We performed a meta-analysis comparing the procedural and outcomes data and related to left atrial appendage occlusion guided by intracardiac echocardiography (ICE) and transesophageal echocardiography (TEE) in nonvalvular atrial fibrillation patients. Technical success with ICE was significantly similar to that of TEE (odds ratio [OR] 1.38, 95% CI [0.62, 3.09], I2 = 0%, P = 0.43). The peri-procedural complications showed no significant difference between the two groups (OR 0.84, 95% CI [0.57, 1.23], I2 = 0%, P = 0.37). Mortality was similar in procedures using ICE vs TEE (OR 0.89, 95% CI [0.51, 1.57], I2 = 0%, P = 0.69). Landing zones, procedural time and fluoroscopic times between ICE and TEE showed no significant differences (MD 1.96, 95% CI [-0.01, 3.94], I2 = 90%, P = 0.05; MD -1.64, 95% CI [-13.45, 10.17], I2 =95%, P = 0.79; and MD 0.49, 95% CI [-2.18, 3.16], I2= 87%, P = 0.72, respectively). Imaging with ICE or TEE is associated with similar outcomes in left atrial appendage occlusion procedures.
Currently, left atrial appendage occlusion (LAAO) or left atrial appendage closure has emerged as an alternative treatment to prevent stroke in patients with chronic nonvalvular atrial fibrillation (NVAF) (Reddy et al., 2011) who are unable to undergo oral anticoagulation (OAC) therapy due to a formal contraindication for OAC or an unacceptable risk of bleeding (Kirchhof et al., 2016). The procedure is mostly performed with fluoroscopic or transesophageal echocardiography (TEE) guidance (Wunderlich et al., 2015). However, TEE requires general anesthesia or profound sedation. Using intracardiac echocardiography (ICE) to guide LAAO, which only requires conscious sedation, may have potential advantages over TEE. Although some studies (Aguirre et al., 2018; Frangieh et al., 2017; Hemam et al., 2019; Ho et al., 2007; Masson et al., 2015; Matsuo et al., 2016) with small samples of patients demonstrated that the feasibility and efficacy of ICE-guided LAAO were not inferior to those of TEE, the findings remain inconsistent, and few meta-analyses have assessed the results. Therefore, we sought to systematically evaluate the extent to which ICE is an alternative to TEE.
This meta-analysis was performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (Moher et al., 2009). The Medline (PubMed), EMBASE and Cochrane databases were electronically searched for primary research papers through Mar 25th, 2019, utilizing combinations of the medical subject heading (MeSH) terms, keywords and word variants of ‘atrial fibrillation’, ‘left atrial appendage occlusion’, ‘transesophageal echocardiography’, and ‘intracardiac echocardiography’. Studies were included regardless of their language and the year of publication. Two independent reviewers conducted the literature search. Disagreements were resolved by consensus with the addition of a third reviewer.
Cohort studies (prospective cohort, retrospective cohort) and consecutive (prospective, retrospective) studies were eligible for inclusion if they investigated ICE and TEE guidance during the PLAAO procedure in NVAF patients. Moreover, TEE was used before the procedure to exclude the presence of thrombi in the left atrium (LA), to assess the ICE guidance and to monitor periprocedural complications and complications during follow-up. The ICE probe was placed in the right atrium (RA), coronary sinus (CS) or LA during the procedure. Studies were excluded if they did not compare ICE with TEE. Additionally, secondary publications, animal experiments, case reports, letters, and editorials were excluded.
The primary endpoints were technical success and complications. Technical success was defined as LAAO implantation guided by ICE when the probe was placed in the RA or LA. Periprocedural endpoints were mainly death, pericardial effusion including tamponade, myocardial infarction (MI), device embolization, stroke, residual leaks, bleeding and hematomas. The secondary endpoints were related to procedural efficacy: measurements of the landing zone (LZ) of the left atrial appendage (LAA), procedural time, fluoroscopic time and contrast usage.
All data from the included studies were extracted by two reviewers independently, and a consensus was reached in case of any disagreement with the involvement of a third investigator. An extraction form was predesigned to obtain the relevant data. Information extracted from eligible studies included the name of the first author or the name of the study, year of publication, original country, proportion of sexes, average age of the patients, number of participants, primary outcomes and numbers.
