Inflammation and oxidative stress are involved in the pathogenesis of cardiovascular diseases such as atherosclerosis, hypertension and ischemic heart disease. Natural products play an important role as nutritional supplements with potential health benefits in cardiovascular diseases. Polygonum minus (PM) is an aromatic plant that is widely used as a flavoring agent in cooking and has been recognized as a plant with various medicinal properties including antioxidative and anti-inflammatory actions. Phytoconstituents found in PM such as phenolic and flavonoid compounds contribute to the plant’s antioxidative and anti-inflammatory effects. We conducted this review to systematically identify articles related to the antioxidative and anti-inflammatory activities of PM. A computerized database search was conducted on Ovid MEDLINE, PubMed, Scopus, and ACS publication, from 1946 until May 2020, and the following keywords were used: ‘Kesum OR Polygonum minus OR Persicaria minor’ AND ‘inflammat* OR oxida* OR antioxida*’. A total of 125 articles were obtained. Another eight additional articles were identified through Google Scholar and review articles. Altogether, 17 articles were used for data extraction, comprising 16 articles on antioxidant and one article on anti-inflammatory activity of PM. These studies consist of 14 in vitro studies, one in vivo animal study, one combined in vitro and in vivo study and one combined in vitro and ex vivo study. All the studies reported that PM exhibits antioxidative and anti-inflammatory activities which are most likely attributed to its high phenolic and flavonoid content.
In recent years, medicinal plants have garnered special interest owing to their pharmacological actions and availability to common people (Ismail et al., 2018; Kamil et al., 2018; Ruszymah et al., 2012). To date, more than 2000 species of medicinal plants with therapeutic effects have been identified in Malaysia. One of the medicinal plants is Polygonum minus Huds. (syn. Persicaria minor [Huds.] Opiz); (PM). PM belongs to the Polygonaceae family and originates from Southeast Asia, particularly from Malaysia, Vietnam, Indonesia and Thailand. It is a creeping plant that grows in small bushes, has an average height of 1 m on lowlands, and is adapted to live in damp areas. It has long and lanceolate leaves and its stems are slender, cylindrical, and light-green to slightly reddish (Fig. 1). Along the stems, numerous nodes are arranged at one cm intervals and are easily rooted when they touch the soil (Bunawan et al., 2011; Christapher et al., 2014).
 Figure 1.
Figure 1.Morphology of Polygonum minus plant. The leaves are long and lanceolate-shaped, while the stems are slender and light-green to slightly reddish.
PM has various vernacular names including Cambodian mint, Vietnamese mint, water pepper and marsh pepper. In Malaysia, this aromatic plant is known as kesum, daun laksa or cenohom and is commonly used in Malay delicacies as a flavoring agent in hot, sour and spicy dishes (Burkill, 1936). Traditionally, PM leaves are boiled with water and drank to treat dyspepsia and used as postnatal tonic (Burkill, 1936). Besides, PM oil is applied to the scalp to treat dandruff (Zakaria and Mohd, 2012).
Gas chromatography-mass spectrometry (GC-MS) has identified 77 metabolites in PM, which are mainly aliphatic aldehydes, organic acids, monoterpenes, and sesquiterpene compounds (Ahmad et al., 2014). Sesquiterpenes in PM contribute to the aroma and flavor of the herb (Rusdi et al., 2018). Owing to the presence of natural aliphatic aldehydes, PM is also a source of essential oils such as dodecanal, 1-decanol and decanal (Baharum et al., 2010). PM is rich in polyphenolic compounds, such as flavone, methyl flavonol, myricetin, quercetin (Christapher et al., 2015), tannin, apigetrin, miquelianin, hyperoside, astragalin, isoquercetin and quercitrin (Abdullah et al., 2017). The methanolic extract of PM leaf is not toxic to rats up to a dose of 2 g/kg (Christapher et al., 2017).
PM has various beneficial pharmacological effects. The aqueous and methanolic extracts of PM leaves showed antioxidative and acetylcholinesterase enzyme inhibition activities (Ahmad et al., 2014; George et al., 2014a). The aqueous extracts showed anti-inflammatory effects and inhibit carrageenan-induced paw edema in rats and the activities of 5-lipoxygenase (5-LOX) and cyclooxygenase-1 (COX-1) in vitro (George et al., 2014b). The n-hexane extracts of PM leaves showed anti-bacterial activity against methicillin-resistant Staphylococcus aureus (Ahmad et al., 2014). The ethyl acetate (EA) fraction of PM is cytotoxic to HepG2 cell lines (Mohd Ghazali et al., 2014). PM extracts have been reported to have anti-hyperlipidemia (Christapher et al., 2016), anti-fungal and anti-ulcer effects (Vikram et al., 2014).
The excessive accumulation of reactive oxygen species (ROS) such as hydroxyl and superoxide anions leads to the oxidative damage of the cell membranes and RNA, protein, and DNA molecules in a process called oxidative stress (Finkel and Holbrook, 2000). Excessive ROS production triggers signaling cascades that contribute to the onset of inflammation. Inflammation is a defense immune system mechanism by a host against pathogens and involves an enhanced ROS production by polymorphonuclear neutrophils (PMNs) at the site of injury. Inflammation has been associated with various diseases, such as cardiovascular diseases (CVD) (Steven et al., 2019), inflammatory bowel disease (Liu and Stappenbeck, 2016) chronic arthritis (Horváth et al., 2017) and asthma (Mishra et al., 2018). An ROS that is produced as part of the inflammatory response plays a role in facilitating the clearance of pathogens. However, when an ROS is persistently present for a long time, it can promote oxidative stress and chronic inflammation-associated disorders. Hence, oxidative stress and inflammation are two processes that are interrelated.
