Exosomes, nanosized lipid bilayer membranous vesicles, are secreted by a variety of cells and contain protein, lipids, mRNA, miRNA, and signaling molecules that participate in intercellular material transfer and information exchange through binding, fusion or endocytosis. Exosomes mediate the gene expression of target cells and regulate pathological and physiological processes, thereby playing a key role in the occurrence and development of various diseases. Accumulated studies has shown that exosomes hold therapeutic potential though their anti-apoptotic and anti-fibrotic roles. They also have been shown to promote angiogenesis, inhibit ventricular remodeling and improve cardiac function, as well as inhibiting local inflammation and regulating the immune response. As such, exosomes represent a new target for the treatment of cardiovascular diseases. This review summarizes the literature in this field to date, including the basic biological characteristics of exosomes, and new progress in the understanding of the mechanisms of their involvement in immune regulation in cardiovascular diseases. In this way, it servrs as a basis for future research and the development of therapeutic exosomes.
Cardiovascular disease is a global epidemic, representing a significant socioeconomic burden with a high rate of morbidity and mortality in both developed and developing countries. According to a report of the world health organization (WHO), 17.5 million people died of cardiovascular diseases in 2012: 31% of the total number of deaths that year, and twice as many as those who died of cancer (Nichols et al., 2014). It is predicted that this figure will reach 25 million by 2030 (Go et al., 2013). The level of diagnosis and treatment of cardiovascular diseases has certainly made considerable progress in recent years with the evolution of coronary interventions and electrophysiological techniques, but there remain limitations and unmet. Contemporary research (Beg et al., 2017; Pan et al., 2019b; Yanan et al., 2019) continues to clarify the role of inflammation in the occurrence and progression of cardiovascular diseases. Therefore, anti-inflammatory therapy may become a new target for the treatment of cardiovascular diseases.
Exosomes are nanosized membranous vesicles of diameter 30-100 nm (Mashouri et al., 2019), secreted by various cells in the body (Bellin et al., 2019; Poe and Knowlton, 2018), carrying proteins, lipids and nucleic acids (DNA, mRNAs, microRNAs) (Barile et al., 2017; Bei et al., 2017). They are involved in ligand-receptor interactions, endocytic pathways (such as endocytosis, pinocytosis, and phagocytosis) and membrane fusion (Khalyfa and Gozal, 2014; Yanan et al., 2019), as well as participating in intercellular communication of information and material exchange, mediating gene expression of target cells, and regulating pathology and pathophysiological processes. Accumulating evidence shows that exosomes have functions in regulating the immune response (Chan et al., 2019), as well as in anti-apoptotic regulation (Wang et al., 2019), and in the promotion of angiogenesis (Ma et al., 2018). As such, they play an important role in the diagnosis, treatment, and prognosis of cardiovascular diseases. In the present review, we provide a brief description of knowledge extablished to date regarding exosomes biological characteristics, biogenesis, composition, isolation, identification, and function. In addition, we summarized and discussed experimental and clinical findings on the role of exosomes in regulating inflammation and immune response in various cardiovascular diseases. A deep understanding of immunomodulation by exosomes may represent a promising therapeutic option for the treatment of cardiovascular diseases.
Exosomes entered our field of vision three decades ago first thought to be a means by which cells remove waste. They have gained increasing research sttention since their discovery (Pathan et al., 2019). Reportedly, these phospholipid bilayer nanovesicles commonly possess diameters of 30-100 nm, although diameter ranges of 20-150nm have also been observed (Fernandez-Llama et al., 2010; Schageman et al., 2013). Almost all types of cells secrete exosomes, including T- lymphocytes, dendritic cells, mast cells, tumor cells, cardiomyocytes, endothelial cells, fibroblasts, and various types of stem cells (Bellin et al., 2019; Kalluri, 2016; Li et al., 2019b; Zhu et al., 2018). They are widely distributed in saliva, urine, bronchial lavage, amniotic fluid, breast milk, plasma, serum and semen in mammals (Caradec et al., 2014; Yanez-Mo et al., 2015), as well as being present in bacteria, fungi, and plants (Baldrich et al., 2019). Observations of exosomes by whole-mount electron microscope revealed them to be “saucer-like” or “deflated-football” shaped (Raposo and Stoorvogel, 2013), while by transmission electron microscope reveal specific “biconcave” or “cup” shapes (Yellon and Davidson, 2014), and this is believed to be due to vesicle collapse during sample preparation. Their buoyant density on the sucrose gradient is 1.10~1.14 g/mL, with this density is related to cell source and varying with protein content (Bobrie et al., 2011). Exosomes can be stored at -20 °C for at least 6 months without loss of biological activity (Konala et al., 2016), which provides a guarantee for scientific research and clinical application.
