Contrast-induced acute kidney injury (CI-AKI) is a serious complication that can affect outcome and prognosis of patients undergoing percutaneous diagnostic and interventional procedures in catheterization laboratories. There have been advancements in case definition and epidemiology. Additionally strategies have emerged that are positioned to have impact in the catheterization laboratory for patients undergoing cardiovascular procedures. The aim of this review is to provide the state-of-the-art of diagnosis, prevention and management of CI-AKI in interventional cardiology.
The first case of contrast-induced acute kidney injury (CI-AKI) was described in 1954, when iodinated contrast media was administered intravenously in a patient undergoing a pyelogram with intravenous injection of iodinated contrast medium (ICM) (Bartels et al., 1954). Over the years the increasing use of diagnostic and interventional procedures requiring the use of ICM has progressively increased the population exposed to the risk of CI-AKI (Figure 1). Over the last 30 years in particular, alongside the spread of endovascular procedures, attention has grown about the possible or established role of ICM in the deterioration of kidney function in patients undergoing diagnostic and interventional cardiovascular investigations and the consequent preventive strategies.
 Figure 1.
Figure 1.Incidence of contrast-induced acute kidney injury (CI-AKI) and new end-stage renal disease requiring dialysis (ESRD) from the ACC Cath-PCI registry, reproduced with permission (McCullough et al., 2016).
There is considerable evidence to show how CI-AKI represents a complication with a serious clinical impact in terms of both mortality and morbidity (Weisbord et al., 2006). Patients managed by catherization laboratories are often subject to the risk of developing deterioration of renal function per se, regardless of exposure to ICM (Nash et al., 2002). The risk of CI-AKI onset frequently leads to conservative treatment being selected in particularly in fragile populations, such as the extremely elderly, despite the proven benefit of endovascular interventional approaches (Chertow et al., 2004). It is therefore obvious that using the correct clinical approach for patients at risk of CI-AKI is of paramount importance, in order to prevent and manage this clinically impacting complication by making the best possible use of the evidence available, yet not ruling out ordinary treatment standards for the most fragile patients.
In the past the clinical condition characterized by deterioration of kidney function following administration of ICM was referred to as "contrast induced acute kidney failure” or "contrast-induced nephropathy ([CIN] or radio contrast-induced nephropathy) with particular reference to loss of function - organ failure secondary to the iatrogenic insult. In developing the RIFLE criteria for the diagnosis and classification of acute kidney failure the Acute Dialysis Quality Initiative (ADQI) group of experts moved the attention from organ failure to the concept of organ risk and injury, with the aim of emphasizing the importance of early diagnosis so as to implement therapeutic measures promptly, within a physiopathological window where loss of function is not irreversible and definitive, but restitutio ad integrum of kidney function is still a possibility (Bellomo et al., 2004). So nowadays it is more correct to refer to “contrast-induced acute kidney injury” (CI-AKI).
Most of the literature on CI-AKI uses diagnostic criteria based on the increase of a biomarker (laboratory criterion), mainly serum creatinine (SCr) in absolute or percentage terms, or on the reduction of diuresis (clinical criterion) in a defined time interval. The reference and cut-off values of these variables have been and still are the subject of debate. The adoption of non-uniform diagnostic criteria for CI-AKI complicates the epidemiological analysis of this clinical condition.
The nephrological guidelines KDIGO 2012 suggest the advisability of standardizing diagnostic criteria for all forms of acute kidney injury (AKI) and they see no reason why CI-AKI should not be subject to the same reference values as other forms of AKI (Levin et al., 2013). They recommend using the criteria promoted by the Acute Kidney Injury Network (AKIN), which updated the RIFLE criteria defining AKI as an increase in SCr of 0.3 mg/dl (26.5 μmol/l), or an increase of SCr of 50% compared to baseline, when this occurs in the first 48h after exposure to ICM (Mehta et al., 2007).
It should be noted that the definition of CI-AKI used most in literature, particularly in studies published before 2012, shows a different cut-off and indicates an increase in SCr of 0.5 mg/dl (44 μmol/l) or 25% compared to baseline, within 48h of exposure to ICM which was originally developed by McCullough and colleagues (McCullough et al., 1997). The 0.5 mg/dl was positioned to be of clinical importance to the practitioner. The 25% minimal increase was developed since that is the level below which could be due to day to day variation in a patient without AKI. More specifically, the absolute increase of at least 0.5 mg/dl of SCr showed, in comparison with the percentage increase of 25%, greater specificity when identifying persons at risk of increased mortality and morbidity (Budano et al., 2011). This definition, which has proved reliable in predicting cardiovascular adverse events in patients subjected to cardiovascular interventional procedures (Harjai et al., 2008), does, however, have the disadvantage of potentially being too restrictive and less sensitive with regard to identifying patients at risk of CI-AKI. In a recent study comparing three different diagnostic criteria of CI-AKI Guillon et al. (2018) demonstrated how the most effective criterion for prognostic purposes in patients undergoing coronarography for acute coronary syndrome, is the absolute increase of SCr of 0.3 mg/dl.
In a minority of cases a characteristic rise in SCr may be encountered up to 5-7 days after exposure to ICM, and many of these cases are referred to as AKI associated with ICM administration rather than a demonstrable cause-effect ratio (contrast-associated AKI). A prospective study has shown how a minimum percentage variation of SCr in the first 12h after administration of ICM is the best predictive factor of CI-AKI and is also closely correlated with the onset of kidney failure at 30 days (Ribichini et al., 2010).
The definition of CI-AKI based on the change in SCr within a time interval has limitations due to the fact that patients undergoing diagnostic and interventional cardiovascular procedures, all the more so if they are hospitalized, represent a population at risk of developing worsening kidney function per se not only because of the direct effect of ICM, but due to baseline clinical conditions and comorbidity predisposing or contributing to AKI. To obtain a correct diagnosis of CI-AKI it is therefore important to exclude other causes of AKI.
New biomarkers, specifically urine L-type fatty acid binding protein and neutrophil gelatinase associated lipocalin, are being validated to allow a more accurate and prompt diagnosis of CI-AKI in the near future (Figure 2). There are still some doubts about the specificity of these biomarkers in the different populations of patients exposed to ICM. Additional data from multicenter clinical trials are required to assess whether these new biomarkers can actually be used in everyday clinical practice.