Quality assessment of the included studies was performed using the nine-star Newcastle-Ottawa Scale (NOS) (Wells et al, 2019), for the included studies. The number of stars represents the quality of the study, which means that higher quality studies have more stars.
Continuous variables are reported as the means and medians, whereas categorical variables are reported as proportions. An odds ratio (OR) for the outcome of interest and the 95% confidence interval were calculated to record the pooled effect. A P-value < 0.5 was considered statistically significant. Heterogeneity between studies was evaluated using the χ2 test and the I2 statistic. A value of P < 0.10 for the χ2 statistic or values of I2 ≥ 50% indicated a substantial level of heterogeneity. Next, we conducted sensitivity analyses to explore the source of heterogeneity. Funnel plot asymmetry analysis was not performed when the total number of studies included for each outcome was < 10. All statistical analyses were performed using Review Manager software (RevMan) [Computer program] Version 5.3. (The Nordic Cochrane Center, The Cochrane Collaboration, 2014, Copenhagen).
A total of 126 articles were identified after duplicates were removed, and 81 studies were excluded based on their titles and abstracts. Next, screening of the full texts of the remaining 27 articles resulted in the identification of 15 studies that met all eligibility criteria, as summarized in the PRISMA chart (Fig. 1).
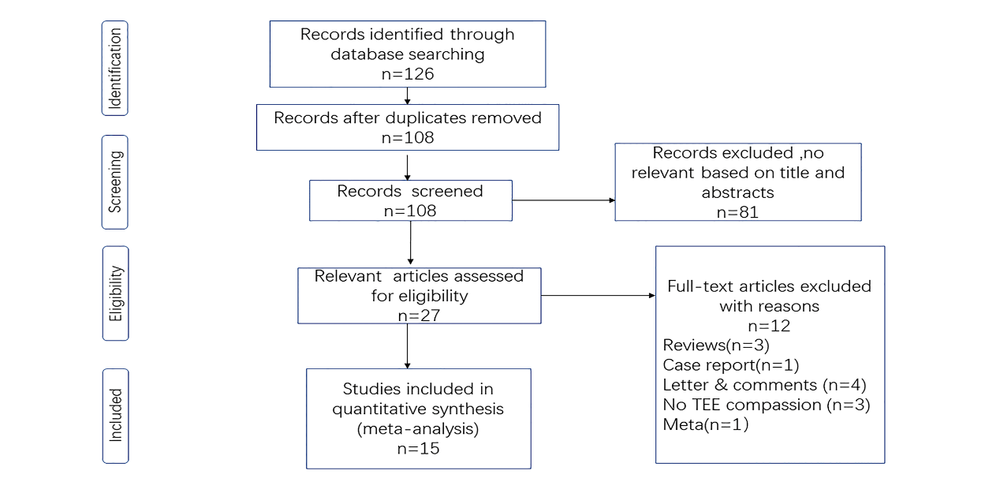 Figure 1.
Figure 1.Flowchart of the processing of the included studies
The baseline information of the included studies is reported in Table 1. Patients were mainly from Europe (7 studies) (Berti et al., 2014; Berti et al., 2018; Clemente et al., 2015; Frangieh et al., 2017; Korsholm et al., 2017; Matsuo et al., 2016; Reis et al., 2018), the Asia-Pacific region and the US (8 studies) (Aguirre et al., 2018; Frikha et al., 2016; Hemam et al., 2019; Ho et al., 2007; Iwasawa et al., 2016; Kim et al., 2018; Masson et al., 2015; Naim et al., 2015). There were 827 patients in the ICE group and 1236 patients in the TEE group. The ages ranged from 67.1 ± 8.4 to 81 ± 7.4 years. We compared the technical success of device implantation, periprocedural complications (Table 2) and procedural endpoints (Table 3).
