Chronic kidney disease is a growing public health problem, as its prevalence and incidence have almost doubled over the last three decades. Chronic kidney disease is defined as the presence of an estimated glomerular filtration rate < 60 ml/min/1.73 m2 and/or proteinuria ≥ 0.150 g/24 h. It has been demonstrated that both proteinuria and reduction in estimated glomerular filtration rate can predict the development of fatal and non-fatal cardiovascular events, regardless of traditional cardiovascular risk factors, namely blood pressure, smoking habit, cholesterol, age, gender. This relationship is found in the general population, high-risk cohorts and in patients referred to Nephrologists (tertiary care). The accuracy by which proteinuria or estimated glomerular filtration rate can predict these events, exceeds that obtained by the combination of all the other traditional risk factors. These important findings have led to chronic kidney disease being considered as a cardiovascular risk equivalent. Although this needs further investigation, a great effort has been made to reduce the cardiovascular risk in chronic kidney disease patients. Indeed, many clinical trials have been carried-out testing the effect of antihypertensive, proteinuria-lowering, lipid-lowering and hypoglycemic agents on cardiovascular risk protection. All these trials reduced, but did not eliminate, the overall cardiovascular risk. Future studies should be undertaken to identify high cardiovascular risk patients and novel therapeutic targets for cardiovascular protection in chronic kidney disease patients.
Chronic kidney disease (CKD) is defined as the presence of an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 as marker of decreased kidney function and/or the presence of abnormal levels of albuminuria as a sign of kidney damage, or both (Levey and Coresh, 2012). The presence of albuminuria (or proteinuria) can be measured on a 24-hour urine sample or, alternatively, by calculating the albumin-to-creatinine ratio (ACR) from a first morning urine void (McIntyre and Taal, 2008; Methven et al., 2011). Current guidelines suggest considering abnormal a 24-hour urine excretion ≥ 150 mg/24 h or ≥ 30 mg/24 h for proteinuria and albuminuria or an ACR ≥ 30 mg/g (Inker et al., 2014). Prevalence of proteinuria varies on the basis of the examined population. In the general population 4.8 to 10.3% of subjects show a degree of proteinuria (Arora et al., 2013; Coresh et al., 2007; De Nicola et al., 2015; Zhang et al., 2012). This frequency is significantly increased in high-risk populations represented by patients with type 2 diabetes or with hypertension and in patients referred to Nephrologists. In this latter setting, up to 70% of patients are diagnosed to have proteinuria. The other parameter that defines CKD is the eGFR, an estimate of GFR per unit of body surface area, mainly based on age, gender and race as surrogates of creatinine generation from muscles. eGFR is often calculated using creatinine-based formulas, such as the Modification of Diet in Renal Disease (MDRD) Study equation and the chronic kidney disease Epidemiology Collaboration (CKD-EPI) equation, the latter being considered more precise and accurate (Ellam and El Nahas, 2011). The Kidney Disease Improving Global (KDIGO) guidelines on the management of CKD classify CKD based on both the assessment of eGFR and proteinuria that allows allocating patients into several risk categories with a progressively worse prognosis. When a low eGFR and the presence of proteinuria have been assessed together in general population, the global prevalence of CKD exceeded 10-15% in most Countries worldwide (De Nicola and Zoccali, 2016). These relevant epidemiologic data have led to recognize CKD as a public health priority. The reason underlying the epidemic diffusion of CKD is related to the change in epidemiology of chronic diseases, in general. Indeed, the prevalence of adults with diabetes has increased fourfold from 1980 to 2014 and those with hypertension has almost doubled from 1975 to 2015 (Zhou et al., 2016; Mills et al., 2016). At the same time, as stated by the Global Burden of Kidney Disease, the prevalence and incidence of CKD have risen by 87% and 89 (Xie et al., 2018). This trend can also be explained with the decrease in mortality from cardiovascular and infectious diseases (Mensah et al., 2017; Murray and Lopez, 2013) and with the progressive aging of the population (Provenzano et al., 2019).
CKD patients referred to Nephrologists constitute a peculiar setting (also defined nephrology care) that is characterized by an elevated prevalence of comorbidities, such as hypertension, previous cardiovascular (CV) disease, diabetes and obesity (Lash et al., 2009; Provenzano et al., 2018). With respect of clinical outcomes, CKD patients are at increased risk of mortality, CV events and progression to End-Stage-Kidney-Disease (ESKD), a terminal phase of the disease that requires the recurrence to renal replacement treatments i.e. dialysis or renal transplantation. It has been demonstrated that age and kidney function are the two main mediators of mortality and ESKD risks in CKD patients (De Nicola et al., 2012; , 2017). In patients up to 60 years of age or 65 years of age (and with eGFR less than 30 ml/min/1.73 m2), the ESKD rate was greater than the rate of death (including CV death), the risk of death being more frequent in patients older than 60 years with mild reduced kidney function.