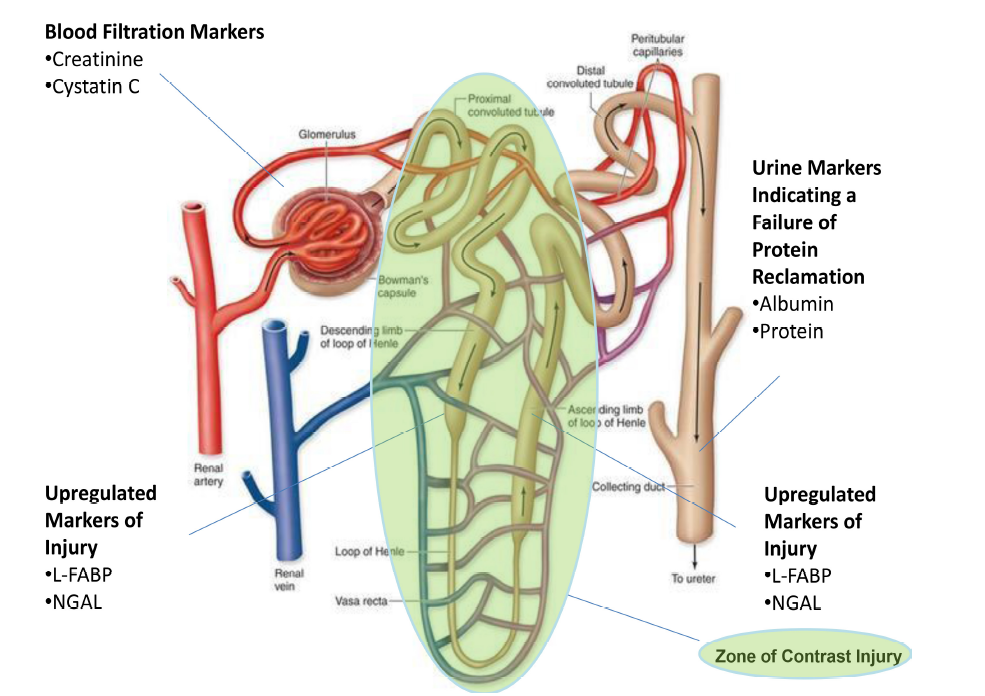 Figure 2.
Figure 2.Anatomy of a single nephron and location of novel and conventional biomarkers of renal filtration and tubular epithelial cell damage, NGAL = neutrophil gelatinase associated lipocalin, L-FABP = L-type fatty-acid binding protein. Markers of filtration indicate transient reductions in glomerular function while cell damage markers indicate cellular injury, and the two together have a poorer prognosis than either alone. Not all of these markers have been validated for contrast-induced AKI.
Pending validation of new biomarkers, we suggest the following definition of CI-AKI: increase of SCr of 0.3 mg/dl (26.5 μmol/l), or ≥ 50% compared with baseline in the first 48h after administering ICM
The incidence of CI-AKI in patients who undergo endovascular diagnostic and interventional procedures is very variable in literature mainly due to the use of numerous definitions of CI-AKI, the heterogeneity of the populations investigated and the different procedures under examination. It has actually been shown that in the same population of patients undergoing coronary angioplasty (PCI), the incidence of CI-AKI may vary from 3.3% to 10.2% (Figure 1) depending on whether the absolute increase in SCr of 0.5 mg/dl or a relative increase in SCr of 25% compared to the baseline is used as a diagnostic criterion. Most available epidemiological data is taken from studies that used an increase of SCr of 0.5 mg/dl or 25% compared with the baseline in the 48h following exposure to ICM as a diagnostic criterion.
CI-AKI is estimated as constituting the third cause of AKI in hospitalized patients, following prerenal kidney failure caused by reduced perfusion and that due to the administration of nephrotoxic medications, representing about 10% of all cases of AKI (Nash et al., 2002). In recent years, albeit with the aforesaid limitations of an epidemiological analysis, the incidence of CI-AKI appears to be in decline, in particular due to those involved in patient management having improved their knowledge and awareness in relation to the reduction of doses of ICM, the use of preventive strategies and the use of less nephrotoxic ICM (McCullough, 2008).
In patients undergoing PCI there is increasingly robust evidence of the greater safety of the radial approach compared with the femoral approach, particularly in relation to reducing complications associated with bleeding. Recent studies have shown that the advantages of the radial approach also include reducing the risk of CI-AKI (Feldkamp et al., 2018; Pancholy et al., 2017). More specifically, in patients undergoing percutaneous procedures within the context of acute coronary syndromes, the population treated with the radial approach has shown a lower incidence of CI-AKI compared with the femoral approach (Andò et al., 2017).
In patients with acute myocardial infarction with ST-elevation (STEMI) percutaneous revascularization with primary PCI (pPCI) represents the mainstay of acute treatment. The incidence of CI- AKI in patients treated with pPCI during STEMI varies from 10.5% to 18.3% depending on the definition used for CI-AKI, with a significant impact on mortality (Silvain et al., 2018).
However recent evidence indicates that the role of ICM in the deterioration of kidney function may be over-estimated. A comparison study between patients exposed to ICM during pPCI and patients treated with fibrinolysis actually showed that the incidence of AKI in the two groups is similar and mainly depends on age, the baseline estimated glomerular filtration rate (eGFR), the presence of heart failure and hemodynamic instability rather than exposure to ICM (Caspi et al., 2017).
Numerous studies have shown that the diagnosis of CI-AKI is correlated with lower survival in the medium and long term (Lindsay et al., 2003). For example, a study by McCullough et al. (1997) on a population of 1826 consecutive patients who underwent interventional coronary procedures, showed how intrahospital mortality for the 264 patients (14.4%) who had developed CI-AKI (defined as a 25% increase of SCr within 5 days of the procedure) was significantly higher than that of the patients who had not developed CI-AKI (7.1 vs. 1.1%, P < 0.0000001; odds ratio [OR] for intrahospital mortality associated with CI-AKI 6.56, confidence interval [IC] 95% 3.34-12.92; P < 0.00001). In a retrospective study on 14 782 patients, James et al. (2013) , demonstrated how CI-AKI has a significant clinical impact even over a long period with increased mortality in the long term, deterioration of kidney failure up to the point of requiring dialysis and increased hospitalization for cardiovascular and kidney events.
CI-AKI may result in chronic kidney failure (chronic kidney disease, CKD) or, as occurs in the majority of cases, there may be a transient worsening of kidney function followed by functional restitutio ad integrum. In patients with pre-existing CKD of least moderate level, defined by eGFR < 60 ml/min, the persistent deterioration of kidney function after CI-AKI is associated with a worse prognosis at 5 years compared with patients experiencing transient CI-AKI (P < 0.015) (Maioli et al., 2012). In a retrospective study on patients undergoing PCI, after 1 year kidney function had standardized in most patients with CI-AKI. The prognosis is worse for those patients (approximately 1.3% of the total population investigated) incur persistent kidney damage after 1 year (Abe et al., 2017).
