Renal congestion is becoming recognized as a potential contributor to cardiorenal syndromes. Adequate control of congestion with simultaneous preservation of renal function has been proposed as a central goal of the management of heart failure. We report our care of a 48-year-old woman suffering from right heart failure and massive fluid overload due to severe pulmonary hypertension secondary to a combination of left-heart disease and status after recurrent pulmonary embolisms. Alterations in Doppler-derived intrarenal venous flow patterns and a novel renal venous stasis index were used to evaluate improvement in renal venous congestion during decompensation. Due to refractory congestion despite optimal medical treatment and continuous veno-venous hemodialysis, a peritoneal dialysis catheter was placed to relieve the massive ascites. The paracentesis of ascites led to a significant loss of weight, normalization of hydration status with subsequent termination of continuous veno-venous hemodialysis, and a significant improvement in clinical and echocardiographic parameters. Renal Doppler ultrasonography showed continuous improvement in intrarenal venous flow patterns and the renal venous stasis index indicative of effective decongestion up to a normal intrarenal venous flow pattern and renal venous stasis index. Furthermore, residual renal function increased during follow-up. This case demonstrates the feasibility of renal Doppler ultrasonography as a simple, non-invasive, and integrative measure of renal congestion. The renal venous stasis index and intrarenal venous flow patterns may be useful to evaluate the treatment response and to guide therapy in patients with right heart failure.
Deterioration of renal function is common in acute and chronic heart failure (HF) settings and has been classified as “cardiorenal syndrome type 1 and 2”, respectively (Ronco et al., 2008). In addition to low cardiac output, persistent venous congestion is becoming recognized as a potential contributor to renal dysfunction in cardiorenal syndromes. Also, adequate control of congestion with simultaneous preservation of renal function has been proposed as a central goal of HF (Damman et al., 2009; Jessup and Costanzo, 2009; Mullens et al., 2009). Congestion can lead to a vicious circle of renal dysfunction, increases in intra-abdominal pressure with ascites formation, neurohormonal activation, excessive reabsorption of sodium in renal tubules, fluid overload, and diuretic resistance, which lead to further HF progression (Mullens et al., 2008). Ultimately, renal replacement therapy (RRT) may be required to relieve congestion in the setting of diuretic-resistant fluid overload and acute kidney injury (Bart et al., 2012).
Recently, renal Doppler ultrasonography has been proposed to evaluate renal venous congestion with different intrarenal venous flow (IRVF) patterns to predict adverse outcomes and the diuretic response in HF (Iida et al., 2016; Nijst et al., 2017; Puzzovivo et al., 2018). However, this approach does not reflect the continuum of renal congestion, and changes within an IRVF pattern category may be missed.
We recently described a new, dimensionless continuous ratio, the renal venous stasis index (RVSI), to reflect the full continuum of renal venous congestion (Husain-Syed et al., 2019). The RVSI, based on Doppler renal venous flow, indicates the proportion of the cardiac cycle during which there is no renal venous outlet flow. The RVSI, by measuring each variable separately, is calculated as:
RVSI = (cardiac cycle time [ms] - venous flow time [ms])/cardiac cycle time [ms]
The example detailed below describes alterations in IRVF patterns and the RVSI as markers of an improvement in renal venous congestion during decompensation of a patient with right heart failure and severe fluid overload.
In June 2017, a 48-year old Caucasian woman was admitted to the intensive care unit of our hospital with hypoxic respiratory failure due to right heart failure and massive fluid overload (ascites, pleural effusion, and anasarca) necessitating non-invasive ventilation.
Renal Doppler ultrasonography revealed a RVSI of 0.74 (denoting an absence of renal venous outlet flow during 74% of the cardiac cycle time) and a monophasic IRVF pattern (Fig. 1). A recent right heart catheterization had shown severe pre- and postcapillary pulmonary hypertension, which was suggested to result from a combination of heart failure with preserved ejection fraction (relevant coronary artery disease and valve disease were excluded, an increased ratio of mitral inflow velocity to lateral annular relaxation velocity of 19 was measured), fluid overload and status after recurrent and distal thromboembolic disease (Galie et al., 2016). Within the previous 3 months, she had been admitted to another hospital due to similar symptoms, but was recompensated with loop diuretics (i.v.). However, this time she remained anuric despite administration of loop diuretics (i.v.) and a thiazide diuretic. Another invasive hemodynamic assessment showed an increase in mean pulmonary arterial pressure and pulmonary wedge pressure with a reduced cardiac index. Sildenafil (i.v.) and continuous RRT using the GENIUS dialysis system were initiated. However, a negative fluid balance could be achieved only slowly due to low ultrafiltration rates because of low blood pressure values necessitating vasopressor use. In addition, the patient stayed anuric.