To combat the harmful effects of excessive ROS, the body has specific defense mechanisms in the form of endogenous antioxidants, such as glutathione and antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT) (Liguori et al., 2018; Španinger and Bern, 2020). The consumption of exogenous natural antioxidants is beneficial for minimizing the deleterious effects of ROS through several mechanisms, including inhibiting the initiation of oxidative chain reaction or directly neutralizing free radicals (Baiano and Del Nobile, 2016). PM is regarded as a potential source of natural antioxidants owing to its high content of polyphenols (Ahmad et al., 2018). Polyphenols exhibit antioxidative, anti-inflammatory and anti-carcinogenic properties (Brglez Mojzer et al., 2016).
The ethnopharmacological uses of PM and published data on its bioactivities provide justifications for the present study. In this study, we systematically reviewed up-to-date research to further characterize the antioxidative and anti-inflammatory activities of PM. This review may provide scientific insights into its benefits and justification for its ethnopharmacological uses.
The study aims to search and identify relevant studies on the antioxidative and anti-inflammatory activities of PM. The relevant studies were retrieved from four online databases, namely, PubMed, Scopus, Ovid MEDLINE, and ACS Publication from 1946 to May 2020. The following keywords were used: (1) Kesum OR Polygonum minus OR Persicaria minor AND (2) inflammat* OR oxida* OR antioxida*. Articles that might be missed during the database search were identified from the reference list of review articles retrieved from the initial search and were added to the list of selected articles (Abdullah et al., 2017; Almey et al., 2010; Huda-Faujan et al., 2007, 2009; Maizura et al., 2011; Nurul et al., 2010; Saputri and Jantan, 2011; Sumazian et al., 2010).
The articles that were retrieved from the database following the keywords were reviewed independently by two authors (A.A.H and A.U) according to the following criteria: (1) Only full-length original articles published in English language were considered in this review (2) articles that reported the antioxidative and anti-inflammatory activities of PM, regardless whether the studies focus on PM alone or include other plants and PM (3) in vitro, in vivo, ex vivo and any combined studies that reported the antioxidative and/or anti-inflammatory activities of PM. Review articles, news, case report, book chapters, conference proceedings, and editorial letters were excluded from this study.
Article screening was conducted in three phases. First, the articles that were not in the selection criteria were excluded according to the title alone. Second, the articles that were not relevant to the antioxidative and anti-inflammatory activities of PM were excluded by reading through the abstracts. Finally, the remaining articles that did not match the inclusion criteria were excluded by reading the full texts thoroughly. The study design, plant source, plant part, types of extract, phytoconstituents, results, outcomes, and reference of each study are recorded in Table 1.
| Study design | Plant source(s) | Plant part(s) | Type(s) of extract | Phyto-constituents | Results | Outcomes | Reference |
|---|---|---|---|---|---|---|---|
| In vitro studies | |||||||
| Chemical assay study. Antioxidative activity measured using FRAP, FTC and TBA assays. TPC was determined. |
Bandar Baru Bangi, Selangor, Malaysia | Edible portion | Aqueous | Phenols | TPC of PM was 44.35 mg TAE/100 g FW). PM extract at 200-1200 ppm had the same FRAP value as synthetic antioxidant, BHA. FTC value of PM was 63.2% of linoleic acid oxidation inhibition. TBA value of PM was 0.060 (absorbance reading at 532 nm). |
PM is a potential source of natural antioxidant. | Huda-Faujan et al., 2007 |
| Chemical assay study. Antioxidative activity measured using FRAP, FTC and TBA assays. TPC was determined. |
Bandar Baru Bangi, Selangor, Malaysia | Edible portion | Methanol | Phenols | TPC of PM was 16.73 mg TAE/100 g FW). PM extract at 600-1200 ppm had the same FRAP value as synthetic antioxidant, BHA. FTC value of PM was 63.66% of linoleic acid oxidation inhibition. TBA value of PM was 62%. |
PM exhibits antioxidant capacity and TPC has positive correlation with antioxidant capacity. | Huda-Faujan et al., 2009 |
| Chemical assay study. Antioxidative activity measured using TBARS level and peroxide value (POV). |
Penang, Malaysia | Leaf | Aqueous | - | Duck meatballs mixed with PM extract had lower TBARS level compared to duck meatballs mixed with BHT and control substance. No significant change in POV. |
PM has antioxidative activity and is a natural resource for enhancing the shelf life of duck meatballs. | Nurul et al., 2010 |
| Chemical assay study. Antioxidative activity measured using DPPH radical scavenging assay. TPC was determined. |
Kuantan, Pahang, Malaysia | Leaf | Methanol Ethanol |
Phenols | Methanol extract of PM had higher TPC (31.38 ± 0.13 mg/g) than ethanol extract (21.06 ± 0.44 mg/g). EC50 of DPPH radical scavenging activity for methanol extract of PM was higher (0.005 ± 0.001 mg/ml) than ethanol extract (0.004 ± 0.001 mg/ml). Weak and negative correlation between TPC and EC50 of DPPH radical scavenging activity for both extracts. |
PM is a natural source of antioxidants. Extracting solvents affect TPC and antioxidative activity of PM. | Almey et al., 2010 |
| Chemical assay study. Antioxidative activity measured using FRAP, DPPH and BCL bleaching assays. Total vitamin C (TVC), total flavonoid content (TFC) and TPC were determined. |
Serdang, Selangor, Malaysia | Leaf | Aqueous Boiled aqueous |
Phenols Flavonoids Ascorbic acid |
Higher FRAP value and DPPH scavenging activity in the aqueous than in the boiled aqueous extract of PM. Higher TFC and TPC in the boiled aqueous than in the aqueous extract of PM. Aqueous extract of PM had BCL bleaching assay value of 12.55% and TVC of 0.54 mg/g FW. |