The onset of CI-AKI seems to have a significant impact not only on mortality from all causes but also on the outcome of these interventional procedures. In a registry of 5967 patients undergoing PCI, the diagnosis of CI-AKI was associated with a significantly increased risk of acute myocardial infarction and revascularization of the target lesion after 1 year (Lindsay et al., 2003). Secondly, the onset of CI-AKI in patients treated with PCI in the context of acute coronary syndrome may occur later and is associated with an increase of ischemic events as well as bleeding events in both the long and short term (Giacoppo et al., 2015).
The incidence of CI-AKI in patients undergoing PCI for chronic occlusion is not significantly higher than in patients undergoing PCI for non occlusive lesions (9.4 vs 12.1%, P = 0.17) (Demir et al., 2018). Patients with pre-existing CKD are the patients most at risk compared with patients with normal kidney function at the time of the procedure. However the need for replacement therapies remains low (0.5% in patients with CKD vs 0 % in patients without CKD) (Azzalini et al., 2018a)and those cases in which renal substitution therapy needs be continued indefinitely are very rare. Patients undergoing percutaneous peripheral revascularization, in particular patients with acute or critical ischemia of the lower limbs, represent a particularly fragile population often with significant comorbidity. In these patients the onset of CI-AKI is also associated with a significant increase in mortality (Zlatanovic et al., 2018). The different definitions of CI-AKI used and the heterogeneity of patients with peripheral atheroma undergoing angiographic procedures and percutaneous revascularization, complicate reliable epidemiological analysis. In the systematic review of literature in 2016 the incidence of ci-AKI in this population was estimated to be approximately 11%, but is probably underestimated (Prasad et al., 2016). The data obtained from a prospective registry of approximately 450 patients undergoing carotid stenting indicate a rather high incidence of CI-AKI (approximately 34%) in this population of patients (Pucciarelli et al., 2018). It should be noted that this study used diagnostic criteria indicating an increase of SCr of 0.3 mg/dl or an increase of SCr of 1.5 times compared to baseline or an increase of SCr > 50% compared to the baseline in the 48h following the procedure. In patients affected by severe aortic valvular stenosis with an indication for valve replacement, transcatheter aortic valve implantation (TAVI) proved safer than traditional surgical replacement as regards the incidence of AKI (Gargiulo et al., 2016). However, the incidence of CI-AKI in patients undergoing TAVI remains rather high, at approximately 22%, particularly in high-risk patients, with a particular impact on prognosis (Gargiulo et al., 2015). The incidence of CI-AKI in patients undergoing endovascular repair of the thoracic aorta with an endoprosthesis is estimated at approximately 14%, with a significant impact on hospitalization times and intra-admission mortality (Piffaretti et al., 2012). The incidence of CI-AKI seems to be lower in patients treated with an endoprosthesis implant in the abdominal aorta, at approximately 7%, once again associated with a proven reduction in survival (Kawatani et al., 2018). In a multicenter registry of 355 patients undergoing percutaneous left atrial appendage occlusion (pLAAO) a 9% incidence of CI-AKI was found, defined as SCr ≥ 0.3 mg/dl or ≥ 50% compared with the baseline during the 48h after the intervention.
Patients undergoing pLAAO complicated by CI-AKI present with high mortality of approximately 23% after 1 year, over twice as high as in patients who do not present CI-AKI (about 9.8%) (Nombela-Franco et al., 2018).
The lack of any standardization of diagnostic criteria for CI-AKI makes an accurate epidemiological analysis particularly complex. CI-AKI is relatively frequent in clinical practice and is associated with a worse prognosis. On the other hand, given the high incidence of AKI from other causes in hospitalized patients suffering from cardiovascular pathologies, it is likely that a non-negligible share of patients labeled as “CI-AKI” in clinical studies may incur deterioration of kidney function not necessarily related solely to the administration of ICM.
The physiopathology of CI-AKI is complex and most of the related concepts are taken from in vitro and animal studies. The kidney receives approximately 25% of arterial blood from each cardiac output. Most of this blood flow flushes the cortex, while the marrow is vascularized by low pressure circulation mainly formed by the vasa recta. It is really the kidney marrow which is the portion most vulnerable to ICM induced injury. There are believed to be three main mechanisms that contribute to kidney injury after administration of ICM (Persson et al., 2005). These include: 1) the direct cytotoxic damage of ICM on the tubular cells; 2) the damage mediated by free oxygen radicals after ICM mediated intrarenal vasoconstriction and hypoxia. In the most serious cases the final outcome of the combined effect of these physiopathologically synergistic mechanisms is acute tubular necrosis (Geenen et al., 2014).
All types of ICM, particularly ICMs with high osmolarity, have a cytotoxic effect on in vitro cultures of kidney tubular cells. The mechanism of direct toxicity of the ICM is not at all clear but several harmful effects have been described: apoptosis, redistribution of membrane proteins, reduction of intracellular calcium, DNA fragmentation, impairment of intercellular junctions, reduction of cell proliferation and impaired mitochondrial activity.
The free radicals known as reactive oxygen species (ROS), such as H2O2, play a physiologically important role in the normal homeostasis of the kidney marrow; they are actually involved in the transmission of intercellular signals, regulation of the microcirculation and control of tubular transport (Figure 3). The hypoxia which is created in the tubular cells of the marrow after administration of ICM, characterized by the discrepancy between increased demand and reduced supply of oxygen, leads to a pathological increase of ROS that, in excess, triggers the damage caused by oxidative stress at the expense of cell membranes, nuclear DNA and mitochondria. In turn, the ROS finally cause vasoconstriction mediated by the increase of endothelin 1 and angiotensin II and reduction of circulating nitric oxide, aggravating the hypoxia.
 Figure 3.
Figure 3.Pathogenesis of contrast-induced acute kidney injury.
The ICM have a direct biphasic effect on renal vascularization: there is brief, transient, vasodilation followed by prolonged vasoconstriction which significantly reduces arterial blood flow. The hypoxia which is created in the marrow has an even more serious effect because the tubular cells increase their oxygen demand at the same time. The result is ischemic damage leading to loss of physiological regulation of mediators involved in vasodilation and vasoconstriction thus establishing a vicious circle which prolongs the hypoxia.
It is commonly accepted that added to these mechanisms is the ischemic insult involving microemboli, not due directly to the ICM but secondary to the invasive endovascular maneuvers, in patients with widespread atheroma of the thoracoabdominal aorta (McCullough et al., 2016). As already mentioned in the previous paragraphs, patients hospitalized for cardiovascular problems have a rather high incidence of AKI even if not exposed to ICM, due to underlying disease (e.g. pre-renal injury caused by low flow) or the concomitant administration of nephrotoxic drugs. Clearly it is often complex to differentiate in detail the direct role of ICM in the AKI of the patient treated endovascularly, from any other concurrent causes.