However, CV risk, in addition to mortality and ESKD, is far from being trivial. In a pooled cohort analysis of CKD patients referred to Italian nephrology clinics that included fatal/non-fatal CV events (myocardial infarction, stroke, congestive heart failure, revascularization, peripheral vascular disease or non-traumatic amputation), ESKD and mortality rates were 4.52, 5.26 and 3.76 per 100/pts/year, respectively (Minutolo et al., 2018).
The aim of this narrative review article is to elucidate the main epidemiologic evidence regarding the association between kidney damage and cardiovascular disease, the mechanisms responsible for this association, available strategies to reduce CV risk in CKD patients and future directions to answer the unmet needs around this topic.
In the past few years, several studies have demonstrated how the presence of CKD is a risk factor for the development of CV events. In this regard, in a recent meta-analysis, which enrolled more than 100.000 individuals in the general population, both a reduction of eGFR and the presence of albuminuria were independently and strictly associated with an increased risk of death (including CV death), regardless of many potential confounders, such as gender, age, and traditional CV risk factors (Matsushita et al., 2010). In particular, the albuminuria-related CV risk doubled with an increase in the urinary albumin/creatinine ratio from 5 mg/g to 100 mg/g, and when evaluating the role of eGFR, the mortality risk was increased for eGFR level ≤ 60 ml/min/1.73 m2 and was 2-fold higher at 30-45 ml/min/1.73 m2, when compared with normal eGFR levels. Similar results were also obtained in studies from patients at elevated risk for CV disease, as in those with type II diabetes and hypertension (Bolignano et al., 2008; Buemi et al., 2007; Coppolino et al., 2008; , 2017; Fox et al., 2012; Mahmoodi et al., 2012). In this context, a great contribution came from the Kidney Early Evaluation Program (KEEP). The KEEP was a community-based study, started in the United States in the early 2000s, which enrolled patients at high risk for developing CKD, i.e. patients with a personal and/or family history of hypertension and diabetes. The aim of this program was to improve early detection of CKD, where prompt and effective prevention strategies can be adopted (Whaley-Connell et al., 2013). At the same time the KEEP cohort has contributed to our understanding on CV risk stratification, prognosis and treatment (Whaley-Connell et al., 2012). In KEEP patients, the overall crude rate of CV disease was higher in patients with CKD as compared to those without CKD. Moreover, CKD has been found at multivariable analysis, to be a significant predictor (Odds Ratio: 1.44; 95% CI, 1.27-1.63) of premature CV death, defined as the occurrence of myocardial infarction or stroke before 55 years in males and 65 years in females. Importantly, the achievement of all CV risk factors target was observed only in a minority of patients (up to 27%), thus suggesting that awareness of CV risk is still low and need further efforts to be improved in the future (McCullough et al., 2011). In a longitudinal analysis restricted to the subgroup of KEEP cohort with CKD, lower eGFR, increased albuminuria and diabetes have been found as significant predictors of mortality, all these factors being considered markers of CV damage (McCullough et al., 2010).
The observational investigation of the Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) clinical trial proved that high albuminuria and low eGFR were independent risk factors for CV events in patients with type II diabetes (Ninomiya et al., 2009). The novel finding of the ADVANCE analysis, as compared with the previous reported studies is that, in the ADVANCE study, CV event risks were estimated by separately considering stroke, myocardial infarction and chronic heart failure.
Thus, the two main kidney measures, i.e. albuminuria and eGFR, are both strongly associated with the risk of CV events. This association is independent from diabetes and hypertension, thus confirming the significant role of CKD per se.
The mechanisms that may explain the association between kidney measures and CV damage have always attracted attention and are not completely elucidated. Researchers from the Steno Memorial Hospital, in Denmark, were the first to hypothesize the deleterious effect of the role of albuminuria on CV risk, in the so-called Steno hypothesis (Deckert et al., 1989). Owing to the finding that in diabetic patients with albuminuria, this biomarker was associated with increased levels of von Willebrand factor and an increased transcapillary escape rate of fibrinogen, they proposed considering albuminuria as a general marker of endothelial dysfunction and systemic vascular damage. The Steno hypothesis was supported by other studies, which demonstrated the association between the presence of albuminuria and the onset of retinopathy (De Nicola et al., 2015; Liang et al., 2013), abnormalities in endothelial glycocalyx as well as other endothelial structures (Perticone et al., 2015; Stehouwer et al., 1992; , 2016). The link between kidney function and cardiovascular risk is not completely understood. In a Japanese survey, samples of heart tissue obtained from 482 individuals, who underwent autopsies, were examined. The severity of coronary atherosclerosis correlated with the grade of kidney damage (Bolignano et al., 2010; De Nicola et al., 2006; Nakano et al., 2010). Furthermore, severe coronary stenosis was found in about 50% of the pre-dialysis patients, correlating with low levels of eGFR (Ohtake et al., 2005). Interestingly, early vascular abnormalities, such as the reduction in renal perfusion and the absence of renal functional reserve, were found as significant predictors of kidney function decline even in patients with normal eGFR levels (Fuiano et al., 2005). The importance of all these findings can be summarized with the evidence that severity of vascular damage, measured by the extent of coronary calcifications, is associated with an increased risk of CV events and CKD progression (Russo et al., 2011). Improving management of CV risk factors before reaching advanced CKD stage is therefore becoming the main target of nephrology care.