| Study | Year | Country | ICE | TEE | Follow-up | Primary endpoints | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men, N (%) | Age (years) | Men, N (%) | Age(years) | ||||||||
| Aguirre | 2018 | Chile | 13 (59.1) | 74 ± 9.3 | 13 (59.1) | 74 ± 9.3 | 30 days | death, pericardial effusion, MI, stroke, renal failure, length of hospital stay | |||
| Berti | 2014 | Italy | 69 (57) | 77 ± 7.6 | 69 (57) | 77 ± 7.6 | within 24 h | pericardial effusion, device embolization, stroke, bleeding, hematoma, others | |||
| Berti | 2018 | Italy | 123 (66) | 76 ± 8 | 271 (65) | 74 ± 7 | 6, 12 months | death, tamponade, stroke, TIA, systemic embolism, major bleeding, device embolization | |||
| Clemente | 2015 | Italy | 78 ± 6 | 78 ± 6 | 5.2 ± 6 months | residual leaks | |||||
| Frangieh | 2017 | Switzerland | 26 (81) | 76 ± 8.9 | 25 (57) | 81 ± 7.4 | in hospital | death, tamponade, pericardial effusion, device embolization, stroke, peridevice leak, bleeding, renal failure | |||
| Frikha | 2016 | Canada | 74.4 ± 7.6 | 74.4 ± 7.6 | 3 months | tamponade, MI, device embolization, stroke, hematoma | |||||
| Hemam | 2019 | USA | 33 (62.3) | 77 ± 10 | 31 (60.8) | 76 ± 7 | 45-120 d | death, tamponade, device embolization, stroke, hematoma, peridevice flow | |||
| Ho, I | 2007 | USA | 8 (80) | 67.1 ± 8.4 | 8 (80) | 67.1 ± 8.4 | 12 months | strokes, transient ischemic attacks | |||
| Iwasawa | 2016 | USA | n | n | n | n | n | death, tamponade, bleeding, hematomas | |||
| Kim | 2018 | Korea | 24 (58.5) | 71.4 ± 9.3 | 51 (49.5) | 72.3 ± 9.2 | 2 weeks, 3 months | death, tamponade, pericardial effusion, stroke, MI, device embolization, air-embolization, thrombus | |||
| Korsholm | 2017 | Denmark | 68 (62) | 73 ± 7.8 | 79 (74) | 73 ± 9.7 | 8 weeks | death, pericardial effusion, device embolization, stroke, bleeding, hematoma, pseudoaneurysm | |||
| Masson | 2015 | Canada | 25 (67.6) | 74.7 ± 8.2 | 25 (67.6) | 74.7 ± 8.2 | 3 months | tamponade, MI, bleeding | |||
| Matsuo | 2016 | Czech | 11 (40.7) | 77.0 ± 8.5 | 11 (40.7) | 77.0 ± 8.5 | 45 d | death, pericardial effusion, stroke, hematoma, others | |||
| Naim C | 2015 | Canada | 75 ± 8 | 75 ± 8 | 3 months | tamponade, device embolization, stroke, hematoma | |||||
| Reis | 2018 | Portugal | 53 (64.6) | 74 ± 8 | 53 (64.6) | 74 ± 8 | 6-12 months | death, tamponade, pericardial effusion, stroke, hematoma, bleeding | |||
| Study | Year | ICE | TEE |
|---|---|---|---|
| n = 823 | n = 1236 | ||
| Aguirre | 2018 | renal failure (1), Length of hospital (1) | renal failure (1), Length of hospital (1) |
| Berti | 2014 | Serious pericardial effusion (3), Procedure-related ischemic stroke (1), Femoral hematoma (2) | Serious pericardial effusion (3), Procedure-related ischemic stroke (1), Femoral hematoma (2) |
| Berti | 2018 | TIA (1), Device embolization (1), Pericardial tamponade (3), Major bleeding (3) | Ischemic stroke (1), TIA (1), Device embolization (1), Pericardial tamponade (8), Major bleeding (16) |
| Frangieh | 2017 | pericardial effusion (1) | esophageal erosion with bleeding (1), non-LAAO related death (1) |