There are three main mechanisms that contribute to kidney injury after administration of ICM: 1) direct cytotoxic damage of ICM on the tubular cells, 2) damage mediated by free oxygen radicals, and 2) hypoxia due to vasoconstriction. Understanding the physiopathological mechanisms at the basis of CI-AKI is of paramount importance when investigating strategies for prevention and treatment.
The possibility of identifying patients at increased risk of CI-AKI means that the risk/benefit ratio of proposed therapeutic options can be explained and shared with the patient, so then preventive pre-procedural strategies can be implemented and post-procedural follow-up programmed.
Most of the information about the predictors of CI-AKI is taken from studies in patients undergoing coronary interventional procedures. The predisposing or risk factors of CI-AKI which have been identified to include: 1) the clinical characteristics of the patient (eGFR 60 ml/ min/1.73 m2, age, diabetes, anemia, congestive heart failure, reduced left ventricular ejection fraction); 2) the clinical context of presentation (shock, hypovolemia, urgent/emergent procedure, concomitant use of nephrotoxic drugs, acute kidney failure from other causes); and 3) procedural aspects (use of aortic counterpulsation device, dose and type of contrast medium, arterial access). It can be seen that the presence of multiple variables in the same patient exponentially increases the risk of CI-AKI. Various models and risk scores have been developed on this basis via retrospective studies on populations of patients undergoing coronary interventional procedures.
The best known risk score is that proposed by Mehran et al. (2019) based on the detection of 8 variables linked to the patient and the procedure. This model has proven to be reliable in identifying patients with a higher and lower risk of CI-AKI (incidence of CI-AKI 8.4% in patients with a score of ≤ 5 vs 55.9% in patients with a score of ≥ 16). This score is not applicable for pre- intervention risk stratification, since, to obtain the score, some procedural data is required, such as the volume of ICM used and use of intra-aortic balloon counterpulsation. The model described by Laskey et al. (2007), based on the ratio between the volume of ICM/creatinine clearance (CrCl) (if > 3.7 it is an independent predictor of post-PCI CI-AKI) has the limitation of only being able to identify patients at the highest risk of CI-AKI after the procedure. Some authors have raised doubts about the applicability of the risk score in everyday clinical practice because in most cases these models have not been validated in prospective studies. The same authors suggest a simplified risk stratification, based on the presence of two variables such as CKD and diabetes as originally proposed by McCullough and co-workers (McCullough et al., 2006; McCullough et al., 1997).
Brown et al. (2008) stressed the importance of estimating the risk of CI-AKI prior to the procedure, with the particular aim of identifying those patients at risk of developing severe, clinically significant CI- AKI (defined as an increase of blood creatinine of ≥ 2 mg/dl or ≥ 50% compared to baseline or even as a requirement for kidney replacement therapy). Amongst the 7 variables subject of the model, pre-existing kidney dysfunction, congestive heart failure and diabetes are the most important, constituting 76% of predictive capability. The other variables investigated are the urgent/emergent nature of the procedure, the pre-procedural need for an aortic counterpulsation device, age ≥ 80 years and female sex. Since this score identifies patients at risk of more severe CI- AKI, which in turn has a significant correlation with mortality, the authors stress that the model can be used to identify patients at a higher risk of post-PCI adverse events in general.
Another risk model based on exclusively pre-procedural variables is the one proposed by Gurm et al. (2013a), again for patients who are candidates for PCI. The model is based on 15 variables, with a PC or smart device being required to obtain the score. The advantage of this model is its greater accuracy in differentiating the patients most at risk compared, for example, with the Mehran risk score. The relative complexity of using this model has, however, limited its use in clinical practice.
Capodanno et al. (2016) demonstrated the validity of stratifying the CI-AKI risk of the ACEF model, already used as a prognostic tool in patients undergoing surgical or percutaneous coronary revascularization, on the basis of 3 simple variables to be checked, such as age, baseline SCr and left ventricular ejection fraction.
Risk stratification of CI-AKI for patients who are candidates for endovascular diagnostic and interventional procedures with ICM is of great importance. There is no current evidence to recommend the systematic/routine use of the CI-AKI risk scores. The suggestion is to pay particular attention in the pre- and post-procedural clinical management of patients with the clinical characteristics associated with an increased risk of CI-AKI such as pre-existing kidney failure (defined as eGFR ≤ 60 ml/min/1.73 m2), diabetes, anemia, advanced age (≥ 75 years), heart failure, depressed ejection fraction, hemodynamic instability
In accordance with the physiopathological background of CI-AKI, numerous pre-pharmacological treatment protocols have been studied and proposed, with the aim of reducing the incidence of CI-AKI (Figure 4). The heterogeneity of the data available in literature, still under debate, does not allow any conclusive indications to be reached about the best pharmacological strategy for preventing CI-AKI. The most significant evidence is listed and discussed below.
 Figure 4.
Figure 4.Algorithm for the prevention and management of contrast-induced acute kidney injury, ALARA = as low as reasonably achievable, Cr = serum creatinine, CI-AKI = contrast-induced acute kidney injury, eGFR = estimated glomerular filtration rate, NGAL = neutrophil associated lipocalin, L-FABP = L-type fatty-acid binding protein, TIMP2*IGFBP-7 = tissue inhibitor of metalloproteinase-2 concentration multiplied by insulin like growth factor binding protein-7 concentration, NSAIDS=nonsteroidal anti-inflammatory agents, ACS = acute coronary syndromes, CKD = chronic kidney disease, DM = diabetes mellitus, HF = heart failure, TAVI = transcatheter aortic valve insertion, RASi = renin angiotensin system inhibitors, LVEDP = left ventricular end-diastolic pressure.
Administration of liquids is the most widespread and apparently simplest and most economical preventive strategy for CI-AKI in patients who are candidates for invasive percutaneous procedures. The guidelines of the European Society of Cardiology (ESC) recommend that all patients in class I (level of evidence C) undergoing coronarography should receive adequate hydration and, in particular, they recommend that patients with moderate or severe renal insufficiency (National Kidney Foundation stages 3b and 4) should receive 1 ml/kg/h of isotonic saline solution in the 12h before the procedure and over the subsequent 24h (0.5 ml/kg/h if ejection fraction ≤ 35%) with recommendation class IIa, level of evidence C (Neumann et al., 2018).