In the clinical setting, a clear demonstration of the strict relationship between the kidney and the heart is represented by a variety of disorders, where an acute or chronic dysfunction in one organ may induce an acute or chronic dysfunction of the other (Rangaswami et al., 2019). These entities have been called cardiorenal syndromes (CRS) (Ronco et al., 2010). The main clinical aspect of these syndromes is represented by the scarce response to treatment of congestive symptoms of heart failure (HF) due to the reduced renal function. The onset of CRS can be attributed to a multitude of hemodynamic and non-hemodynamic risk factors. The pre-renal hypoperfusion that is seen in the presence of HF explains only in part the decreased kidney function in patients with CRS. Indeed, an eGFR reduction can be observed in HF patients with or without systolic dysfunction (Adams et al., 2005). It has been demonstrated that other factors, increasing the central venous pressure and intra-abdominal pressure are associated to a worsening in renal function since, in these cases, the compensatory increase in intraglomerular pressure is lost and eGFR is reduced. The activation of Renin-angiotensin-aldosterone-system (RAAS) and neurohumoral axis cooperates in the development of CRS by increasing water and sodium reabsorption in the renal proximal tubule, thus resulting in oliguria and congestion worsening (Ljungman et al., 1990). Interestingly, several non-hemodynamic risk factors are effective in CRS, such as reactive oxygen species/nitric oxide production, inflammatory factors (Tumor Necrosis Factor-α, Interleukin-1 and Interleukin-6), being responsible for the structural changes that are simultaneously present in the heart and the kidney. Although the CRS often presents overlap and recognizing their primum movens can be challenging, the Acute Dialysis Quality Initiative consensus approach (ADQI) identified 2 major groups, the cardiorenal and renocardiac syndromes. These groups have been further subdivided into 5 categories, based on the acuity of disease and the sequence in organ involvement. The ADQI remarked that a crucial step for the clinician is distinguishing the presence of a fluctuation of kidney function (i.e. worsened renal function) from a renal injury in the context of CRS, since this may guide a specific diagnostic and therapeutic approach (Damman et al., 2014). To this aim, the measurement of several biomarkers of cardiac (B-type Natriuretic Peptide, Suppressor of Tumorigenicity 2) and renal damage (Neutrophil Gelatinase-Associated Lipocalin, Tissue Inhibitor of Metalloproteinase-2 and Insulin-like Growth Factor-Binding Protein 7) may improve, if correctly used, risk stratification of CRS patients and are promising arms for future research (Rangaswami et al., 2019).
Several CV risk assessment techniques are available for use in clinical practice (Piepoli et al., 2016). The Framingham risk score is a algorithm formulated to estimate the 10-year individual risk of developing coronary heart disease (CHD), based on a combination of predictors, such as age, gender, systolic blood pressure, total and high density lipoprotein (HDL) cholesterol and smoking status. Once the score has been calculated, it should modify the clinical decision, mainly with respect to when and how to start treatments, in accordance with the 10-year risk: individuals with low risk have < 1% or less CHD risk at 10 years; those with intermediate risk have a risk between 1 and 5%; high and very high risk categories are defined by a 10-year risk of 5-10% and ≥ 10%, respectively. Starting from the Framingham risk score, a recent score has been developed by expanding the 10-year risk prediction estimates to all atherosclerotic CV events (coronary death or nonfatal myocardial infarction, fatal or nonfatal stroke). Risk factors considered for developing and computing the ASCVD score equation are age, sex, race, total cholesterol, HDL cholesterol, systolic blood pressure, blood pressure lowering drugs, diabetes and smoking status. Interestingly, to guarantee the generalizability of the score, Investigators used other community-based populations, other than the Framingham Original cohort: the Atherosclerosis Risk in Communities (ARIC) community, the Cardiovascular Health Study and the CARDIA (Coronary Artery Risk Development in Young Adults) study (Goff et al., 2014). However, when these predictive models have been applied to CKD patients they do not work. The Framingham risk score equation showed poor calibration and the suboptimal accuracy in CKD patients, mainly because the observed CV events (%) were completely different between CKD patients as compared to patients selected from community (Weiner et al., 2007). Therefore, research has recently focused on assessing whether adding the CKD measures (i.e. albuminuria and eGFR) can improve the predictability of the existing models. As demonstrated in a recent meta-analysis, albuminuria, eGFR or both should be taken into consideration for prediction of cardiovascular events, being able to improve model performance for the prediction of all CV endpoints: CV mortality, coronary heart disease, stroke, heart failure (Cedillo-Couvert and Ricardo, 2016; Coppolino et al., 2014; Matsushita et al., 2015; Coppolino et al., 2018). Moreover, when the CV events prediction accuracy of eGFR and albuminuria has been evaluated, it has been noted that the contribution of both these factors was similar, or even greater, than any traditional risk factor (Matsushita et al., 2015). However, it is not clear how to incorporate these measures for actual risk prediction. A CV risk score, built specifically on CKD patients, thus accounting for basal risk factors (including eGFR and proteinuria) of this population, is highly awaited (Ballew and Matsushita, 2018).