PM exhibits antioxidative activity. | Sumazian et al., 2010 |
| Chemical assay study. Antioxidative activity measured using DPPH and FRAP assays. TPC was determined. |
Selangor, Malaysia | Leaf | Aqueous | Phenols | Antioxidative activity of PM was 82.6% ± 0.7% from DPPH assay and 46.3% ± 1.2% from FRAP assay. TPC in PM was 165.3 ± 1 mg GAE/100 g extract. Positive correlation between TPC with FRAP value and DPPH radical scavenging effect of PM. |
PM has high TPC and antioxidative activity and the antioxidant power may be contributed by the phenolic content. | Maizura et al., 2011 |
| Chemical assay study. Antioxidative activity measured using DPPH and FRAP assays. TPC was determined. |
Selangor, Malaysia |
Leaf | Aqueous Ethanol |
Phenols | Ethanol extract had higher FRAP value, DPPH radical scavenging activity, and TPC than the aqueous extract of PM. Positive correlation between TPC with FRAP and DPPH scavenging effect. |
Ethanol extract of PM had higher antioxidative activity and TPC. Aqueous and ethanol extracts of PM did not show any cytotoxicity against a normal human lung fibroblast cell line (Hs888Lu). | Qader et al., 2011 |
| Chemical assay study. Antioxidative activity measured using DPPH assay and TBARS assay with LDL as the oxidation substrate. TPC was determined. |
Peninsular Malaysia | Leaf | Methanol | Phenols |
With increasing concentration of PM, DPPH radical scavenging activity increased (IC50 value: 14.6 ± 3.3 μg/mL) and LDL oxidation was inhibited (IC50 value: 1.2 ± 0.1 μg/mL). TPC was 122.1 ± 1.6 mg GAE/g DW. TPC showed positive correlations with DPPH scavenging activity and LDL antioxidative activity. |
PM inhibits LDL oxidation and has antiplatelet activity that could partly be due to its TPC. | Saputri and Jantan, 2011 |
| Chemical assay study. Antioxidative activity measured using DPPH and FRAP assays. TPC was determined. |
Ulu Yam, Malaysia | Leaf Stem Root |
Aqueous Methanol Ethanol Dichloromethane n-Hexane Essential oil |
Monoterpenes Sesquiterpenes Aliphatic compounds Organic acids |
PM polar extracts (methanol, ethanol and aqueous) had high antioxidative activity whereas non-polar extracts (DCM and n-hexane) did not exhibit good antioxidative activity. Ethanol extract had the highest DPPH radical scavenging activity and FRAP value for all parts (leaf, stem and root), followed by methanol extract for all parts. Methanol and ethanol extracts from the stem had the highest antioxidative activities. Essential oil from PM leaf had a higher DPPH scavenging activity than essential oil from the stem, but no antioxidative activity was found in essential oil from the root. In all the polar extracts, leaf contained the highest TPC, whereas root had the lowest TPC. A positive correlation was found between the DPPH scavenging activities and the FRAP values of the extracts. |
Different solvent extracts significantly affect antioxidative activity, with ethanol extract showing highest antioxidative activity in both leaf and stem extract. Essential oil of PM has antioxidative properties. | Ahmad et al., 2014 |
| Chemical assay study. Antioxidative activity was determined using DPPH scavenging and FRAP assays. TPC was measured and qualitative phytochemical analysis was conducted. |
Selangor, Malaysia. | Leaf | Aqueous Petroleum ether Methanol Ethyl acetate Seven different fractions (F1, F2, F3, F4, F5, F6, and F7) obtained from ethyl acetate extract. |
Flavonoids Alkaloids Saponins |
Ethyl acetate extract had the highest DPPH scavenging activity, FRAP and TPC followed by aqueous, methanol and petroleum ether extracts. Ethyl acetate extract contained the most abundant amount of flavonoids, alkaloids and saponins. Ethyl acetate extract was fractionated into seven fractions (F1, F2, F3, F4, F5, F6, and F7). F7 had the highest DPPH scavenging activity, FRAP and TPC followed by F6, F5, F4, F3, F2, and F1. There was a positive correlation between the DPPH scavenging activity and the FRAP value. |
PM ethyl acetate extract shows high antioxidative capacity and its fraction, F7, has selective antiproliferative activity against HepG2 cells. This activity is correlated to antioxidant capacity. | Mohd Ghazali et al., 2014 |
| Chemical assay study. Antioxidative activity measured using DPPH and BCL bleaching assays. TPC and TFC were determined. |
Selangor, Malaysia | Leaf | Aqueous Ethanol |
Phenols Flavonoids |
Ethanolic extract had higher TPC, TFC and DPPH radical scavenging activity than aqueous extract. Aqueous extract had better BCL inhibition activity than ethanolic extract. Strong positive correlation between TPC and DPPH radical scavenging activity. Low negative correlation between TFC and antioxidative activities. |
PM is a good source of natural antioxidants. | Othman et al., 2014 |
| Chemical assay study and cell culture study using HCT116 cells. Antioxidative activity measured using FRAP, ABTS, DPPH, FIC, CAA, superoxide anion and nitric oxide scavenging assays. TPC and TFC were determined. |
Kuala Lumpur, Malaysia | Leaf | Aqueous Methanol Ethyl acetate Hexane |
Tannins, Flavonoids (apigetrin,hyperoside, isoquercetin,astragalin, miquelianin, quercetin, and quercitrin) | Methanolic extract of PM exhibited the highest values for TPC (174.00 ± 0.18 mg GAE/g), TFC (53.19 ± 0.71 mg QE/g), FRAP (1728.33 ± 0.96 µmol Fe2+/g), ABTS (226.25 ± 4.25 µmol TE/g), DPPH (1276.81 ± 7.08 µmol TE/g) and nitric oxide scavenging assays (IC50, 675 ± 32.33 µg/mL). In the CAA assay, the EC50 value of PM methanolic extract was 263.92 ± 21.60 µg/mL. PM methanolic extract protected HCT116 cells from oxidative damage, with an EC50 value of 263.92 ± 21.60 µg/mL. |
PM methanolic extract has the highest antioxidant potential, which is most likely attributed to its relatively high phenolic and flavonoid contents. | Abdullah et al., 2017 |
