, 2019; Castiglioni et al., 2012; Cho et al., 2019; Chourdakis et al., 2017; Citro et al., 2013; Comoglio et al., 2009; Dapas et al., 2016; García-Pardo et al., 2012; Gonzalez et al., 2017; Gotzmann and Mugge, 2011; Gupta et al., 2019; Gürtler et al., 2019; Halapas et al., 2014; Head et al., 2011; Kesimci et al., 2014; Lane et al., 2015; Lee et al., 2014, 2019; Loh et al., 2013; Loverix et al., 2013; Montero-Cruces et al., 2019; Mori et al., 2019; Morioka et al., 2019; Nguyen et al., 2019; Ochiai et al., 2016; Ohori et al., 2018; Olsthoorn et al., 2019; Pabilona et al., 2015; Pichard et al., 2017; Rafiq et al., 2011; Ruchonnet et al., 2019; Russo et al., 2019; Santarpino et al., 2013; Sari et al., 2016; Seok Koh et al., 2014; Sugimura et al., 2019; Sulženko et al., 2014; Takimoto et al., 2015; Wilbring et al., 2014; Wong et al., 2009). The baseline characteristics of the pooled cohort are presented in Table 3. In all patients, the diagnosis was established according to modified Duke criteria. Echocardiographic data were available in 89 out of 157 patients. In 6 patients, additional computer tomography was performed. Most patients received antibiotic prophylaxis before the procedure according to the local protocols with cephalosporin group or Vancomycin.
| Baseline characteristics | OR | 95% CI | P-value | |
|---|---|---|---|---|
| Age (y), mean ± SD | 79.1 ± 7.63 | 0.97 | 0.92-1.02 | NS |
| Gender | ||||
| Male | 91/61.1% | 0.67 | 0.28-1.61 | NS |
| Female | 58/38.9% | - | - | - |
| Time TAVI to IE (m), |
5.4 (1.6-12.2) | NS | ||
| Valve type | ||||
| Balloon-expandable | 72/47.4% | - | - | - |
| Self-expandable | 80/52.6% | 0.39 | 0.16-0.98 | < 0.05 |
| Type of microorganism | ||||
| Gram-positive | 125/79.6% | 0.62 | 0.13-2.97 | NS |
| Gram-negative | 10/6.4% | 1.76 | 0.20-15.47 | NS |
| Fungi | 6/3.8% | 0.53 | 0.03-10.28 | NS |
| Polymicrobial | 2/1.3% | - | - | - |
| Unknown | 14/8.9% | - | - | - |
| Possible source of bacteremia | 78/49.7% | 1.28 | 0.55-3.01 | NS |
| Affected cardiac structure | ||||
| TAVI prosthesis | 102/73.4% | - | - | - |
| Mitral valve | 24/17.3% | 0.95 | 0.30-2.97 | NS |
| Complex | 11/7.9% | 0.95 | 0.15-5.84 | NS |
| Others | 2/1.4% | - | - | - |
| Treatment | ||||
| Surgery | 35/22.3% | 0.15 | 0.04-0.62 | < 0.05 |
| Antibiotic therapy | 122/77.7% | - | - | - |
| Total mortality | 59/38.3% | - | - | - |
Median time from TAVI to IE for overall group was 5.5 months with IQR 1.8-12.5, for Gram-positive IE it was 5.4 (2.3-12.52), Gram-negative - 1.5 (0.9-6.2), mycotic infection - 5.15 (2.0-9.0) and unknown etiology- 12.0 (5.75-16.32), polymicrobial - 21.0 (9.0-33.2). The highest incidence was during the first seven months after implantation. There was no difference in IE's onset between self-expandable and balloon-expandable devices groups (Fig. 6).