Interestingly, two independent analyses, carried-out in the Alberta Kidney Disease and ARIC populations, proved that in patients with CKD (eGFR < 60 ml/min/1.73 m2) with or without diabetes, the risk of cardiovascular events was similar (Tonelli et al., 2012; Weiner et al., 2007). These results, that noted the importance of albuminuria and eGFR for CV risk stratification, have led guidelines to define CKD as CV risk equivalent, analogously to type II diabetes (Ponikowski et al., 2016; Stevens et al., 2013). Nevertheless, some concerns were raised about this definition: 1) by stating CKD a CV risk equivalent, all patients who match the CKD definition are considered at increased CV risk regardless of age, previous CV (such as myocardial infarction or stroke), smoking habit status, blood pressure and cholesterol level; a patient with eGFR of 45 ml/min/1.73 m2, who is currently a smoker and with a blood pressure levels of 150/100 mmHg will be classified in the same CV risk category as another patient with eGFR of 45 ml/min/1.73 m2, who is not a smoker and who is normotensive. 2) In the aforementioned studies, the ARIC and Alberta Kidney Disease, even if the rates of CV events were similar in diabetic and non-diabetic patients, a major determinant of CV events was the presence of previous cardiovascular events (which is the principal risk factor for new CV events). The discussion is still ongoing as to whether CKD be considered a risk equivalent for CV disease, but more evidence must be obtained to reach this conclusion. One of the main problems of CKD patients is the coexistence of many comorbidities and the resulting complexity in terms of basal risk, management and prognosis. In a multi-cohort analysis of about 4,000 patients referred to Nephrology Units in Italy, we have previously described the main features and prognosis of CKD patients under regular nephrology care. The population was characterized by old average age (67 years), and a high prevalence of diabetes (29%) and CV disease (34%). Therefore, this CKD population is at increased risk of developing CV events. A detailed illustration of the frequency of CV disease in patients with and without CKD has been provided by the United States Renal Data System (USRDS) and is depicted in Fig. 1. Moreover, in patients under regular nephrology care, a higher prevalence of CV disease was found among CKD patients who were diagnosed with diabetic nephropathy or hypertensive nephropathy, thus demonstrating the coexistence of multiple risk factors in these patients (Fig. 2) (Provenzano et al., 2018). On the other hand, among over 400,000 Italian patients followed by general practitioners, the frequency of diabetes and CV disease was 4.7% and 7.9%, respectively, testifying wide difference of basal risk factors between settings (Minutolo et al., 2008).
 Figure 1.
Figure 1.Prevalence of common cardiovascular diseases in patients with (Red area) or without (Blue area) chronic kidney disease (CKD) in the United States, in the year 2015.
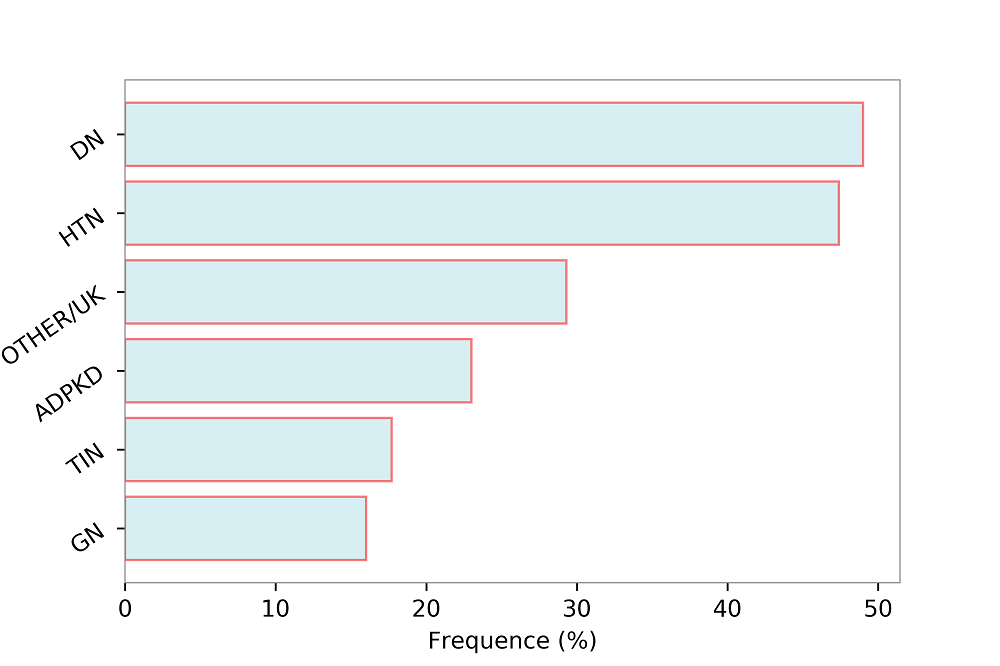 Figure 2.