| Chemical assay study. Antioxidative activity measured using a conventional DPPH assay and a new Lab-on-a-Disc (LoD) method that integrated conventional DPPH test and the use of a microfluidic compact disc. |
Selangor and Perak, Malaysia | Leaf | Ethanol | - | DPPH radical scavenging activity measured using the LoD method was consistently higher than the conventional method for all doses of PM used (25-100 mg/mL). | LoD method is a rapid and simple technique to measure antioxidative activity in plants including PM. | Rahman et al., 2018 |
| Chemical assay study. Antioxidative activity measured by DPPH, ORAC and TEAC assays. TPC was determined. |
Pahang, Malaysia |
Leaf | Ethanol | Phenols | TPC of PM was 1.63 ± 0.02 mg GAE/L sample, DPPH scavenging activity was 719.89 ± 0.73 mg TE/L sample. ORAC value was 5.81 ± 0.05 TE/L sample. TEAC value was 27.16 ± 0.82 TE/L sample. Meat patties wrapped in different concentrations of PM extract had lower TBARS values than the control. |
PM has high antioxidative activity and can be used as a source of natural antioxidants to extend the shelf life of meat products. | Wan Yahaya et al., 2019 |
| In vivo study | |||||||
| In vivo animal study. Sprague Dawley rats were divided into seven groups (negative control received carboxymethyl cellulose, positive control received omeprazole and remaining five groups were pre-treated for 1 h with five different fractions (F1,F2, F3, F4, and F5, obtained from PM ethanolic extract). Gastric ulcer in the rats was subsequently induced by administering 95% ethanol. Antioxidative activity in each rat's stomach tissues was measured using superoxide dismutase (SOD) activity. |
Selangor, Malaysia | Leaf | Five different fractions (F1, F2, F3, F4, and F5) obtained from ethanolic extract. |
Gallic acid, coumaric acid, rutin, and quercetin | Pre-treatment of rats with ethyl acetate:methanol fraction (1:1) of PM ethanolic extract (F2) increased SOD activity in the gastric wall mucosa of the rats. F2 contained the highest amount of phenolic compounds, such as gallic acid, rutin, coumaric acid, and quercetin. Gallic acid and coumaric acid are the most abundant compounds in the fraction. |
The ethyl acetate: methanol fraction (1:1) of PM ethanolic extract confers protection against ethanol-induced gastric ulcer by enhancing antioxidative activity and prostaglandin E2 synthesis. |
Qader et al., 2012 |
| Combined in vitro and in vivo study | |||||||
| Chemical assay and in vivo animal study. In vitro anti-inflammatory activity measured using COX-1, COX-2, 5-LOX, and sPLA2 inhibition assays. In vivo anti-inflammatory assay measured using carrageenan-induced paw edema test. Thirty-two male and female Wistar albino rats aged 8-10 weeks were injected with carrageenan at the foot pads and randomly divided into four groups of eight rats (n = 8). The rats in the four experimental groups were fed with the test agents 30 min after the carrageenan injection; (i) vehicle control (0.5% carboxymethyl cellulose); (ii) positive control (10 mg/kg bw diclofenac sodium); (iii) 100 mg/kg bw PM (iv) 300 mg/kg bw PM. The paw volume of each rat was measured at different time intervals (0, 2, 4, and 6 h) after carrageenan injection. |
Selangor, Malaysia | Aerial parts (stem and leaf) | Aqueous Ethanol |
- | PM ethanolic extract at a dose of 100 μg/mL showed 100% inhibition on COX-1 and 5-LOX, 25% inhibition on COX-2, and no inhibition on sPLA2. PM ethanolic extract at a dose of 30 μg/mL showed 100% inhibition on 5-LOX, 35% inhibition on COX-1, and no inhibition on COX-2 and sPLA2. Rats fed with aqueous extract of PM (100 and 300 mg/kg bw) showed reduction of paw edema volume after 4 h compared with the control group. |
PM exerts an anti-inflammatory effect by inhibiting COX and 5-LOX activities. | George et al., 2014b |
| Combined in vitro and ex vivo study | |||||||
| Chemical assay and ex vivo study using red blood cells. Antioxidative activity measured using ORAC and CAP-e assays. |
Selangor, Malaysia | Leaf | Aqueous | - | The total ORAC value of PM was 16,964 μmole TE/g. For the inhibition of cellular oxidative damage, IC50 of PM was 0.58 g/L as derived from CAP-e assay. |
PM has antioxidative activity and can reduce oxidative stress in a dose-dependent manner. | George et al., 2014a |
DPPH: 1-diphenyl-2-picrylhydrazyl; 5-LOX: 5-lipoxygenase; ABTS: 2,20-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid diammonium salt; BCL: ß-carotene linoleate; BHA: butylated hydroxyanisole; BHT: butylated hydroxytoluene; bw: body weight; CAA: cellular antioxidant activity; CAP-e: cellular antioxidant protection of erythrocytes; COX-1: cyclooxygenase-1; COX-2: cyclooxygenase-2; DM: dried matter; EC50: effective concentration at 50%; FIC: ferrous ion-chelating; FTC: ferric tyhiocyanate; FRAP: ferric-reducing antioxidant power; FW: fresh weight; GAE: gallic acid equivalent; IC50: inhibition concentration at 50%; LoD: Lab-on-a-Disc MDA: malondialdehyde; SOD: superoxide dismutase; TAE: tannic acid equivalents; TBA: thiobarbituric acid; ORAC: oxygen radical absorbance capacity; PM: Polygonum minus; POV: peroxide value; sPLA2: secretory phospholipase A2; TBARS: thiobarbituric acid reactive substances; TE: trolox equivalent; TEAC: trolox equivalent antioxidant capacity; TFC: total flavonoid content; TPC: total phenolic content; TVC: total vitamin C content.
A total of 125 articles were retrieved from four online database, of which 17 articles were from ACS Publication, 18 articles were from Ovid MEDLINE, 19 articles were from PubMed, and 71 articles were from Scopus. Another eight additional articles were retrieved from the list of studies cited in review articles and through Google Scholar. Subsequently, 22 articles were removed because of duplication. After the titles and abstracts were reviewed, 94 articles were excluded. The full-length articles for the remaining 17 studies were obtained and reviewed thoroughly. In total, 17 articles fulfilled the inclusion criteria and included in this review. The article selection process is shown in Fig. 2.