It has been demonstrated that intravenously controlled hydration, started 12h before the procedure and continued for a total of 24h, is more effective than oral administration (Bader et al., 2004; Trivedi et al., 2003), even if this approach is difficult to apply in acute patients or those admitted under the day-hospital regime. The intravenous administration of isotonic saline solution 0.9% proved to be superior in terms of efficacy in the prevention of CI-AKI, compared with other solutions such as saline 0.45% combined with glucose 5% (in 1620 randomized patients, incidence of CI-AKI 0.7% for saline solution 0.9% vs 2.0% for saline solution 0.45% glucose + 5%, P = 0.04) (Mueller et al., 2002). In the absence of evidence about the optimum hydration regime, Brar et al. (2014) demonstrated the superiority in terms of efficacy of a volume expander adjusted to the hemodynamics of the individual patient (in the case in question the monitored parameter was left ventricular end diastolic pressure, measured with a pigtail in the left ventricle), in comparison with a standard hydration regime. However, from a practical point of view this approach is difficult to implement in standard clinical practice. In the study of (Qian et al., 2016), 264 patients with heart failure and CKD were randomized in a hydration protocol adjusted to central venous pressure vs a standard hydration regime: also in this case the volume expander based on hemodynamic parameters proved to be superior in preventing CI-AKI in comparison with the control group. (Maioli et al., 2014; Maioli et al., 2018) demonstrated the efficacy of an adequate volume expander, adjusted to reflect the bioelectrical impedance analysis.
In all studies in which hydration was correct according to hemodynamic/bioelectrical impedance analysis parameters larger volumes of saline solution were administered compared to controls with incorrect volume expander, indicating that the standard hydration regimes are probably below what is necessary for obtaining adequate nephroprotection. This assumption can explain why, in the meta-analysis produced by Giacoppo et al. (2017), which compared 10 different preventive approaches with each other, hydration which is not adjusted to hemodynamic parameters, was found to be the least effective strategy for preventing CI-AKI. Probably for the same reason, in the AMACING study, hydration administered with two incorrect protocols according to the patient response (0.9% NaCl 3-4 ml/kg/h 4h before and 4h after administration of ICM or 0.9% NaCl 1 ml/kg for 12h before and 12h after the angiographic procedure), proved not to be effective in preventing CI-AKI, compared with the non-hydrated control group of patients (Nijssen et al., 2017).
N-acetylcysteine was proposed as a pharmacological pre- treatment to reduce the incidence of CI-AKI, given its antioxidant and vasodilator effect (Tepel et al., 2000). Studies on the potential benefit of N- acetylcysteine in terms of prevention of CI-AKI, however, have provided contrasting results over the years (Fishbane et al., 2004). In a randomized trial on 183 patients with CKD undergoing percutaneous coronary or peripheral procedures, in which pre-medication with hydration combined with 600 mg of N-acetylcysteine twice a day vs hydration alone was tested, the absence of any benefit from pre-treatment The PRESERVE trial recently confirmed the clinical ineffectiveness of N-acetylcysteine in the prevention of the CI-AKI (Weisbord et al., 2018). It should be noted that the vast majority of studies with negative results on the nephroprotective effect of N-acetylcysteine indicated oral administration, which is characterized by poor bioavailability of the drug due to first pass metabolism. On the other hand, even after intravenous administration, the plasma concentration of free circulating N-acetylcysteine is very low because of plasma protein and tissue binding.
It has been suggested that pre-treatment with sodium bicarbonate is superior in terms of efficacy in reducing the incidence of CI-AKI than hydration with saline alone, due to the alkalizing effect on tubular urine with a reduction in the formation of free oxygen radicals (Merten et al., 2004). However, there are contrasting data in literature in terms of the real efficacy in clinical practice of pre-treatment with bicarbonate in the prevention of CI-AKI. In a review of the literature (Brar et al., 2009) discovered how larger and more methodologically correct clinical trials did not demonstrate any superiority of pre- medication with bicarbonate compared with hydration with saline solution in the prevention of CI-AKI. In the PRESERVE trial pre- treatment with sodium bicarbonate did not show any benefit in the prevention of the CI-AKI, compared with protocols involving the administration of N-acetylcysteine, saline or placebo (Weisbord et al., 2018).
Studies in vitro and in animal models have suggested that statins may have a "nephroprotective" action on ICM, due to the anti- inflammatory effect and the capacity of statins themselves to reduce cell apoptosis. In the meta-analysis of (Thompson et al., 2016) out of 19 randomized clinical trials involving a total of 7161 patients, this protective effect of statins towards CI-AKI seems to be confirmed even if it is less evident in patients with CKD. In a meta-analysis on 124 trials (28 240 patients), comparing the 10 most studied pre-treatment strategies, pre-treatment with statins proved to be the only effective one in reducing the risk of CI-AKI (Giacoppo et al., 2017). On the basis of these data, ESC guidelines recommend the administration of statins at high doses (atorvastatin 80 mg or rosuvastatin 20 or 40 mg) in naive patients prior to exposure to ICM (class of recommendation IIa, level of evidence A) (Neumann et al., 2018).
On the basis of the evidence available, we recommend that patients at risk of CI-AKI are pre-treated with adequate volume expansion with saline solution 0.9% (at least 3 ml/kg/h in the 4h before the procedure), possibly with customized approach based on clinical data and possibly with the help of hemodynamic /bioelectrical impedance analysis monitoring parameters. Prescribing high doses of statins is also suggested. There is no evidence for implementing protocols indicating the use of N-acetylcysteine or bicarbonate.
Non-steroid anti-inflammatory drugs (NSAIDS), angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists may contribute to a deterioration of kidney function in certain clinical situations. Metformin is potentially associated with an increased risk of lactic acidosis in situations in which excretion is reduced. There is conflicting data about the advisability of temporarily withdrawing these therapies in patients undergoing procedures that involve the use of ICM. The principal evidence is provided below.
Metformin is the most widespread oral antidiabetic agent, from the category of biguanides. It is 90% eliminated via the kidney and has a half-life of between 4 and 9 h in patients with preserved kidney function. Metformin is not nephrotoxic per se but it may accumulate in the presence of a picture of acute kidney failure, with the potential risk of causing lactic acidosis, secondary to an increase of anaerobic glycolysis and inhibition of hepatic gluconeogenesis. The risk of metformin induced lactic acidosis increases in the presence of serious comorbidities such as sepsis, heart failure or liver impairment, and is associated with a mortality rate of up to 50%. On the basis of these circumstances withdrawing metformin 48 h before administering ICM in elective patients was recommended, reintroducing it 48 h after the examination (Goergen et al., 2010). In reality in clinical practice metformin induced lactic acidosis is a rare event (10 cases out of 100 000 patients treated per year) and is mainly described in clinical cases. Recently doubts have been raised about the soundness of the evidence used as the basis for recommendations about withdrawing metformin in all patients undergoing angiographic procedures, and the need to investigate this aspect in randomized trials or large multicentre registers has been underlined(Maznyczka et al., 2012). ESC guidelines advise withdrawing metformin in patients with CKD prior to a procedure with ICM, and if this is not possible, they recommend controlling kidney function post- procedure in patients who have not suspended the drug, monitoring the onset of any signs of lactic acidosis (Neumann et al., 2018). In a recent update ESUR guidelines recommend withdrawing metformin at the time of administering ICM in patients with eGFR < 30 ml/min/1.73 m2 (van der Molen et al., 2018).