Figure 2.Prevalence (%) of cardiovascular disease (myocardial infarction, stroke, peripheral vascular disease, chronic heart failure, angina) by primary renal disease categories. HTN, Hypertensive Nephropathy; DN, Diabetic Nephropathy; OTHER/UK, Other or Unknown; ADPKD, Autosomal Dominant Polycystic Kidney Disease; TIN, Tubulo-Interstitial Nephropathies; GN, Glomerulonephritis. Data source: 3.957 patients selected from the Italian multicenter cohort of CKD patients referred to nephrologists (Provenzano et al., 2018).
A further important finding from previous studies is that, in CKD patients, CV disease is poorly controlled (De Nicola et al., 2015; , 2012; Russo et al., 2005). The TArget Blood Pressure LEvels (TABLE-CKD), a multicenter observational study, showed that in a cohort of CKD patients with CKD stage 3-5, followed for at least 6 months, less than 15% of patients were within the target blood pressure ( < 130/80 mmHg). In an observational study of patients under nephrology care, who were treated with at least one RAAS inhibitor (RAAS-I), Minutolo and colleagues found that 69.5% of patients still had proteinuria. In this study, residual proteinuria was an independent predictor of fatal and non-fatal CV events, with the risk starting to increase from 0.500 g/24 h and from 0.150 g/24 h onward, in non-diabetic-CKD and diabetic-CKD patients, respectively (Minutolo et al., 2018).
Thus, CKD population is characterized by multiple risk factors that, overall, increase the individual CV risk. However, the definition of CKD as an equivalent of cardiovascular risk needs further observational and intervention studies to be accepted. In addition, since non-traditional CV risk factors appear to be scarcely controlled in CKD patients, strategies aimed at improving treatments and prognosis are necessary.
Although the reduction of cardiovascular risk is a primary target in CKD patients, in the past, high-risk patients, including those with impaired kidney function, were regularly excluded from the randomized clinical trials (Zoccali et al., 2019). Within the last twenty years, this trend has fortunately changed, and a growing number of intervention studies have been completed, with the aim of achieving a better control of CV risk in CKD patients (Asselbergs et al., 2004; Anand et al., 2009; Baigent et al., 2011; Berl et al., 2003; Heerspink et al., 2010; Brugts et al., 2007; Mann et al., 2001; Norris et al., 2006; Orth and Hallan, 2008; Parving et al., 2012; Perkovic et al., 2007; Rahman et al., 2006; Shiga et al., 2010; Solomon et al., 2006; Tonelli et al., 2012; Wanner et al., 2016; Xia et al., 2017) . The main studies assessing the CV risk reduction in CKD patients are recorded in Table 1. The CV benefits of RAAS-I are well investigated. The Heart Outcomes Protection Evaluation (HOPE) and Perindopril Protection against Recurrent Stroke Study (PROGRESS) have shown that, in patients with CKD, the use of RAAS-I not only offers benefits for the control of blood pressure, but also protects from major CV outcomes, such as stroke, coronary heart disease and CV death (Bolignano et al., 2016; Mann et al., 2001; Perkovic et al., 2007). It is attractively that the magnitude of the absolute CV risk reduction, achieved with RAAS-I in these studies, was greater in patients with CKD than in those without CKD, testifying that the obtained benefit is more pronounced in patients with kidney dysfunction, particularly in presence of proteinuria (Sarafidis et al., 2007). Indeed, RAAS-Is act by reducing intraglomerular pressure that is considered a trigger for the development of proteinuria. An increase in intraglomerular pressure is responsible of the wall stress and the expansion of glomerular diameter that, together with the mesangial-cell inflammatory response, induce glomerular injury over time (Cortes et al., 1999).