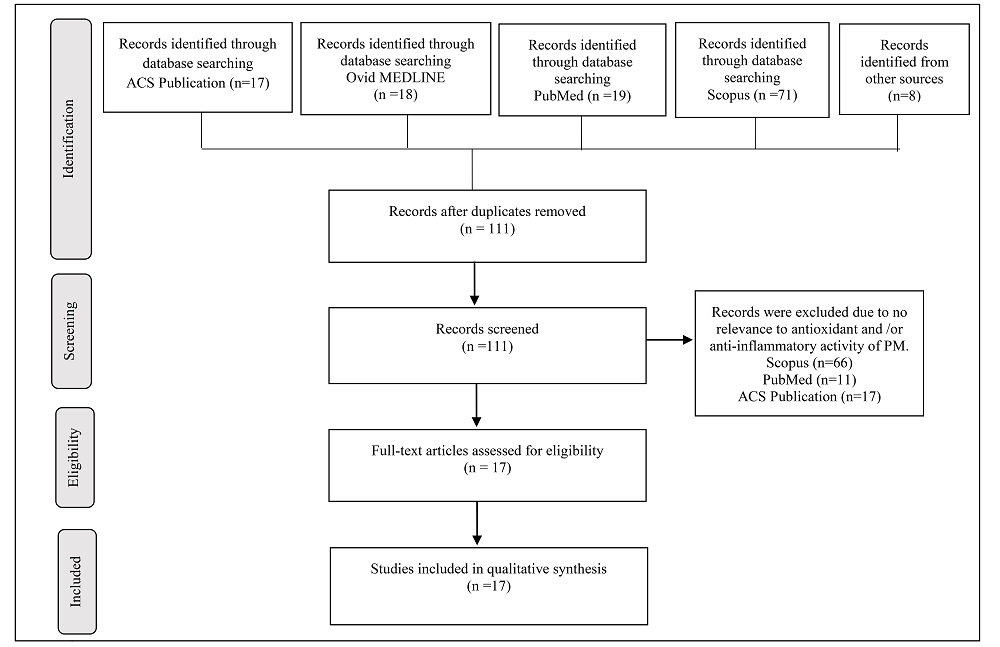 Figure 2.
Figure 2.PRISMA flow diagram. Summary of the literature search and the study selection process according to the PRISMA guidelines. Overall, 17 studies met the search criteria. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
All studies are listed in Table 1. A total of 17 studies met the inclusion criteria and were published between 2007 and 2019. These studies consisted of 14 in vitro studies, one in vivo study, one combined in vitro and in vivo study, and one study combining in vitro and ex vivo assays. According to the type of activities, 16 studies investigated antioxidative activity and only one assessed the anti-inflammatory activity of PM. The types of animal used in the animal studies were Wistar albino rats (George et al., 2014b) and Sprague Dawley rats (Qader et al., 2012). The former were used in antioxidant study whereas the latter were used in anti-inflammatory study. In an in vitro study described in this review, enzyme assays measuring the activities of COX-1, cyclooxygenase-2 (COX-2), 5-LOX and secretory phospholipase A2 (sPLA2) were used in assessing the anti-inflammatory action of PM (George et al., 2014b). Meanwhile, an in vitro cell culture study assessed the antioxidative activity of PM using HCT116 cells (Abdullah et al., 2017). In one study using an ex vivo model, erythrocytes treated with oxidizing agent were used in determining the cellular antioxidant protection of PM (George et al., 2014a). The chemical assays used in this study included 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging, ferric reducing antioxidant potential (FRAP), oxygen radical absorbance capacity (ORAC), β-carotene linoleate (BCL), ferric thiocyanate (FTC), superoxide anion scavenging, thiobarbituric acid-reactive substances (TBARS), thiobarbituric acid (TBA) and trolox equivalent antioxidant capacity (TEAC) assays. A new Lab-on-a-Disc (LoD) method was reported, which integrates the conventional DPPH test with the use of a microfluidic compact disc (Rahman et al., 2018). Furthermore, carrageenan-induced paw edema test was used to determine in vivo anti-inflammatory activity of PM (George et al., 2014b). In most of the studies reviewed in this study, antioxidative activity was assessed under varying experimental conditions including different types of PM extract and concentrations.
The phytoconstituents of PM used in all the studies reviewed are listed in Table 1. In most studies, PM samples were collected from Selangor, Malaysia (George et al., 2014a; George et al., 2014b; Huda-Faujan et al., 2007, 2009; Maizura et al., 2011; Mohd Ghazali et al., 2014; Othman et al., 2014; Qader et al., 2011; Qader et al., 2012; Rahman et al., 2018; Sumazian et al., 2010). In other studies, PM samples were collected from Kuala Lumpur (Abdullah et al., 2017), Pahang (Almey et al., 2010; Wan Yahaya et al., 2019), and Perak (Ahmad et al., 2014). The majority of the studies used leaf extracts (Abdullah et al., 2017; Almey et al., 2010; George et al., 2014a; Maizura et al., 2011; Mohd Ghazali et al., 2014; Nurul et al., 2010; Othman et al., 2014; Qader et al., 2011, 2012; Rahman et al., 2018; Saputri and Jantan, 2011; Sumazian et al., 2010; Wan Yahaya et al., 2019), one study used the aerial parts of the plant (leaf and stem) (George et al., 2014b), and only one study used all parts of PM (leaf, stem and root) (Ahmad et al., 2014). Two studies used the edible portions of PM (Huda-Faujan et al., 2007, 2009). Various solvents were used for PM extraction. Four studies used aqueous extracts (George et al., 2014a; Huda-Faujan et al., 2007; Maizura et al., 2011; Nurul et al., 2010), two studies used ethanol extracts (Rahman et al., 2018; Wan Yahaya et al., 2019) and two studies used methanol extracts (Huda-Faujan et al., 2009; Saputri and Jantan, 2011). In other studies, more than one type of extracts was tested, and the total phenolic compound (TPC) and antioxidative activities of different types of PM extracts were compared. One study used aqueous and boiled aqueous extracts (Sumazian et al., 2010), one study used methanol and ethanol extracts (Almey et al., 2010), and three studies used aqueous and ethanol extracts (George et al., 2014b; Othman et al., 2014; Qader et al., 2011). Meanwhile, one study used aqueous, methanol, petroleum ether and ethyl acetate extracts (Mohd Ghazali et al., 2014), one study used aqueous, methanol, ethanol, dichloromethane and n-hexane extracts as well as PM essential oil (Ahmad et al., 2014) and one study used aqueous, methanol, petroleum ether and ethyl acetate and hexane extracts (Abdullah et al., 2017). Phytoconstituents that were identified in PM include flavonoids, phenolic acids, ascorbic acid, aliphatic compounds, organic acids, essential oils and alkaloids. Flavonoids identified in PM are apigetrin, hyperoside, isoquercetin, astragalin, miquelianin, quercetin and quercitrin (Abdullah et al., 2017), and phenolic acids identified are gallic acid and coumaric acid (Qader et al., 2011). The compounds of essential oils, such as monoterpenes and sesquiterpenes, were identified in one study (Ahmad et al., 2014).