ACE-inhibitors and sartans are regarded as "nephroprotective" drugs since, by inhibiting the conversion of angiotensin I to angiotensin II, or the angiotensin II receptor, they cause vasodilatation of the efferent arteriole and consequently reduce intraglomerular pressure. They also reduce the production of free oxygen radicals and increase the concentration of nitric oxide, a powerful vasodilator that potentially opposes the vasoconstrictor effect of ICM. Secondly, ACE-inhibitors inhibit the formation of transforming growth factor-b, which has been shown to prevent damage and necrosis of the proximal tubule. Consequently, there are conflicting physiopathological assumptions about the possible protective or harmful effect of ACE inhibitors and sartans on the nephron exposed to ICM. The evidence about the advisability of withdrawing these drugs or maintaining them in patients undergoing angiographic analyses is conflicting. Studies found in literature have heterogeneous populations, they use diverse criteria to define CI-AKI and they investigated different molecules and dosages. Data about sartans is scarce. Cirit et al. (2006) showed a significantly increased incidence of CI-AKI in patients taking ACE- inhibitors on a chronic basis prior to a coronarography, compared with the group of patients not receiving treatment with ACE- inhibitors, while in a randomized clinical trial Gupta et al. (1999) showed that pre-treatment with captopril reduced the risk of CI-AKI by 79%. The CAPTAIN randomized trial compared the strategy of withdrawing treatment with ACE-inhibitors/sartans starting at least 24h before administration of ICM vs maintenance of treatment; a trend was identified, even if not statistically significant, of lower incidence of CI-AKI in the group of patients in which therapy with ACE-inhibitors/ sartans had been withdrawn with a significantly reduced increase of post-procedural SCr; the authors therefore recommend considering withdrawing these treatments in anticipation of an angiographic examination, given the simplicity of applying this preventive strategy (Bainey et al., 2015). In a review of the literature on studies about the effect of ACE inhibitors on the incidence of CI- AKI, Kalyesubula et al. (2014) conclude that the suspicion of a potentially damaging impact of maintaining therapy with ACE-inhibitors and sartans in terms of increased incidence of CI-AKI is not balanced by robust evidence about a nephroprotective effect and that therefore withdrawing such therapies should be considered in patients at risk of CI-AKI starting from 24h prior to the procedure, administering them again 3 days after the angiographic examination.
NSAIDS have a potential nephrotoxic effect due to the reduction of renal perfusion mediated by inhibition of prostaglandins, which regulate vasodilation at glomerular level. There is little evidence in literature about the advisability of withdrawing treatment with NSAIDS in patients undergoing investigations with ICM to reduce the risk of CI-AKI. In a prospective study Weisbord et al. (2008) did not find any significant reduction of the incidence of CI-AKI in patients whose NSAIDS had been withdrawn but the sample investigated was limited and no conclusive opinions can be reached. The recommendations about withdrawing or restricting the prescription of NSAIDS in the risk of CI-AKI are based on expert opinions with a partial contribution from surgical experience.
There is no sufficient evidence to recommend withdrawing metformin in patients with eGFR > 30 ml/min/1.73 m2 who are candidates for procedures using ICM. It’s recommended monitoring kidney function pre- and post-procedure in high risk patients (e.g. those with comorbidities such as heart failure, low flow rate or advanced age). In the event of eGFR ≤ 30 ml/min/1.73 m2 temporary withdrawal is recommended 24h before the procedure in patients at high risk of CI- AKI until kidney function has stabilized post-procedure. It is recommended restricting and, if possible, avoiding the use of NSAIDS in patients at risk of CI-AKI over the 24h before the angiographic procedure and up until stabilization of kidney function.
The volume of ICM is considered to be a modifiable risk factor of CI-AKI (Dangas et al., 2005) and represents one of the variables of the Mehran risk score for CI-AKI (Mehran et al., 2004). Previously some authors had shown that it was not the volume of ICM in absolute terms to be correlated with the risk of CI-AKI but its correction based on the pre-procedural SCr and body weight (Freeman et al., 2002), according to an estimate of the maximum usable dose of contrast calculated using the formula proposed by Cigarroa et al. (1989): [5 ml di ICM/body weight in kg (max 300 ml)]/SCr in mg/dl. More recently, the ICM/CrCl < 3.7 volume ratio was a cut- off with good sensitivity and specificity in identifying patients at risk of developing CI-AKI in a population of 3179 non-selected patients not undergoing PCI, in the 24h post-procedure (Laskey et al., 2007). Other authors have suggested a lower cut-off (MCI/CrCl < 2.7) for elderly patients undergoing TAVI, seemingly justified by the greater fragility of this particular population (Yamamoto et al., 2013).
Further studies have shown that the amount of ICM administered during endovascular diagnostic and interventional procedures represented a risk factor for developing CI-AKI, particularly under an urgency/emergency regime (Marenzi et al., 2009).
The analysis of data relating to 1.3 million patients, obtained from the National Cardiovascular Data Registry Cath PCI Registry reveals significant inter operator variability in the average quantity of ICM used per procedure. This variability does not seem to be related to the complexity of the procedures, and there does not seem to be any trend towards reducing the amount of ICM in patients at increased risk of CI-AKI (Amin et al., 2017). Providing education about the importance of limiting the amount of ICM administered during the procedures may, according to the authors, offer an important opportunity for reducing the incidence of CI-AKI. On the basis of these findings the ESC guidelines on myocardial revascularization recommend that in patients with moderate to severe CKD (CKD stages 3b and 4) the volume of ICM administered should be limited as far as possible (Neumann et al., 2018).
Some simple precautions have been proposed to reduce the volume of ICM as much as possible during diagnostic and interventional procedures: favor 5-6F catheters without "side holes", limit "test" injections in fluoroscopy, reduce the volume injected in the various acquisitions to the minimum essential for a correct view, remove the contrast from the catheter using "back bleeding" for example before injecting drugs or when changing materials used in the intervention, take advantage of images captured previously (in particular if this concerns “staged” interventional procedures), choose alternative methods to angiography for characterizing lesions (e.g. intravascular ultrasound or functional assessment)(Nayak et al., 2010). There are currently no studies capable of identifying a minimum time interval to be considered "safe" in terms of preventing CI-AKI in higher risk patients.
It is recommended limiting as far as possible the volume of ICM used particularly in patients at high risk of CI-AKI and in general in patients with moderate or severe CKD. The recommendation is also to postpone any further “staged” procedures, waiting for kidney function parameters to stabilize, when clinically possible.