| Study | Population | Intervention | Outcome | Results |
|---|---|---|---|---|
| PROGRESS Study (Perkovic et al., 2007) | Cerebrovascular disease and CKD | Perindopril vs. placebo | Total stroke (fatal or non-fatal) and major vascular events. | In patients treated with perindopril, risks of major vascular events and stroke were 30% and 35% lower then placebo. This evidence is found in patients with CKD, where the effect of treatment was greater as compared to patients without CKD. |
| CV outcomes in the Irbesartan Diabetic Nephropathy Trial (Berl et al., 2003) | Type 2 diabetic nephropathy and hypertension | Irbesartan, amlodipine, or placebo. | Doubling of serum creatinine, end-stage renal disease, death from any cause. | Cardiovascular risk was unchanged with the addition of irbesartan, amlodipine, or placebo to conventional antihypertensive therapy in patients with type 2 diabetes and nephropathy. |
| Risk of coronary events in people with chronic kidney disease compared with those with diabetes (Tonelli et al., 2012) | CV events in CKD and diabetes | Demonstrating whether chronic kidney disease should be considered as a coronary heart disease risk equivalent. | The rate of incident myocardial infarction was lower in diabetic patients than in patients with CKD defined eGFR < 45 mL/min per 1.73 m2 and severe proteinuria | |
| ALLHAT Study (Rahman et al., 2006) | CKD and hypertension | Chlorthalidone vs. Amlodipine vs. Lisinopril. | Rates of coronary heart disease (CHD) and ESRD; Predictors of CHD; |
Older patients with hypertension and reduced eGFR developed more frequently CHD than ESRD. A low GFR was and independent predictor of increased CHD risk. Neither amlodipine nor lisinopril is superior to chlorthalidone in reducing risks for CHD, stroke or combined CVD. Chlorthalidone was superior to amlodipine and lisinopril for preventing heart failure. |
| ADVANCE Study (Heerspink et al., 2010) | CKD and type 2 diabetes | Perindopril and Indapamide vs. placebo | Major adverse cardiac event or MACE (cardiovascular death, non-fatal myocardial infarction, unstable angina, heart failure, stroke and other cardiovascular events requiring hospitalization). | The treatment with perindopril-indapamide in patients with type 2 diabetes reuduced the risk of cardiovascular, renal outcomes and death across all stages of CKD. Absolute risk reductions were higher in patients with CKD, thus demonstrating the importance of blood pressure control in this population. |
| EMPA-REG OUTCOME Study (Wanner et al., 2016) | Type 2 diabetes at increased cardiovascular risk | 10 mg Empaglifozin vs. 25 mg of Empagliflozin vs. placebo | Progression of CKD (development of macroalbuminuria, doubling of the serum creatinine, ESRD, or death from renal disease) and incident albuminuria. | Empagliflozin slowed the progression of kidney disease as compared to standard care. |
| HIJ-CREATE Study (Shiga et al., 2010) | High-risk hypertensive patients with CHD and CKD | Candesartan vs. non-ARB treatment | Major adverse cardiac event (MACE) | There was no difference in MACE between the two treatment groups in patients without CKD. However, there was a lower incidence of MACE in the candesartan-based treatment group than in the non-ARBs treatment group in patients with CKD. |
| ALTITUDE Study (Parving et al., 2012) | Type 2 diabetes and CKD, CVD, or both | Aliskiren vs. placebo in addition to an ACE inhibitor or an ARB | Major adverse cardiac event (MACE); ESRD, death due to kidney failure, need for renal-replacement therapy with no dialysis or transplantation available or initiated, doubling of serum creatinine. | The addition of aliskiren to standard RAAS therapy with renin-angiotensin system blockade in diabetic patients was not demonstrable and was potentially dangerous. |
| HOPE Study (Mann et al., 2001) | CKD and non CKD patients | Ramipril vs. Vitamina E vs. Placebo and Vitamina E vs. Placebo | Major adverse cardiac event (MACE); effect of Ramipril on CV risk reduction. | In patients with previous cardiovascular disease and diabetes, mild renal impairment significantly increased the risk for subsequent cardiovascular events. Ramipril showed to be effective in reducing cardiovascular risk. |
| EUROPA Study (Brugts et al., 2007) | CKD and non CKD patients with stable CHD | Perindopril vs. Placebo | Major adverse cardiac event (MACE). | Treatment benefits of perindopril were present in both patient groups with low or high eGFR ( ≥ 75 or < 75). |
| PEACE Study (Solomon et al., 2006) | CKD and non-CKD patients with stable CHD |
Trandolapril vs. placebo | Major adverse cardiac event (MACE). | Trandolapril determined a reduction in mortality risk in patients with CKD. Conversely, this result was not confirmed in non-CKD patients. |
| Val-HeFT Study (Anand et al., 2009) | CKD and non CKD patients with Hearth failure (HF) | Valsartan vs. placebo | Death and first morbid event (death, sudden death with resuscitation, hospitalization for HF, or administration of intravenous inotropic or vasodilator drugs for 4 hours or more). | Valsartan reduced the risk of the first morbid event in patients with CKD and HF. |
| AASK Study (Norris et al., 2006) | African Americans with hypertensive nephrosclerosis | Metoprolol vs. Ramipril vs. Amlodipine | Major adverse cardiac event (MACE). |
Neither randomized antihypertensive drugs nor blood pressure level had a significant effect on the occurrence of CV events. |
| PREVEND IT Study (Asselbergs et al., 2004) | Microalbuminuric subjects | Fosinopril vs. placebo | Major adverse cardiac event (MACE). |
In microalbuminuric subjects, fosinopril significantly reduced albuminuria. In addition, fosinopril treatment appeared to reduce the risk for cardiovascular events. |
The evidence from several trials that the efficacy in cardiovascular and renal risk reduction of RAAS-I as compared to other antihypertensive drugs (i.e. Calcium Channel Blocker, Beta Blockers) is present in patients with high proteinuria levels also confirms the mechanistic benefit of RAAS-I in CKD patients. (Asselbergs et al., 2004; Norris et al., 2006). The large part of intervention studies showed that reducing blood pressure leads to a reduction in albuminuria and consequently a reduction in CV risk (Asselbergs et al., 2004; Berl et al., 2003; Klahr et al., 1994; Norris et al., 2006; Solomon et al., 2006; Wanner et al., 2016). However, it is also true that the decrease in proteinuria per se, protects against CV events. In a post hoc analysis of the Reduction in Endpoints in Non-insulin dependent diabetes mellitus with the Angiotensin II Antagonist Losartan (RENAAL) study, patients that were at lower risk for CV events were those with a higher decrease of albuminuria after the first months of treatment (de Zeeuw et al., 2004). Later on, a meta-analysis of 32 RCTs, provided more robust evidence for this by showing a 13% to 29% risk reduction of CV endpoints for each 10% decrease in albuminuria following different treatments (Savarese et al., 2014). Moreover, RAAS-Is have also shown to decrease proteinuria, regardless of blood pressure, through mechanisms that involve: the improvement in permselective properties of the glomerulus and anti-fibrotic effect (Remuzzi et al., 2006, 1991).