| Frikha | 2016 | Tamponade (1), silent MI (1), hematoma (3) | Tamponade (1), silent MI (1), hematoma (3) |
| Iwasawa | 2016 | groin hematoma (2) | cardiac tamponade (1), retro-peritoneal bleed (1), groin hematoma (2) |
| Kim | 2018 | Pericardial effusion (1) | Cardiac tamponade (1), Pericardial effusion (2), Device embolization (3), Vascular complications (1) |
| Korsholm | 2017 | Pericardial effusion with tamponade (2), Access-site hematoma (3), Pseudoaneurysm (1) | Device embolization n (1), Ischemic stroke (1), Hemorrhagic stroke (1), Major extracranial bleeding (2), Access-site hematoma (1) |
| Masson | 2015 | Tamponade (1), Silent myocardial infarction (1), bleed (1) | pulmonary embolism (1), heart failure (1), bleed (1) |
| Matsuo | 2016 | pericardial effusion (1), hematoma (3), transient dizziness (1) | Hematoma (3) |
| Naim C | 2015 | Tamponade (2), hematoma (2) | residual leak (2), bleeding (2) |
| Reis | 2018 | pericardial effusion (2), hematoma (1) | residual leak (5), bleeding (4) |
| Total | 5.6% | 6.5% |
| Study | Year | Landing zone (mm) | Procedural time (min) | Fluoroscopic time (min) | Contrast usage (ml) | ||||
|---|---|---|---|---|---|---|---|---|---|
| ICE | TEE | ICE | TEE | ICE | TEE | ICE | TEE | ||
| Berti | 2018 | 108 ± 33 | 92 ± 34 | 25 ± 12 | 20 ± 11 | ||||
| Clemente | 2015 | 21 ± 2.8 | 16.9 ± 3.7 | ||||||
| Frangieh | 2017 | 48 ± 14.8 | 34.5 ± 12.6 | 9.8 ± 4.6 | 7.9 ± 6.74 | 85 ± 21.7 | 90 ± 63.7 | ||
| Hemam | 2019 | 21.27 ± 3.27 | 21.5 ± 4.68 | 46 ± 24 | 46 ± 30 | 4.8 ± 2.7 | 7.3 ± 4.7 | ||
| Ho, I | 2007 | 22.6 ± 3.4 | 19.5 ± 1.5 | ||||||
| Iwasawa | 2016 | 53 ± 31 | 57 ± 30 | ||||||
| Kim | 2018 | 26.0 ± 1.5 | 25.9 ± 1.4 | 58.0 ± 4.44 | 80.0 ± 27.41 | 7.6 ± 3.78 | 8.3 ± 10.81 | ||
| Korsholm | 2017 | 44 ± 11.9 | 55 ± 28.1 | 15 ± 5.9 | 14 ± 8.9 | 60 ± 17.7 | 70 ± 20 | ||
| Masson | 2015 | 105 ± 34 | 25 ± 10 | 155 ± 69 | |||||
| Matsuo | 2016 | 23.7 ± 3.8 | 20.4 ± 5.4 | 2.98 ± 0.8 | |||||
| Naim C | 2015 | 91.0 ± 28.1 | |||||||
| Reis | 2018 | 65.8 ± 15.2 | 69.9 ± 13.6 | 30.4 ± 17.0 | 35.1 ± 16.5 | ||||
ICE, intracardiac echocardiography. TEE, transesophageal echocardiography.
All included studies were nonrandomized trials, and we used the NOS for quality assessment (Table 4).
| Study | Selection | Comparability | Outcome |
|---|---|---|---|
| Aguirre | ★★★ | ★ | ★★ |
| Berti 2014 | ★★★ | ★★ | |
| Berti 2018 | ★★★ | ★★ | ★★ |
| Clemente | ★★★ | ★ | ★ |
| Frangieh | ★★★ | ★★ | |
| Frikha | ★★★ | ★ | ★★ |
| Hemam | ★★★ | ★★ | ★★ |
| Ho, I | ★★★ | ★★ | ★★ |
| Iwasawa | ★★ | ★ | |
| Kim | ★★ | ★★ | ★★ |
| Korsholm | ★★★ | ★★ | ★★ |
| Masson | ★★★ | ★ | ★★ |
| Matsuo | ★★★ | ★ | ★★ |
| Naim C | ★★★ | ★ | ★★ |
| Reis | ★★★ | ★★ | ★★ |
NOS, Newcastle-Ottawa Scale
In total, 99.0% of patients in the ICE group versus 98.7% of patients in the TEE group completed the procedure successfully (Fig. 2, OR 1.38, 95% CI [0.62, 3.09], I2 = 0%, P = 0.43). There was no significant difference between ICE guidance and TEE guidance during the closure procedure.
 Figure 2.
Figure 2.Forest plot of the technical success of ICE vs TEE.