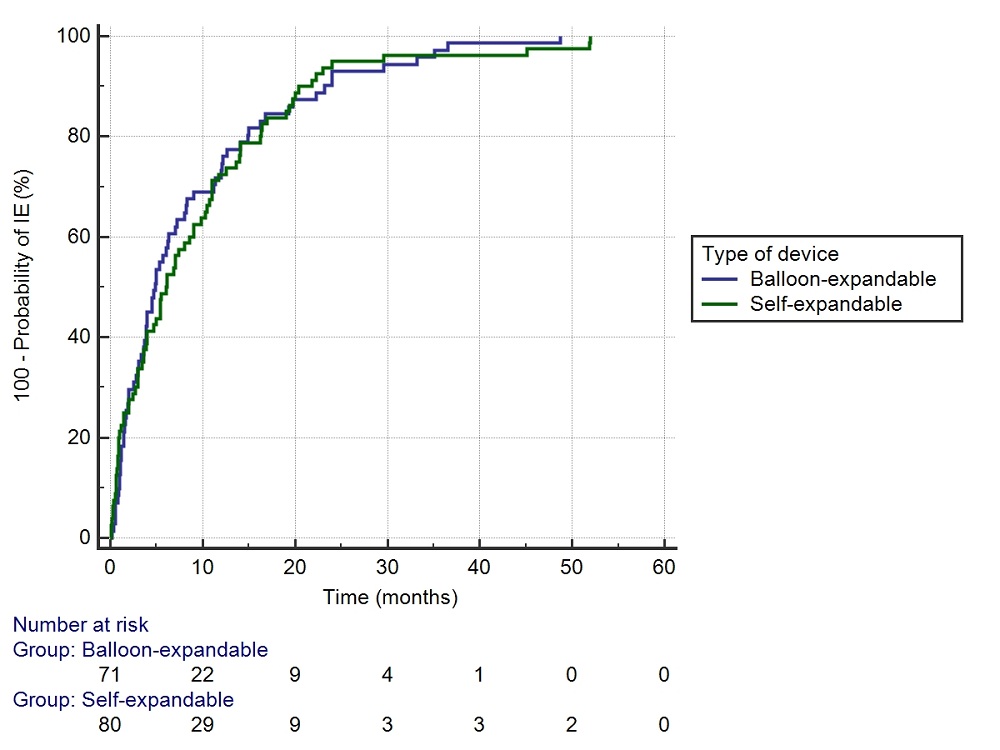 Figure 6.
Figure 6.Comparison of time from TAVI to IE according to the type of valve. Kaplan-Meier analysis comparing the time from transcatheter aortic valve implantation procedure to the onset of infective endocarditis according to the type of the used device.
The most frequently isolated pathogens in blood culture were as follows: streptococci (25.3%), staphylococci (25.3%), enterococci (24.1%). Blood culture was negative in 12.7%; in three cases, it was polymicrobial. The highest prevalence in the staphylococci group had Staphylococcus aureus (60%); for the enterococci group, it was Enterococcus faecalis (65.8%). There were 6 cases of mycotic IE, 5 with Candida spp. (Amat-Santos et al., 2015; Carrel and Eberle, 2019; Martinez-Selles et al., 2016; Morioka et al., 2019; Russo et al., 2019) and 1 with Histoplasma capsulatum (Head et al., 2011). Gram-negative flora was represented by 3 cases of IE with Escherichia coli (Gallouche et al., 2018; Olsen et al., 2015; Puls et al., 2013), 2 cases of Acinetobacter spp (Amat-Santos et al., 2015; Martinez-Selles et al., 2016), Pseudomonas aeruginosa (Dapas et al., 2016; Scisło et al., 2019) and 1 case of Serratia (Amat-Santos et al., 2015), Salmonella enteritidis (Martinez-Selles et al., 2016), and Moraxella nonliquefaciens (Rafiq et al., 2011). A Source of bacteremia was identified in 49.7% of the patients. In staphylococci TAVI-IE, an entry door was found in 58%, soft tissue infections being most common (6 cases), for enterococci group in 44.7% (10 cases of urinary origin).
TAVI prosthesis was affected by 73.4%; this included vegetation on prosthetic leaflets, frame, and peri-annular complications. In 17.3%, only the mitral valve was interested. Multiple valves IE was presented in 7.9%, 9 cases of TAVI prosthesis and mitral valve, and 2 cases of TAVI prosthesis and tricuspid valve. In 86 patients were possible to identify the severity of intracardiac lesions, thus in 66.3%, it was simple injury with a single valve or prosthesis implication. In 33.7% of the cases, it was complex injury with periannular extension, intracardiac shunts, or multiple valves lesions.
Surgical treatment was performed in 22.3%; it included isolated SAVR or in combination with mitral valve replacement/repair, minimally invasive approach, replacement of aortic root, TAVI in TAVI procedure (only 2 cases), or pacemaker removal.