A total of 16 studies demonstrated that PM had antioxidative activity. These studies included 14 in vitro studies, one in vivo study (Qader et al., 2012) and one combined in vitro and and ex vivo study (George et al., 2014a). Meanwhile, one combined in vitro and in vivo animal study showed the anti-inflammatory activity of PM (George et al., 2014b). All types of studies (in vivo and ex vivo animal studies, in vitro cell culture and chemical assay studies) demonstrated the positive effects of PM extracts (parts or whole plant) on oxidative and inflammatory conditions.
This is the first paper that reviews systematically current evidence related to the antioxidative and anti-inflammatory activities of PM. Sixteen studies showed the positive antioxidative activities of PM. Most of these studies were chemical assay studies, and FRAP and DPPH scavenging assays were the most common tests performed because of their straightforward method and reliability. In the FRAP assay, reduction power of Fe3+ to Fe2+ in the presence of antioxidant was measured. Colorless Fe3+ is converted to a blue-colored Fe2+ tri-pyridyl triazine (TPTZ-reduced form, which is due to the action of the electron donation from antioxidants) (Vijayalakshmi and Ruckmani, 2016). Meanwhile, DPPH scavenging assay measures the reducing ability of antioxidant toward DPPH, which is a stable radical. DPPH reacts with compounds that can donate hydrogen atoms and decolorize the DPPH solution, causing a decrease in absorbance. The FRAP values of aqueous and methanol extracts of PM at 200-1200 and 600-1200 ppm, respectively, are equivalent to the FRAP value of the synthetic antioxidant BHT (Huda-Faujan et al., 2007, 2009). The results of other antioxidant assays, such as ABTS, BCL, FIC, CAA and superoxide anion scavenging assays, followed the trends of the results of the DPPH and FRAP assays.
Oxidizing agents cause lipid peroxidation that results in the formation of malondialdehyde (MDA), which can be measured because it reacts with TBARS. In some studies, the term TBA was used (Huda-Faujan et al., 2007, 2009). Other studies used the term TBARS, which refers to the same assay (Nurul et al., 2010; Saputri and Jantan, 2011; Wan Yahaya et al., 2019). Four out of 16 studies used TBARS to determine the antioxidative effect of PM extracts. The TBARS values of aqueous and ethanolic PM extracts were comparable to BHT (Huda-Faujan et al., 2007, 2009). In one of the studies using duck refrigerated meatball, TBARS level was lower in samples treated with PM aqueous extract than in the control (no antioxidant treatment) and BHT-treated samples, suggesting that PM is a potential natural shelf life enhancer in commercial food industry (Nurul et al., 2010). In one study, methanolic extract of PM was investigated for its ability to inhibit copper-mediated oxidation in isolated human LDL which was then measured using the TBARS assay (Saputri and Jantan, 2011). The results from this study indicated that the methanolic extract of PM contained compounds that can inhibit LDL oxidation and this effect is comparable to that of probucol. Together, evidence from the studies showed that aqueous, ethanol, and methanol extracts of PM can inhibit lipid peroxidation.
In some studies, the antioxidative activities of different types of PM extracts were compared. Solvents used to extract PM can be divided into three groups, namely, polar solvent, semipolar and nonpolar solvents. Aqueous, methanol and ethanol are polar solvents while ethyl acetate is a semipolar solvent. Petroleum ether, dichloromethane and n-hexane belong to the nonpolar solvent group. Essential oils are solvent with a mixture of polar and nonpolar molecules. Three studies compared the antioxidative activity of PM extracted using polar and nonpolar solvents (Abdullah et al., 2017; Ahmad et al., 2014; Mohd Ghazali et al., 2014). The results indicated that PM polar extracts (ethanol and methanol) had higher antioxidative activity than nonpolar extracts, as demonstrated by DPPH scavenging activities and FRAP (Ahmad et al., 2014). Meanwhile, PM extracted using semipolar solvent (ethyl acetate) had the highest DPPH scavenging activity, FRAP and TPC followed by polar solvents (aqueous and methanol), and nonpolar solvent (petroleum ether) (Mohd Ghazali et al., 2014).
The higher antioxidative activity of PM polar extracts may be attributed to the high TPC obtained from this extraction method. High phenolic compounds are often extracted in polar solvents because polyphenols solubility mainly depends on the presence and position of hydroxyl groups and on the molecular sizes and lengths of constituent hydrocarbon chains (Iloki-Assanga et al., 2015). It was also noted that for the similar polar solvent used, different plant parts gave different extraction yield. According to extraction yield, ethanolic extract of PM leaves had the highest TPC, whereas root had the lowest TPC. The results from the selected studies are consistent with other studies using other medicinal plants, indicating that the extraction efficiency favors highly polar solvents (Iloki-Assanga et al., 2015).