Fig. 3 shows the pooled effect of periprocedural complications. A total of 5.6% of patients in the ICE group and 6.5% of patients in the TEE group had periprocedural complications. Periprocedural complications were not significantly different between the procedures guided by ICE and those guided by TEE (Fig. 3: OR 0.84,95% CI [0.57, 1.23], I2 = 0%, P = 0.37). The funnel plot showed no publication bias (Fig. 4).
 Figure 3.
Figure 3.Forest plot of periprocedural complications.
 Figure 4.
Figure 4.Funnel plot of periprocedural complications.
Periprocedural mortality was not observed in ICE patients, but periprocedural mortality was observed in one TEE patient (Frangieh et al. 2017); this patient died due to non-LAAO-related comorbidities after multiple other interventions. The deaths during follow-up were similar to periprocedural deaths.
LZ measurements: The LZs of the LAA were measured by ICE and TEE, as shown in Fig. 5. The sizes were significantly similar between ICE and TEE (MD 1.96, 95% CI [-0.01, 3.94], I2 = 90%, P = 0.05). Next, we carried out the sensitivity analysis using fixed effects due to the substantial heterogeneity. The forest plot showed a large amount of variance in the overall effect (MD 0.77, 95% CI [0.32, 1.23], I2 = 90%, P = 0.0009). When we excluded each of the five studies one at a time, the heterogeneity did not change substantially. The heterogeneity might have been affected by confounding factors.
 Figure 5.
Figure 5.Forest plot of the measurements of the LZ for ICE vs TEE. Random effects model.
The procedural times are presented in Fig. 6. The duration results showed no significant difference between the ICE procedure and the TEE procedure (MD -1.64, 95% CI [-13.45, 10.17], I2=95%, P = 0.79). Similarly, the sensitivity analysis was conducted with a fixed effects model due to the high degree of heterogeneity. The fixed effects model showed minor variance (MD -2.15, 95% CI [-4.71,0.41], I2=95%, P=0.10). When we excluded each of the 7 studies sequentially, the heterogeneity did not change substantially.
 Figure 6.
Figure 6.Forest plot of the procedural time for ICE vs TEE. Random effects model.
Fluoroscopic time: Fig. 7 shows that the fluoroscopic time guided by ICE was significantly equivalent to that guided by TEE (MD 0.49, 95% CI [-2.18, 3.16], I2 =87%, P = 0.72). Considering the heterogeneity (I2 = 87%), we performed a fixed-effects sensitivity analysis. The fixed effects model indicated no significant variability compared to the previous effects. Next, we excluded each of the six studies in a stepwise manner to assess the heterogeneity. When Berti’s 2018 study (Berti et al., 2018) was excluded, the heterogeneity was reduced from 87% to 70%.
 Figure 7.
Figure 7.Forest plot of the fluoroscopic time for ICE vs TEE. Random effects model.
Compared to Velagapudi et al. (2019) , we included more studies due to the search strategy and eligibility criteria. We searched Cochrane databases instead of SCOPUS and Google Scholar, and there were no limits on the types of devices for closure and no language limits in the key words. Some cohort studies were also included.
The use of procedural echocardiography is an essential requirement for safe and successful PLAAO. ICE is an imaging technique applied in various interventional cardiac procedures, such as catheter ablation of atrial fibrillation, percutaneous closure of atrial and ventricular septal defects, and mitral valvuloplasty (Biermann et al., 2012; Hijazi et al., 2009). More recently, ICE has been adopted to guide LAAO because of its advantages.
Our findings: First, the technical success in the ICE group was similar to that in the TEE group. No heterogeneity or publication bias was found. In the 15 eligible studies, the technical success rates, which were not estimated, between the ICE and TEE groups were equal to 100% in 7 studies (Aguirre et al., 2018; Berti et al., 2018; Clemente et al., 2015; Frangieh et al., 2017; Hemam et al., 2019; Ho et al., 2007; Matsuo et al., 2016). The pooled effects were driven by the remaining 8 studies (Berti et al., 2014; Frikha et al., 2016; Iwasawa et al., 2016; Kim et al., 2018; Korsholm et al., 2017; Masson et al., 2015; Naim, Potvin et al., 2015; Reis et al., 2018).