Overall mortality for the pooled cohort was 38.3%. There were no statistically significant differences for mortality between streptococci, staphylococci, and enterococci groups - 35%, 30%, and 21% (P > 0.05). Mortality in surgery and antibiotic treatment groups was 16.7% and 37.4% (P < 0.05) (Fig. 7). In a multivariate logistic regression model surgical treatment (OR 0.15 95% CI 0.04 to 0.62 P < 0.05) and self-expandable device (OR 0.39 95% CI 0.16 to 0.98 P < 0.05) were factors linked to lower mortality in TAVI-IE (Table 3).
 Figure 7.
Figure 7.Survival rate in TAVI-IE according to treatment strategy. Bar chart depicting the comparison of the survival rate in transcatheter aortic valve implantation infective endocarditis according to the treatment strategy.
PVE is the most severe form of infective endocarditis and accounts for up to 20% of IE (Cahill and Prendergast, 2016), with an incidence of 0.54% per person-years for SAVR (Valdes et al., 2008). Incidence of TAVI-IE ranges from 1.1 to 3.4 per person-years (Kaur et al., 2020; Mangner et al., 2016; Puls et al., 2013; Regueiro et al., 2016). The present study reported no significant difference for in-hospital, early, mid-term, or late incidence of IE between patients with TAVI or SAVR. Summers et al. (2019) reported a PVE incidence rate of 5.06 [95% CI, 4.19 to 6.12] per 1,000 person-years in PARTNER trials with no statistical difference in the incidence of TAVR and SAVR-PVE (Incidence rate ratio [IRR] 1.27 [95% CI, 0.70 to 2.32; P = 0.44]).
Younger age emerged to be one of the risk factors for TAVI-IE. A possible explanation for this finding may be the selection criteria of patients for TAVI, specifically the more severe comorbidities, which made them eligible candidates for a percutaneous approach. In a pooled analysis, male gender, OTI, new pacemaker implantation, and CKD were identified as risk factors for TAVI-IE. Although the higher prevalence of IE among males is yet to be elucidated, several factors like dental and urologic hygiene could be speculated to influence this relation. (Olsen et al., 2015). General anesthesia with OTI compared to local anesthesia has a higher risk of pulmonary complications and can be a source of bacteremia (Valdes et al., 2008). An increased rate of IE in this group can be triggered by associated severe comorbidities and cardiac insufficiency, making it challenging to tolerate a conscious supine position. Pacemaker implantation can create entry routes for pathogens and cause endocardial lesions. CKD can induce persistent bacteremia due to frequent intravascular manipulations. In our meta-analysis, paravalvular leak (PVL) was not identified as a risk factor for TAVI-IE, despite the general opinion that the mechanical injury resulted from regurgitation jet can create necessary conditions for bacterial fixation and proliferation (Ambrosioni et al., 2017).
Diagnosis of infective endocarditis necessitates an integration of clinical findings, microbiological analysis, and imaging results (Harding et al., 2020). The modified Duke clinical diagnostic criteria incorporate these three domains and weigh findings as either major or minor criteria (Cahill and Prendergast, 2016). It can be particularly challenging to make a diagnosis of PVE after TAVI. Detection of small vegetations by TEE is challenging due to the shadowing effect and reflectance of prosthesis material. Some degree of PVL and regurgitation are joint after TAVI due to calcifications, which do not allow the valve to expand properly (Puls et al., 2013).
Median time from TAVI to IE in our study group was 5.4 months, Kolte et al. reported a median of 66 days (Kolte et al., 2018) while others indicated a median of 4.6 to 9.6 months (Amat-Santos et al., 2015; Gallouche et al., 2018; Martinez-Selles et al., 2016; Regueiro et al., 2016). The onset for gram-negative IE was much earlier, with a median of 1.1 months. These pathogens seem to be responsible for severe cases of in-hospital and early endocarditis. This suggests immediate device contamination during implantation. Gram-negative multidrug-resistant organisms could frequently colonize groin region (Weintrob et al., 2010). Thus a shift towards broader spectrum antibiotics as prophylactic agents is recommended. Regueiro et al., showed no difference in the onset of IE according to the type of implanted device (Regueiro et al., 2019), the same results were observed in our analysis.