Exosomes are continuously secreted within the physiological and pathophysiological milieu of tissue cells (Yanan et al., 2019) generation is accompanied by multiple mechanisms. Firstly, a vesicle close to the closed cell membrane is formed by budding of the cell membrane, termed an early endosomes (EEs). In the process of their maturation, early endosomes bud inward again and form late endosomes (LEs) close to the nucleus. At the same time, they selectively encapsulate proteins and nucleic acids in the cytoplasm, forming a series of intraluminal vesicles (ILV). Such late endosomes containing ILV are referred to as multivesicular bodies (MVB) (Maas et al., 2017; Record, 2014). The biological characteristics of the multivesicular body determine its path, namely: (1) it is degraded by binding to lysosomes; or (2) it fuses with the cell membrane to release the inner vesicles into the extracellular environment. It is these internal vesicles entering the extracellular space that are called exosomes (Kowal et al., 2014; Pant et al., 2012). Studies have shown that the production of exosomes is regulated by four types of endosomal sorting complexes required for transport (ESCRT). ESCRT-0 is responsible for the loading of the cargo in a ubiquitin-dependent manner, ESCRT-I and ESCRT-II induce membrane budding, and ESCRT-III participates in the splitting of the vesicle from the membrane (Ailawadi et al., 2015; Zhang et al., 2019). The release of exosomes is regulated and occurs through one or two mechanisms involving Rab GTPases (Hsu et al., 2010): either the constitutive or inducible release pathway. The constitutive pathway is regulated by several types of proteins such as RAB GTPases (Rab27a/b, Rab11 and Rab35), WNT5A, glycosphingolipids, heterotrimeric G-protein and flotillins (Hsu et al., 2010; Ostrowski et al., 2010). The inducible release pathway can be triggered by, for example, DNA damage, hypoxia, aberrant intracellular calcium release, cytokines, or other cellular stresses (Chen et al., 2011; Lespagnol et al., 2008; Savina et al., 2003).
Exosomes are rich in proteins, lipids, and nucleic acids, which play an important role in cell-to-cell communication and provide a basis for the detection and identification of exosomes. Exosomes are highly enriched in proteins with various functions. These include: tetraspanins (CD9, CD63, CD81, CD82), which take part in cell penetration, invasion, and fusion events; heat shock proteins (HSP70, HSP90), which, as part of the stress response that are involved in antigen binding and presentation; MVB formation proteins that are involved in exosome release (Alix, TSG101); and as well as proteins responsible for membrane transport and fusion (annexins and Rab). Other proteins include clathrin, cytoskeletal element (ezrin, tubulin), MHC molecule, intercellular adhesion molecule 1 (ICAM-1), co-stimulatory T-cell molecule (CD86), other transmembrane protein aM (dendritic cells), A4b1 (reticulocyte), immunoglobulin A33 (enterocytes), P-selectin (platelets), etc. (Vlassov et al., 2012). Exosomes also contain different types of RNA molecules, including messenger RNAs (mRNAs), circular RNAs (circRNAs), long noncoding RNAs (lncRNAs), and microRNAs (miRs). These RNA molecules are involved in the regulation of a variety of biological processes and, once loaded into exosomes, can be transported between cells, eliciting transient or persistent phenotypic changes in recipient cells (Coumans et al., 2017; Li et al., 2018). In addition, lipids, such as phosphatidylserine (PS), phosphatidic acid, cholesterol, sphingomyelin (SM), arachidonic acid, prostaglandins, and leukotrienes has also been reported as to be present on exosome membranes, which are stable structures and are protected from degradation and dilution in transportation (Zhang et al., 2019).