The periprocedural complications of patients under ICE guidance were similar to those of patients under TEE guidance. When using TEE, the complications were attributed to esophageal, throat or respiratory tract injuries, such as esophageal erosion or bleeding, laryngospasm, and bronchospasm, yet complications caused by anesthesia were rarely reported in the eligible studies. Vascular complications were caused by puncture and included groin hematomas and pseudoaneurysms. These adverse events increased when using ICE. Moreover, when the ICE catheter was placed into the LAA after the transseptal puncture, it often caused cardiac injuries, such as pericardial effusions and even tamponades. Some high risks of complications were observed in the study of Berti published in 2018 (Berti et al., 2018); these adverse events included pericardial tamponade and major bleeding, especially in the TEE groups. Mortality during the periprocedural period was not significantly different between the ICE groups and TEE groups.
As ICE guidance provides access for a catheter operator, its imaging is used to guide transseptal (TS) puncture, confirm the absence of LAA thrombi, identify the LAA dimensions, verify the delivery sheath position, confirm the location and stability of the device before and after release and monitor procedural complications such as cardiac tamponade or device embolization. During the procedure, the ICE probe can be placed in the RA, CS, LA, or even the right ventricular outflow tract (RVOT) (Ren et al., 2013) to obtain an optimal view for guiding or monitoring. Positions in the RA, CS, and ROVT (Berti et al., 2014; Berti et al., 2018; Clemente, et al., 2015; Ho et al., 2007; Matsuo et al., 2016) are the conventional positions and are more often used to guide TS puncture, detect thrombi, and measure the LAA dimension. Moreover, the ICE probe is also placed into the right ventricular inflow tract (RVIT) (Moradkhan et al., 2012) and pulmonary artery (PA) (Ren et al., 2013) to obtain more detailed imaging of the LAA. Recently, the ICE probe was placed into the LA, LAA, and left superior pulmonary vein (LSPV) (Aguirre et al., 2018; Berti et al., 2018; Frangieh et al., 2017; Frikha et al., 2016; Hemam et al., 2019; Iwasawa et al., 2016; Kim et al., 2018; Korsholm et al., 2017; Masson et al., 2015; Naim et al., 2015; Reis et al., 2018) to guide the procedure, especially to obtain optimal imaging of the structure of the LAA, including the ostium, LZ diameters and length, and even the LAA emptying flow velocity. There are two types of approaches for inserting the ICE probe into the LA. One is the TS approach, in which the ICE is first placed in the home view of the mid-RA and then rotated downward to advance into the sheath through the septum into the LA. In the other approach, the ICE probe is directly inserted into the LA through the left femoral artery. Berti’s 2018 study used both right and left intracardiac imaging to guide the procedure. Compared with TEE, ICE can provide more clear and detailed imaging of the structure of the LAA, but the manipulation of the ICE is more complicated. We must consider the learning curve of the operators because the procedure requires rigorous training and expertise. In contrast, TEE is classic, feasible and rather straightforward; however, it requires sedation or anesthesia and may present artifacts or equivocal images in some patients. Two figures demonstrate the implantation of the closure device as contrasted between ICE and TEE (Fig. 8, 9) (Frangieh et al., 2017).
 Figure 8.
Figure 8.ICE guidance for LAAO. (A) Interatrial septal puncture (ICE probe in the right atrium); (B) visualization of the LAA and anatomy assessment (ICE probe in the left atrium); (C) device position assessment; (D) check for peri-device flow/leak with color Doppler-flow imaging. LAA: left atrial appendage; LA: left atrium; RA: right atrium; W: Watchman device.
 Figure 9.
Figure 9.TEE guidance for LAAO. (A) Interatrial septal puncture; (B) visualization of the LAA and anatomy assessment; (C) device position assessment; (D) check for peri-device flow/leak with color Doppler-flow imaging (the red color indicates the flow toward the left atrium and not a leak since the flow is a lower velocity and no peri-device penetration detected). LAA: left atrial appendage; LA: left atrium; MV: mitral valve; RA: right atrium; W: Watchman device.
Second, we addressed procedure-related endpoints.