All these findings lead to a very important “public health” message, namely the priority of identifying CKD patients at increased CV risk that need more intensive management in term of surveillance and a stricter control of the main risk factors such as proteinuria and blood pressure (Mann et al., 2001). The approach of reducing blood pressure target in CKD patients has been investigated in clinical trials. In the African American Study of Kidney Disease and Hypertension (AASK) study, patients randomized to the lower blood pressure arm (125/75 mmHg) showed a reduce risk of mortality as compared to those assigned to the higher blood pressure target (140/90 mmHg) regardless of proteinuria levels (Norris et al., 2006). In the Systolic Pressure Intervention Trial (SPRINT), an intensive blood pressure lowering ( < 120 mmHg vs. < 140 mmHg) significantly reduced mortality and the composite endpoint of myocardial infarction, acute coronary syndrome, stroke, heart failure, or cardiovascular death. However, in this study intensive blood pressure lowering increased the risk of a 30% decline in eGFR (Cheung et al., 2017). The same adverse event, derived from an aggressive blood pressure regimen, has been reported in the Modification of Diet in Renal Disease (MDRD) study (Klahr et al., 1994).
Achieving a better control of proteinuria is mandatory. In fact, as previously discussed, despite the spread use of the RAAS-I in patients with CKD, the proportion of patients with residual proteinuria is elevated and a large part of patients remain at increased CV risk. One of the reasons that explain this trend is the variability of individual responses to RAAS-I. It has been demonstrated that 30 to 40% of patients do not respond to RAAS-I (Bolignano et al., 2015; Minutolo et al., 2000; Petrykiv et al., 2017). For this reason, a further effort should be done to unravel the underlying mechanisms of drug response variability and to find alternative albuminuria lowering drugs in order to optimize treatment for each individual (Wanner et al., 2016). This is feasible by testing the efficacy of new drugs, possibly different from RAAS-I. Two promising examples are represented by the sodium-glucose cotransporter 2 (SGLT-2) inhibitors and selective endothelin A receptor antagonists. SGLT-2 inhibitors act by reducing the reabsorption of glucose in the proximal tubule of the kidney. They elicit a regularization of the glomerular hyperfiltration, a mechanism that is shared between diabetic and non-diabetic kidney diseases, and significantly reduce proteinuria. They have also shown to considerably decrease cardiovascular and renal risk in type 2 diabetes with and without CKD (Coppolino et al., 2018; Garofalo et al., 2019). Differences in CV and renal outcomes between SGLT-2 inhibitors clinical trials have been observed and attributed to the different basal characteristics of patients enrolled, in particular albuminuria and eGFR levels (Kluger et al., 2019; Neal et al., 2013; Perkovic et al., 2019; Wanner et al., 2016; Wiviott et al., 2019). Indeed, patients enrolled in the Dapagliflozin Effect on CardiovascuLAR Events (DECLARE-TIMI 58) trial, CANagliflozin CardioVascular Assessment Study (CANVAS) Program and Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose (EMPA-REG OUTCOME) showed consistently lower albuminuria and higher eGFR levels than patients included in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial (Fig. 3). However, despite these differences, the CV risk reduction due to SGLT-2 inhibitors, observed in these trials, was related to the basal albuminuria and eGFR levels more than to the presence of previous CV disease. This testifies the importance of eGFR and albuminuria in establishing CV risk and predicting response to SGLT-2 inhibitors in CKD patients (Zelniker et al., 2019). Intriguingly, the Dapa-CKD clinical trial (ClinicalTrials.gov Identifier: NCT03036150) is currently ongoing, extending the use of the SGLT-2 inhibitors also in patients without diabetes and results, which (Wanner et al., 2016) included effects on CV outcomes, are eagerly expected at the end of 2020. The selective endothelin receptor antagonists have demonstrated to reduce blood pressure and proteinuria in clinical studies, by contrasting the deleterious effect of endothelin-1 on renal vasculature (increase in vasoreactivity) and podocytes (nephron shedding, cytoskeletal disruption) (Kohan and Barton, 2014). The Study of Diabetic Nephropathy with Atrasentan (SONAR) has also shown that the selective endothelin receptor antagonist Atrasentan reduces risk of renal events such as ESKD (Heerspink et al., 2010). Future studies should address the potential benefit of these drugs on CV outcomes in CKD patients. Another adopted strategy in clinical research with the aim of understanding and reducing the variability in proteinuria-lowering drug response consists in increasing the number of crossover studies, where more treatment periods with different drugs are used, and correlations between responses to one drug with each other are measured. The Rotation for Optimal Targeting of Albuminuria and Treatment Evaluation (ROTATE) clinical trial is an example of such design, where patients receive in random order 4 weeks of: the RAAS-I telmisartan, the SGLT-2 inhibitor empagliflozin, the dipeptidil-peptidasi-4 (DPP4) inhibitor linagliptin, and the glycosaminoglycan sulodexide, with 4-weeks wash-out periods in between (NCT03504566). This study will provide additional insight into which patients respond best to which drug in the next early future.