The ideal characteristics of intra-arterially injected ICM are the ability to effectively opacify the vessels under investigation and patient tolerability. ICM for angiographic use are hydrosoluble compounds derived from triiodobenzoic acid classified on the basis of their physical- chemical characteristics (molecular structure, ionicity and osmolarity) as monomers/dimers, ionic/non-ionic, with high/low iso- osmolarity. The first ICM used were ionic compounds with high osmolarity (high osmolar contrast media, HOCM). They frequently caused adverse reactions and were poorly tolerated by patients (Klein et al., 2009). Nowadays the ICM used intra-arterially are predominantly non- ionic compounds, with lower osmolarity than those used in the past (low osmolar contrast media, LOCM). Iodixanol is the only iso- osmolar compound (iso-osmolar contrast media, IOCM) available and has the same osmolarity as the blood (280-295 mOsm/kg H2O). As a comparison LOCM have an osmolarity between 2 and 3 times that of the blood (521-915 mOsm/kg H2O).
Another chemical-physical characteristic that varies between the different molecules is viscosity. This is particular to each molecule and depends on the dimensions of this molecule and the concentration of iodine. It is questionable whether the high viscosity should be considered to be a disadvantage when comparing the various ICM. Viscosity also depends on temperature: heating the ICM to bring the temperature close to that of the body considerably reduces viscosity (Brunette et al., 2008).
In literature, numerous studies have compared the safety and tolerability of the various contrast media. Whether IOCM are less nephrotoxic has been evaluated in many randomized trials.
The NEPHRIC randomized multicenter study compared the nephrotoxicity of iodixanol vs iohexol in diabetic patients with CKD, encountering a significant reduction of the incidence of CI-AKI in patients randomized to receive IOCM (Aspelin et al., 2003). In the RECOVER study, 300 patients with CrCl < 60 ml/min were randomized to receive iodixanol or the LOCM ioxaglate: in this case the lower incidence of CI-AKI in the IOCM group compared with the population in which LOCM was administered was demonstrated (7.9 vs 17%, P = 0.021) with 0.415 OR (IC 95% 0.194-0.889) for iodixanol (Jo et al., 2006).
Nie et al. (2008) compared the incidence of CI-AKI in patients with CKD, who underwent coronarography with or without percutaneous revascularization, randomized to iodixanol vs iopromide, experiencing an incidence of CI-AKI significantly lower in the IOCM group compared with the LOCM patients (5.7 vs 16.7%; P = 0.011). Song et al. (2017) randomized 220 patients with heart failure and reduced left ventricular systolic function (with or without PCI) to iodixanol or iohexol. The primary endpoint was the incidence of CI-AKI 72h after the coronary procedure. Amongst the secondary endpoints there was the measurement of the spike of a biomarker of kidney injury, cystatin C. In this study the IOCM also showed a lower incidence of CI-AKI compared with the LOCM (12.7 vs 29.1%; P = 0.041). The increase in cystatin C was also significantly lower in the group of patients receiving iodixanol than in the patients in a coronarography study with iohexol.
Other randomized trials either small and underpowered, without head-to-head randomization schemes, or irregular measurement of creatinine afterwards, comparing IOCM vs various LOCM, concluded that there was no statistically significant difference in the incidence of CI-AKI. In the ICON study Mehran et al. (2009) compared the incidence of CI-AKI in patients with CKD randomized to iodixanol (n = 72) vs ioxaglate (n = 74). Even though a trend in favor of iodixanol could be recognized, no statistically significant differences were encountered between the two groups in terms of increase in SCr from 0 to 3 days post-procedure (0.09 mg/dl; interquartile range 0.00-0.30 mg/dl for the Iodixanol group vs 0.15 mg/dl; interquartile range 0.00-0.40 mg/dl; P = 0.07 for the ioxaglate group). The same non statistically significant trend has been found for the percentage of patients with an increase of creatinine ≥ 5 mg/dl (15.9% iodixanol vs 18.2% ioxaglate), ≥ 1.0 mg/dl (1.4% iodixanol vs 4.5% ioxaglate) and ≥ 25% or ≥ 0.5 mg/dl (15.9 and 24.2%, respectively). In the VALOR study approximately 300 patients were randomized to receive iodixanol or ioversol within the context of coronarography investigations. No significant difference was detected in the incidence of CI-AKI between the two groups (21.8% in the iodixanol group and 23.8% in the ioversol group; P = 0.78). In the general population subject of the study the secondary endpoint, i.e. the mean percentage variation of the creatinine peak, was not significantly different between the two groups (14.7% with iodixanol and 20.0% with ioversol; P = 0.06), but in diabetic patients it was significantly lower in the iodixanol group (12.9%) vs ioversol (22.4%, P = 0.01) (Rudnick et al., 2008). The CARE study compared the incidence of CI-AKI in patients with CKD undergoing coronarography or PCI, randomized to iodixanol vs iopamidol. The incidence of CI-AKI (defined as an increase of SCr ≥ 0.5 mg/dl between 2 and 5 days post-procedure with creatinine not measured daily) was not significantly different between the two groups (6.7% iodixanol vs 4.4% iopamidol; P = 0.39). This study reveals quite an interesting aspect, namely the importance of SCr control timing. In those patients in whom the serum creatinine concentration analysis was carried out in the first 3 days there seems to be a trend towards greater safety for iopamidol, but on the contrary measurements taken after 72h showed a lower incidence of CI-AKI in the iodixanol group (Solomon et al., 2007).
In a single center randomized study comparing iodixanol and iopamidol in patients undergoing peripheral diagnosis and intervention no difference was found in the incidence of CI-AKI. It should be pointed out that in the population covered by the study patients with eGFR ≤ 60 ml/min/1.73 m2 were poorly represented (Xiong et al., 2018). Even in the study conducted by Feldkamp et al. (2006) there were no differences in the incidence of CI-AKI in a population at low risk of CI-AKI subjected to coronary diagnosis and intervention, randomized to receive iodixanol vs iopromide. The absence of significant differences in the incidence of CI-AKI between iodixanol and iopromide was encountered in the Shin et al. (2011) even in high-risk patients (on account of presence of CKD, eGFR, ≤ 60 ml/min/1.73 m2). These data are in line with what was previously identified by Laskey et al. (2009) that, in a study of a population of high-risk patients (CKD and diabetes) undergoing coronary diagnostic and interventional procedures, no significant differences were encountered in the incidence of CI-AKI in patients who received iopamidol or iodixanol (9.8% vs 11.2%, P = 0.7).