The measurements of the LZ of the LAA were performed in five studies (Clemente et al., 2015; Hemam et al., 2019; Ho et al., 2007; Kim et al., 2018; Matsuo et al., 2016.) The size measured by ICE was significantly similar to that measured by TEE, with substantial heterogeneity. The sensitivity analyses suggested that the heterogeneity might be affected by confounding factors, including the workflow, manipulation of ICE, puncture, teamwork and other variables. ICE had a better correlation with cardiac computed tomography angiography (CCTA) and conventional cardiac angiography (CCA) than did TEE during LAA measurements. CCTA appears to be a practical and concrete method for determining the size of the LAA occluder device to be implanted and for identifying eventual possible collateral findings. ICE seemed to reduce the risk of high-flow leaks and problems related to undersizing (Clemente et al., 2015; Berti et al. 2014). Berti et al. (2014) reported that the LZ measurements assessed by ICE were significantly correlated with those assessed by angiography and TEE; moreover, the device selected by ICE was usually the same as that selected by TEE. In some studies, the LAA dimensions were assessed by preoperative TEE, and some guidance imaging was performed by ICE and TEE simultaneously. Combining ICE and TEE imaging, these studies concluded that the measurements of the LAA ostial size were consistent between ICE and TEE imaging (Frangieh et al., 2017, Ho et al., 2007).
Procedural times were not significantly different between the ICE procedure and the TEE procedure. The procedural times were defined from femoral venous puncture to closure, including TS puncture in most eligible studies. Using TEE with general anesthesia, endotracheal intubation and post anesthesia care often increased the total in-room time and the turnaround time, but TEE guidance did not prolong the time from puncture to closure. Because of the learning curve, the ICE procedural times were longer than those of TEE in the studies published by Frangieh (2017) and Berti (2018). Similarly, the high degree of heterogeneity in the procedural times might have been affected by confounding factors, as previously described.
The fluoroscopic time associated with ICE guidance was significantly equivalent to that associated with TEE. Berti’s 2018 study (Berti et al., 2018) was the source of heterogeneity, as demonstrated by the sensitivity analyses. That multicenter retrospective study appeared to be the major cause of the heterogeneity.
Two studies (Frangieh et al., 2017; Korsholm et al., 2017) compared contrast usage. The contrast usage associated with ICE was less than that associated with TEE, but more samples are needed to verify this result. Using ICE could avoid fluoroscopic angiography for measurement, thus reducing the fluoroscopic procedure and contrast usage.
The results of our analyses of technical success were similar to the acute procedural success assessed by Velagapudi et al. (2019), although the definition was different. Additionally, although our eligible studies were different from the studies in Velagapudi et al. (2019), our findings regarding complications, procedural time and fluoroscopic time were also similar to the results obtained by Velagapudi et al (2019). Currently, whether the ICE-guided imaging procedure will be another choice in addition to TEE is still uncertain. Most of our eligible studies, including the study by Berti (2014) in which ostium and LZ measurements by ICE were significantly related to those measured by TEE, supported the use of ICE as a replacement for TEE. Berti’s 2018 study (Berti et al., 2018) revealed almost similar results; he noted that TEE is actually the gold standard for LAAO procedure guidance at present, but ICE-guided LAAO may represent an alternative imaging modality. Using ICE guidance, the ICE catheter adds additional cost to the procedure, but high TEE and anesthesia technical fees and recovery room charges in the TEE groups offset the ICE charges; thus, overall hospital costs and charges are similar between the two groups (Berti et al., 2018, Hemam et al., 2019). Our meta-analysis suggested positive evidence supporting ICE as an alternative guidance method.
There are several limitations in our study. No randomized control trials were found regarding PLAAO guided by ICE compared to that guided by TEE in NVAF patients. Additionally, this meta-analysis includes studies with mostly small sample sizes. We did not compare the effects of the two types of occlusion devices (Amplatzer Cardiac Plug and Watchman) or the two types of ICE catheters (Acuson AcuNav and ViewFlex Xtra). Finally, the learning curve and health costs may affect the use of ICE. Further study of this technique is needed in the future to corroborate the feasibility and efficacy of the alternative approach using ICE.
Our meta-analysis suggests that ICE is effective and equivalent to TEE without increasing complications or mortality; moreover, ICE can measure LZs or may reduce contrast usage. ICE may be preferable for imaging guidance for LAAO in NVAF patients.
Thanks to all the peer reviewers and editors for their opinions and suggestions.
The authors declare no conflict of interest.