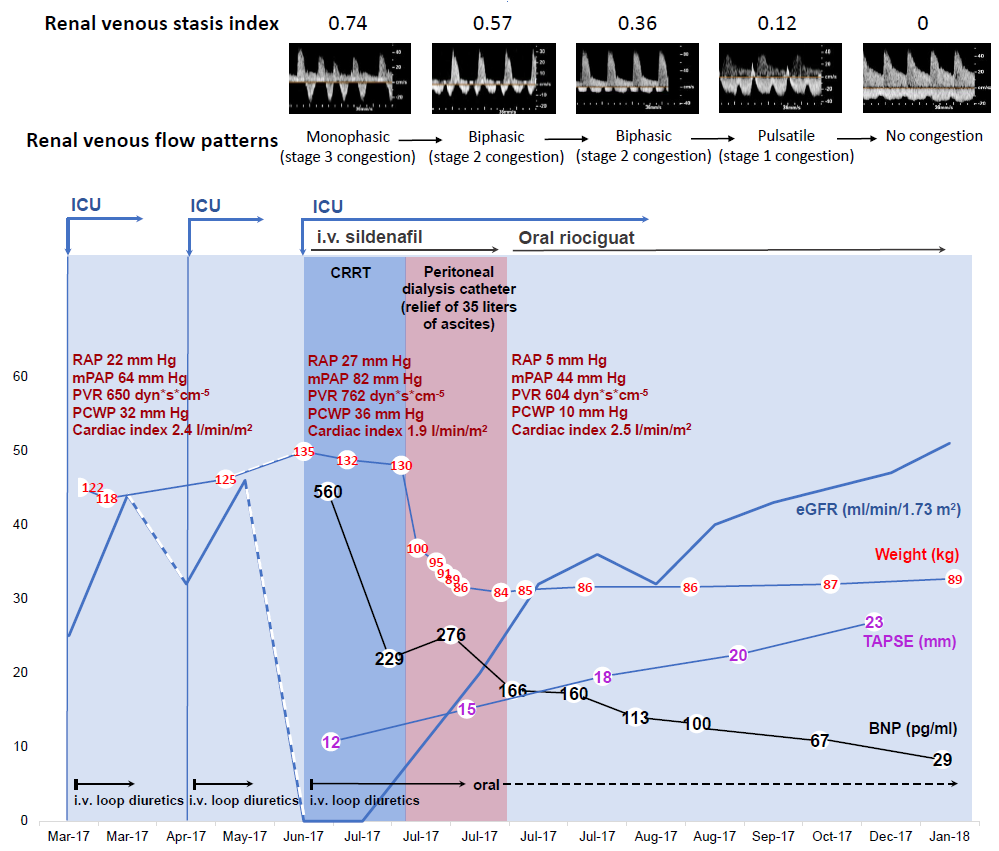 Figure 1.
Figure 1.Time-course of body weight, invasive hemodynamics, right ventricular systolic function (TAPSE), neurohormonal activation (BNP), and residual renal function (eGFR using Chronic Kidney Disease Epidemiology Collaboration (Levey et al., 2009)) during hospital stay. A marked reduction in weight and BNP level, and an increase in TAPSE over time was seen after continuous relief from ascites, followed by progressive improvement in residual renal function. Dark-blue and pink bars denote CRRT and paracentesis of ascites/peritoneal ultrafiltration sessions, respectively. BNP, b-type natriuretic peptide; CRRT, continuous renal replacement therapy; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; i.v., intravenous; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; IRVF, intrarenal venous flow; RVSI, renal venous stasis index; TAPSE, tricuspid annular plane systolic excursion.