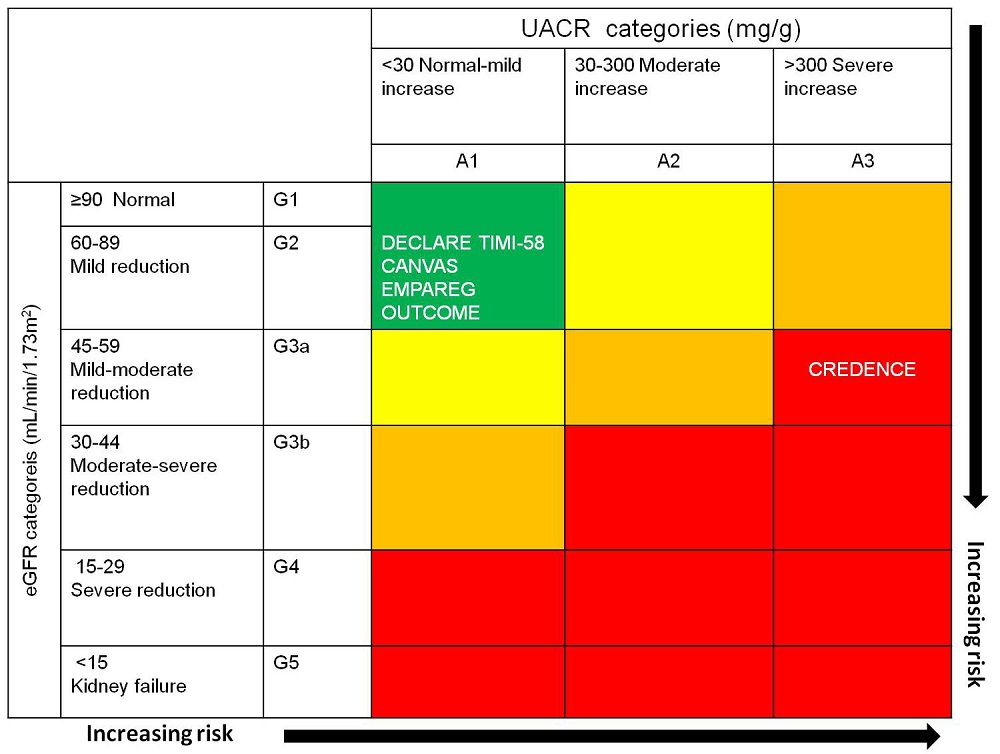 Figure 3.
Figure 3.Baseline renal risk in DECLARE-TIMI 58, CANVAS, EMPA-REG OUTCOME and CREDENCE trials. Horizontal dotted lines and white arrows estimate trials averaged mean eGFRs minus 1 pooled standard deviation; vertical dotted lines and white arrows approximate trials’ quartile 3 of UACR. (Reproduced and adapted from (Kluger et al., 2019))
In conclusion, in the last few years there has been an improvement in our knowledge on the link between CKD and CV risk. CKD has been demonstrated as an independent risk factor of CV outcomes, the “public health” aspect of this concern being considerable, given the increased prevalence of CKD. Identifying high risk patients is a priority; to reach this aim, further studies should be implemented in the future to: 1) gain more insights on the role of the main risk factors for CV disease, specifically assessed in CKD population; 2) computing risk calculators specific for CKD patients since the commonly used CV risk scores, not including renal measures, are suboptimal; 3) designing more intervention studies, which investigate whether it is possible to reduce the variability in drug response and, thus, reduce CV risk.
AF, atrial fibrillation; AMI, acute myocardial infarction; CAD, coronary artery disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; CVD, cardiovascular disease; HF, heart failure; PAD, peripheral arterial disease; HTN, hypertensive nephropathy; DN, diabetic nephropathy; GN, glomerulonephritis; TIN, tubulo-interstitial nephropathies; ADPKD, autosomal dominant polycystic kidney disease UK, unknown.
Thanks to Dr. Paolo Zaffino from the Department of Experimental and Clinical Medicine “Magna Graecia “University of Catanzaro for his support in Figures’ design and editing. Thanks to all the peer reviewers and editors for their opinions and suggestions.
All the authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