In literature there are various meta-analyses comparing LOCM and IOCM in terms of nephrotoxicity. Some studies conclude that there are no significant differences in the incidence of CI-AKI between LOCM and IOCM (Biondi-Zoccai et al., 2014; Eng et al., 2016; Heinrich et al., 2009; Pandya et al., 2017; Reed et al., 2009), but other studies report greater safety of the IOCM compared with LOCM (Laskey et al., 2009; Xiong et al., 2018). Most of the observational studies on "all-comers" patients did not find a higher profile of kidney safety for IOCM compared with LOCM (Azzalini et al., 2018b; Briguori et al., 2005). When the trials are restricted to intra-arterial exposure and with a head-to-head randomization of iodixanol to LOCM and daily creatinine values are measured in a standard fashion for each group, a protective effect is observed for iodixanol with ~50% risk reduction (McCullough and Brown, 2011).
There is no evidence to recommend using IOCM vs LOCM in the general population of patients undergoing endovascular diagnostic or interventional procedures. However, in patients clinically judged to be at high risk of CI-AKI, given that the data express the equivalence or otherwise superiority of IOCM vs LOCM in terms of reducing the risk of CI-AKI, the reported trend of greater safety in favor of iso-osmolar ICM could be taken into account in choosing which ICM administer.
The volume of ICM injected is considered a modifiable risk factor of CI-AKI. The effect of automatic injectors (automated contrast injector system, ACIS) on the amount of ICM used and the incidence of CI-AKI has been studied in the past. Some evidence supports the hypothesis that the use of ACIS reduces the amount of ICM administered and consequently reduces the incidence of CI- AKI. In the Minsinger et al. meta-analysis (Minsinger et al., 2014) almost 80 patients from 10 studies were included: the patients in the ACIS group received on average a lower volume of ICM of 45 ml/case (95% CI from -54 to -35; P < 0.001). The incidence of CI-AKI was reduced by 15%, with OR 0.85 (95% CI 0.78 - 0.93; P < 0.001) in patients treated by using ACIS compared with patients receiving a manual injection. Different conclusions were drawn from the Gurm et al. observational study (Gurm et al., 2013b), in which the data about the procedure and the incidence of CI-AKI were analyzed in over 60,000 patients undergoing PCI with or without ACIS. The difference in the volume of ICM used had statistical significance, presumably explained by the significant sample size (mean 199 ± 84 ml with ACIS vs mean 204 ± 82 ml with manual injection; P < 0.0001), but from a clinical point of view no superiority of ACIS compared with the manual injection was demonstrated (incidence of CI-AKI 3.11 vs. 3.42%; P = 0.15).
With the aim of optimizing the volume of ICM injected, reducing the excess (e.g. ICM flowing back from the coronary ostia), without adversely affecting image quality, a device for adjusting the manual injection of ICM has been developed. Clinical studies have demonstrated the effectiveness of reducing the volume of ICM without compromising the quality of the angiographic display (Kaye et al., 2014; Prasad et al., 2015). This aspect has been recently confirmed by a randomized trial in which the use of the AVERT device allowed a relative reduction of 15.5% of the volume of ICM employed in the general population, with a maximum of 46% in patients undergoing complex PCI on three vessels. Despite the significant reduction in the volume of ICM, no benefits in terms of preventing CI-AKI were demonstrated (incidence of CI-AKI 27.0 vs 26.6%; P = 0.70) (Mehran et al., 2018).
The possibility of removing the ICM from the coronary sinus, in the course of the coronarography investigation was tested for the first time in vivo in a limited series of patients by Danenberg et al. (2008), using a double lumen catheter equipped with a balloon for coronary sinus occlusion. In this study the operators were not able to obtain an adequate position for the catheter used in approximately half of the patients. In the 3 patients in whom the catheter was placed correctly on average 44% of the ICM injected was removed. Subsequently Diab et al. (2017) confirmed the possibility of removing more than a third of the volume of ICI injected in the course of coronarography in a series of approximately 40 patients, in whom a catheter for the transseptal approach or a double lumen balloon was used for coronary sinus aspiration. Duffy et al. (2010) tested a device dedicated to the removal of ICM from the coronary sinus, the CINCOR Contrast Removal System (Osprey Medical, St. Paul, MN, USA), obtaining adequate cannulation of the coronary sinus in 31 of the 41 patients studied, with no complications related to the device, removing on average approximately one third of the ICM administered. Preliminary data on the use of this device seems promising with regard to reducing the risk of CI-AKI but further studies are needed to check the actual clinical impact of removing ICM from the coronary sinus.
The RenalGuard device allows adequate water reintegration, adjusted to the euvolemic control, in the course of forced diuresis caused by diuretics. Consequently, this device has been proposed as an instrument for the prevention of CI-AKI. In the REMEDIAL II randomized trial the use of RenalGuard was better at preventing CI-AKI than treatment with N-acetylcysteine and bicarbonate in high risk patients (Briguori et al., 2011). In a study on 400 patients at high risk of CI- AKI with reduced eGFR, RenalGuard proved to be a safe device, with greater efficacy if used to maintain forced intra-procedural diuresis ≥ 450 ml/h (Briguori et al., 2016).
The use of automatic ICM injectors is recommended where possible, to be favored over manual injections. The evidence about the use of devices to prevent CI-AKI is currently quite scarce, although some devices have promising data on efficacy.
Alongside the spread of endovascular procedures using ICM there has been a growing interest over the years in physiopathological mechanisms, epidemiology and preventive strategies for CI-AKI. From a review of the literature, it has become evident that the findings on this subject re not always in Agreement. For this reason, multiple national and international scientific societies, in the disciplines of radiology, cardiology and nephrology, have come together to define consensus and position papers on this topic.
Recently, the Italian Society of Interventional Cardiology (SICI-GISE) published a consensus document on CI-AKI, in partnership with the Italian Society of Nephrology (SIN), with the aim to increase awareness among the community of interventional cardiologists of the importance of a correct approach to patients undergoing invasive procedures, including in the context of nephroprotection (Ronco et al., 2019).
There are many factors determining the renal outcome of patients treated in hemodynamics laboratories. This aspect complicates the performance of randomized clinical trials on strategies for the prevention of CI-AKI and explains why the data available in literature is often conflicting. As reported in a recent review (Mehran et al., 2019), dissemination of knowledge about the clinical impact of CI-AKI must not in itself restrict the indication for invasive procedures, so as not to preclude patients at high cardiovascular risk from following therapeutic paths of proven effectiveness, through the fear of a deterioration in kidney function often of multifactorial genesis and not only linked to the administration of ICM. Rather, the importance of a correct approach for patients who are candidates for diagnostic and interventional procedures using ICM is emphasized, implementing preventive strategies and clinical monitoring in order to reduce the risk of CI-AKI.
The authors declare no funding, no contribution from other persons.
The authors declare no conflicts of interest statement.