Finally, we decided to implant a peritoneal dialysis catheter under local anesthesia, primarily to relieve the massive ascites. During 15 days, 35 L of ascites were drained. Laboratory testing revealed transudative ascites without signs of infection or malignancy. Finally, when ascites decreased below 500 mL per day, peritoneal ultrafiltration was initiated with empirically 1,000 mL icodextrin twice a day with a dwell time of 12 h. As the patients’ residual renal function recovered quickly during decompensation, a peritoneal dialysis treatment was not required. During the course of intensive care, renal Doppler ultrasonography showed continuous improvement in the RVSI and IRVF patterns indicative of effective decongestion up to a normal RVSI and IRVF pattern. Continuous RRT could be terminated because the patient lost a significant amount of weight. Diuresis increased in parallel, and the estimated glomerular filtration rate remained stable at > 30 mL/min/1.73 m2 with a serum creatinine level of 158 µmol/L and urine output of 2 L/day so that icodextrin treatment could be discontinued. Right-ventricular function (as measured by tricuspid annular plane systolic excursion) improved significantly, and the level of b-type natriuretic peptide fell significantly. Re-assessment of invasive hemodynamics revealed precapillary pulmonary hypertension with a good hemodynamic response to riociguat. Since a catheter-based pulmonary angiography revealed a distal inoperable chronic thromboembolic disease, a targeted pulmonary hypertension therapy with riociguat was initiated. Ascites did not return, so the peritoneal dialysis catheter was removed in September 2017. The patient was discharged soon after that, and she has been in a stable condition since that time.
The case demonstrated three main teaching points. After repeated hydropic decompensation with the necessity of hospitalization, there was a need for an alternative therapeutic approach.
First, implantation of a peritoneal dialysis catheter for relief of ascites led to normalization of hydration status with subsequent termination of continuous RRT, improvement in renal function, and significant improvement in clinical and echocardiographic parameters. We did not measure intra-abdominal pressure continuously. However, we assumed that its progressive decrease due to relief from ascites, in addition to the normalization of fluid overload, was associated with improvement in renal venous congestion (Mullens et al., 2008). Sildenafil was administered as a continuous intravenous infusion in view of the presumed inability to absorb sildenafil orally due to gastrointestinal malabsorption (Lammers et al., 2006). We acknowledge that intravenous sildenafil in patients with right heart failure is an off-label use (Price et al., 2010). However, in patients with pulmonary hypertension due to left heart disease and a pronounced pre-capillary component (high pulmonary vascular pressure and/or high diastolic pulmonary gradient), treatment with pulmonary vasodilators (“targeted pulmonary hypertension therapy”) may be considered as a therapeutic option on a case-by-case basis (Guazzi et al., 2011; Olsson et al., 2018).
Second, re-assessment of invasive hemodynamics after normalization of fluid overload revealed a precapillary pulmonary hypertension, emphasizing the importance of an euvolemic clinical status during right heart catheterization to accurately assign the patient to the underlying pulmonary hypertension group.
Third, renal Doppler ultrasonography showed a parallel and continuous improvement in RVSI and IRVF patterns that indicated effective decongestion up to a normal RVSI and the IRVF pattern. RVSI may be more sensitive to detect changes of renal-venous congestion within an IRVF pattern category.
This case demonstrated the feasibility of using renal Doppler ultrasonography to evaluate the treatment response and to guide therapy in patients with right heart failure. In particular, this case highlighted the potential role of the RVSI as a simple, non-invasive, and integrative Doppler-derived measure of renal congestion (Table 1). Prospective studies are needed to: (i) evaluate the RVSI as a potential new diagnostic measure for predicting outcome in HF; (ii) and assess the utility of the RVSI in HF management.
| • The renal venous stasis index (RVSI) is a new and integrative Doppler measure of renal venous congestion. |
| • RVSI indicates the proportion of the cardiac cycle during which there is no renal venous outlet flow and is calculated as (cardiac cycle time-venous flow time)/cardiac cycle time. |
| • The present case vignette presents the potential usefulness of RVSI as a dynamic marker of alterations in renal venous congestion secondary to right heart failure. |
| • RVSI may provide additional prognostic information in daily clinical practice to better characterize patients with cardiorenal syndromes. |
| • Longitudinal studies are needed to assess the utility of renal Doppler ultrasonography-estimated renal congestion to guide decongestive or heart failure therapy. |
The authors thank the nursing staff of the Division of Pulmonology and Nephrology, Department of Internal Medicine II, University Hospital Giessen and Marburg, Giessen for their hard work and commitment to patient wellbeing. Without their support, this work would not have been possible.
Dr. Seeger discloses personal fees for consulting from Bayer Pharma AG, Liquidia Technologies, Inc, and United Therapeutics Corporation outside the submitted work.
Dr. Gall discloses personal fees and non-financial support from Actelion, AstraZeneca, Bayer, BMS, GlaxoSmithKline, Janssen Cilag, Lilly, MSD, Novartis, Pfizer, and United Therapeutics/OMT outside the submitted work.
Dr. Ghofrani discloses grants from German Research Foundation during the work, and personal fees from Actelion, Bayer, GSK, Novartis, Pfizer, Bellerophon Pulse Technologies, and MSD Merck Sharpe & Dohme outside the submitted work.
None of the other authors declare a conflict of interest.