The primary causative pathogens for TAVI-IE were in the order of frequency: streptococci (25.3%), staphylococci (25.3%), enterococci (24.1%). Mangner et al. (2016) found staphylococci in 38.2%, enterococci in 30.9% and atypical organism in 18.2%, in contrast, Regueiro et al. (2016) presented 24.6% for enterococci and 23.8% for staphylococci etiology of IE. Such a high prevalence of Enterococcus spp. could be explained by frequent use of transfemoral implantation route, this agent being a common groin contaminant. A potential source of bacteremia was identified in about 49.7% of patients; in previous studies, this ranged between 50% to 77.3% (Mangner et al., 2016). Epidemiology of IE changed considerably compared to previous decades (Ambrosioni et al., 2017). The microbiological profile in TAVI-IE is different from the surgical counterpart, and antibiotic prophylaxis suitable for sternotomy may not be the best choice for the transfemoral approach.
The implication of TAVI prosthesis in infectious process varied among studies, vegetations on prosthetic leaflets were found in 39.6%-47.9% of patients, on stent frame in 17%-18.2%, peri annular complications (abscesses, fistulas, pseudoaneurysms) 18%-22.6% (Amat-Santos et al., 2015; Regueiro et al., 2016). We reported a total of 73.4% of isolated TAVI prosthesis IE, including all perivalvular extensions.
Patients undergoing TAVI represent a group with high surgical risk, quantified by different risk scores, like Euroscore II or STS prom. The association of IE and its related complications leads to an escalation of the operative risk. Thus, surgical treatment in patients with TAVI-IE is analyzed with great caution. In a cohort of 224 patients with TAVI-IE, Kolte et al. (2018) reported not a single case of surgical treatment. Despite this, aortic valve replacement can be a viable option for treatment of TAVI-IE, in a series of 250 patients, 37 underwent surgical treatment with 11 related deaths (Regueiro et al., 2016). In the present analysis, the surgical procedure was performed in 22.3% of cases. Only 2 cases in the group where TAVI in TAVI procedures. Surgical removal of the all potentially compromised synthetic material is “a must do” strategy for the treatment success and prevention of further recurrences in space/organ infection. This can explain such un-popular opinion on using TAVI in TAVI for IE. Mortality in surgery and antibiotic group was 16.7% and 37.4%, respectively. The probability of publishing cases with positive outcomes is higher; that's why the results must be interpreted with precaution.
Mortality in IE is one of the highest among heart-related diseases; it can reach up to 24% for native valve IE (Cresti et al., 2017) and surpass 46% for PVE (Akowuah et al., 2003). Moriyama et al. (2019) reported 30-days and one-year mortality in TAVI-IE of 37.7% and 52.5%. In the pooled series, it was 38.3% for overall reported follow up. In multiple logistic regression models, surgical treatment and the use of self-expandable devices proved to be related to lower mortality. Keeping in mind that TAVI was developed as an alternative for surgical treatment in patients with a high or prohibitive operative score, an association of IE in these patients increases, even more, the surgical risk. Despite this, the results of open-heart surgery in this group are inspiring. The findings related to survival according to the type of valve are quite controversial, and it is difficult to judge the advantage of self-expandable over balloon-expandable valves. Regueiro et al. (2019) reported no difference in mortality between balloon-expandable and self-expandable valves. Despite this, further studies must be performed.
PVE remains a rare but devastating complication with no difference in incidence between TAVI and SAVR. Evidence from analyzed papers suggests a higher risk for TAVI-IE among younger males, but future studies are required to confirm these data. Since invasive vascular procedures can trigger bacteremia, exposing the TAVI valve to future infection, antibiotic prophylaxis is mandatory. However, no significant difference in IE rate was noted in our analysis between patients with TAVI and those with SAVR for in-hospital, early, mid-term and late IE. Surgical treatment of TAVI-IE can be a viable option in patients with prohibitive risk score due to the wide variation in outcomes. Still, it should be performed in large valve centers of excellence.
Concept/design: AT, AB, GT;
Data collection: AT, AB;
Data analysis/interpretation: all authors;
Drafting article: all authors;
Critical revision of article: AB, GT;
Approval of article: all authors.
Thanks to all the peer reviewers and editors for their opinions and suggestions
The authors declare no conflict of interest.