Major phytoconstituents identified in PM are phenols which were measured as total phenolic compounds through the Folin-Ciocalteu assay. Specifically, phenolic compounds that were identified in PM are phenolic acids (coumaric acid and gallic acid), flavonoids (apigetrin, astragalin, hyperoside, miquelianin, isoquercetin, quercetin, and quercitrin), saponins, essential oils (monoterpenes and sesquiterpenes), aliphatic compounds, and organic acids. Eight studies reported the correlation between TPC and the antioxidative activities of PM. Out of these eight studies, seven studies reported positive correlation between TPC and the antioxidative activities of PM extracts. Specifically, positive correlations were noted among the TPC of PM extract, DPPH scavenging activity, and FRAP values. One study reported weak and negative correlation between TPC and DPPH scavenging activity in the methanol and aqueous extracts of PM. Three studies determined the TFC that was expressed as gram rutin equivalent per 100 g FW (g RE/100g FW) (Othman et al., 2014), mg catechin/g dry weight (mg CE/g DW) (Sumazian et al., 2010) and mg quercetin per gram dry extract (mg QE/g extract) (Abdullah et al., 2017). Two of these three studies reported the positive correlation between TFC and DPPH scavenging activity, LDL antioxidative activity, and nitric oxide scavenging activity (Abdullah et al., 2017; Sumazian et al., 2010). Meanwhile, a weak correlation between TFC of PM extract and its antioxidative activity was reported (Othman et al., 2014). Overall, these results indicate that phenolic compounds are the major contributors of PM's antioxidant effect.
Most of the studies did not distinguish the phenolic and flavonoid constituents of PM. Only one study identified phenolic compounds in the fractions of PM ethanolic extracts through high performance liquid chromatography (HPLC). High contents of gallic acid, rutin, coumaric acid, and quercetin were identified in one of the fractions. This fraction was able to inhibit gastric ulcer formation and maintain SOD level in an ethanol-induced oxidative stress rat model (Qader et al., 2012). HPLC analysis of methanolic extract of PM revealed the presence of flavonoids, namely, apigetrin, hyperoside, isoquercetin, astragalin, miquelianin, quercetin and quercitrin which contributed to antioxidative activity (Abdullah et al., 2017). Miquelianin, hyperoside, astragalin, isoquercetin and quercitrin were demonstrated to affect inflammation-related diseases and decrease the risk of CVD (Hooper et al., 2008) and CVD-related mortality (Grosso et al., 2017). Apart from polyphenols, PM extracts contain tannins (Abdullah et al., 2017) and gallic acid (Qader et al., 2012), which have anticarcinogenic effects (Faried et al., 2007; Hostnik et al., 2019). Owing to the presence of tannins and gallic acid in the extracts of PM, potential anticarcinogenic effects should be investigated and reviewed in future studies.
Out of the 17 studies, only one study assessed the anti-inflammatory effect of PM. In this study, the anti-inflammatory effects of ethanolic and aqueous extracts of PM were evaluated using in vitro and in vivo methods. In the in vitro study, the anti-inflammatory action of PM ethanolic extract was tested using COX-1, COX-2, 5-LOX and sPLA2 inhibition assays (George et al., 2014b). These are the key enzymes that mediate the inflammatory process activated by the release of various membrane components, including phospholipids, which are then converted to arachidonic acid (AA) by the enzyme phospholipase A2 (PLA2). Arachidonic acids generated in excess are converted to inflammatory substances, such as prostaglandins by cyclooxygenase (COX) and leukotrienes by lipoxygenase (LOX) pathways (Yui et al., 2015).
Results showed that PM ethanolic can inhibit COX-1, COX-2 and 5-LOX but not sPLA2. These findings were further supported by the reduction of carrageenan-induced paw edema in rats fed with PM aqueous extracts (George et al., 2014b). The ability of PM to inhibit COX-1, COX-2, and 5-LOX indicated that PM exerts its anti-inflammatory effect by inhibiting COX and 5-LOX enzymes. Meanwhile, its inability to inhibit sPLA2 indicates that it does not modulate the production of AA from membrane phospholipids. Thus, PM is a potential anti-inflammatory agent. The mechanisms underlying the anti-inflammatory activity of PM is summarized in Fig. 3. Apart from COX-1, COX-2, 5-LOX, and sPLA2 inhibitory activity and reduction of rats paw edema, other parameters have been widely used in measuring the anti-inflammatory activities of medicinal plants but are not tested yet for PM. These parameters include enzymes, cytokines, and transcription factors that are involved in the inflammatory process such as inducible nitric oxide synthase, nuclear factor ĸB, C- reactive protein, IL-1β, IL-6, IL10, and TNF-α (Azab et al., 2016).
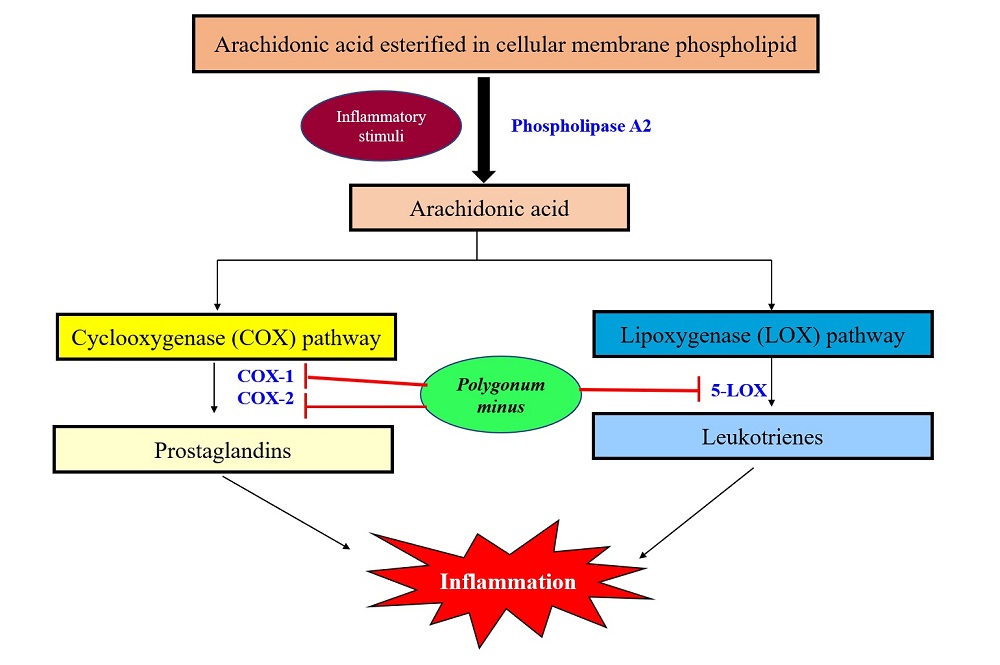 Figure 3.
Figure 3.Schematic representation of the anti-inflammatory mechanisms of Polygonum minus. PM exerts its anti-inflammatory effect by inhibiting COX-1, COX-2 and 5-LOX enzymes. COX-1: Cyclooxygenase-1; COX-2: Cyclooxygenase-2; 5-LOX: 5-lipoxygenase.
Summary of the proposed mechanisms underlying the antioxidative and anti-inflammatory effects of PM and its phytoconstituents is depicted in Fig. 4. Most of the studies that evaluated the antioxidative and anti-inflammatory effects of PM were conducted using in vitro chemical assays. Currently, in vivo studies and clinical trials involving PM are few. In vivo animal study would be beneficial to the understanding of the biological actions of PM. Data from animal studies are essential particularly before clinical trials are performed on human subjects. Other parameters for antioxidative activities, such as antioxidant enzymes (SOD, CAT, and GPX) are not much tested. Data from these additional parameters would provide concrete evidence of the antioxidative activity of PM. As mentioned previously, only two studies identified the specific compounds of PM extracts. Studies assessing the effects of specific active compounds in PM extract should be conducted. Given that parameters and studies that evaluate the anti-inflammatory activity of PM are limited, further study in this area is necessary.
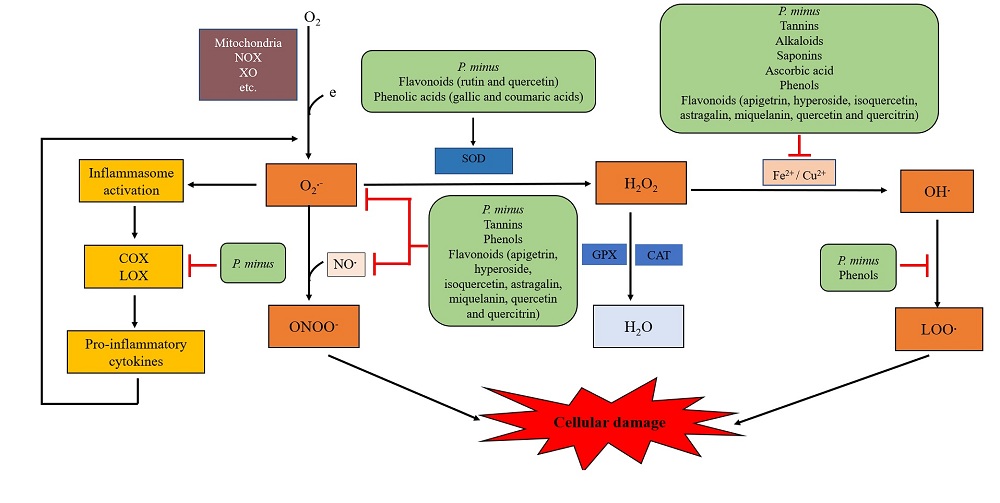 Figure 4.
Figure 4.Summary of the proposed mechanisms underlying the antioxidative and anti-inflammatory effects of Polygonum minus and its phytoconstituents. Superoxide anions (O2●-) produced from the reactions of electrons (e) with molecular oxygen (O2) by several sources, such as the mitochondrial electron transport chain, NADPH oxidase (NOX), xanthine oxidase (XO), etc., act as the primary reactive oxygen species. O2●- activates the inflammatory cascade, hence enhancing more O2●- productions. Besides, O2●- reacts with nitric oxide (NO●) to produce peroxynitrite (ONOO-) or is catalyzed to hydrogen peroxide (H2O2) by superoxide dismutase (SOD). H2O2 is converted to water (H2O) by catalase (CAT) and glutathione peroxidase (GPX). However, in the presence of iron (Fe2+) and copper (Cu2+), H2O2 is transformed into a highly toxic hydroxyl free radical (OH●) via the Fenton reaction. OH● can be converted into lipid peroxyl radical (LOO●). These free radicals target biomolecules such as DNA, protein and lipids, ultimately causing cellular damage. P. minus and its phytoconstituents act by directly scavenging the O2●- and NO●, stimulating the SOD activity, and inhibiting the Fenton reaction and lipid peroxidation. P. minus also inhibits cylclooxygenase (COX) and lipoxygenase (LOX) in the inflammatory pathway.
PM extracts have antioxidative and anti-inflammatory activities that are attributed to its phytoconstituents such as phenolic compounds, flavonoids, ascorbic acid, tannins, and alkaloids. Therefore, PM has the potential to be developed as a natural antioxidative and anti-inflammatory agent for diseases related to oxidative stress and inflammation, such as cardiovascular diseases.
Adila A Hamid and Azizah Ugusman contributed to conception and design; Adila A Hamid, Amilia Aminuddin, Mohd Heikal Mohd Yunus, Jaya Kumar Murthy, Chua Kien Hui and Azizah Ugusman contributed to data acquisition, analysis and interpretation; and Adila A Hamid and Azizah Ugusman were involved in drafting the manuscript and revising it critically for important intellectual content. All authors have given final approval on the version to be published.
This study was funded by Universiti Kebangsaan Malaysia Research University Grant (GUP-2018-145) and UKM Medical Centre Matching Grant (FF-2019-084/1). The authors would like to thank Mrs. Norizam Salamt for her assistance in formatting the manuscript.
The authors have no conflict of interest to declare.