Ensuring quality of isolation and purification of exosomes has been a persistent concern of researchers. The physical features of exosomes are the basis of different isolation techniques. Generally, exosomes can be obtained through hypervelocity centrifugation, immune affinity capture of specific antibodies/proteins, polymer precipitation, ultrafiltration, size exclusion chromatography, and commercially available kits or microfluidic technologies (Hong et al., 2016; Lazar et al., 2018; Li et al., 2017). Each separation method has its own advantages and disadvantages. The centrifugation method is the most mature separation method in vitro.
Exosomes are usually confirmed as such by their size, morphology, and surface specific protein markers. Electron microscope analysis, nanoparticle tracking analysis (NTA), dynamic light scattering (DLS) analysis are used to observe their shape. The western blot technique is used to identify specific proteins on the exosome surface, and flow analysis is used to detect the expression of exosome surface proteins. Real-time reverse transcriptional polymerase chain reaction and oligonucleotide microarray analysis are used to determine the types of miRNAs and miRNAs contained in exosomes (Diaz-Varela et al., 2018; Sahoo and Losordo, 2014).
Exosomes are important mediators of intercellular communication. They shuttle nucleic acids (DNA, mRNAs, microRNAs), proteins, and lipids between donor and target cells through fusion, endocytosis or binding. MicroRNAs (miRNAs), non-coding small RNA molecules, can inversely regulate their target gene expression at the posttranscriptional level by interacting with the 3ʹ-untranslated region (3ʹ-UTR),and play an important role in cell proliferation, differentiation, and apoptosis (Namazi et al., 2018; Yang et al., 2017). Specific manifestations of this are: (1) exosomes fuse with cell membranes to release the cargo into cells (Parolini et al., 2009); (2) are ingested by recipient cells through endocytosis (Khalyfa and Gozal, 2014); and (3) they facilitate binding of ligands to receptors on cell membranes (Segura et al., 2007). Recent studies have shown that exosomes play a role in promoting angiogenesis, anti-apoptosis, maintaining cellular homeostasis, immune regulation, inhibiting the inflammatory response and promoting tissue repair (Gurunathan et al., 2019). Mesenchymal stem cell-derived exosomes have been shown to inhibite the proliferation of lymphocytes, promoted the conversion of T cells from Th1 to Th2, and promoted the expression of anti-inflammatory factor TGF-β (Chen et al., 2016). Furthermore, exosomes can also regulate the phenotype of macrophages, affect the expression of inflammatory factors, and play an immunomodulatory role (Chan et al., 2019). They can also reflect the state of donor cells, as well as holding potential as biomarkers for disease diagnosis and prognosis. In addition, in their capability to load and deliver proteins, nucleic acids, drugs, etc., exosomes offer the potential to mediate the pathophysiological processes of target cells, and hence may be used in the treatment of diseases.
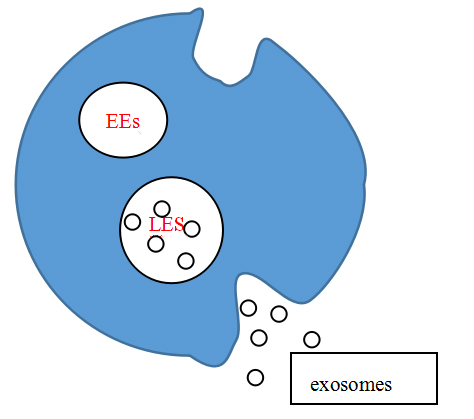 Figure 1.
Figure 1.Exosomes are secreted.
With the increasing morbidity and mortality of cardiovascular diseases, stem cell therapy has been regarded as the most promising treatment method (Abdelwahid et al., 2016). Different stem cells types, such as mesenchymal stem cells (MSCs) (Yuan et al., 2019), cardiac progenitor cells (CPCs) (Bielmann et al., 2015), embryonic stem cells (ESCs) (Khan et al., 2015), and induced pluripotent stem cells (iPSCs) (Yang, 2018), have been proposed for the treatment of cardiovascular diseases due to their contribution to restoration of cardiac function following tissue damage. However, major concerns such as the formation of teratomas (Jeong et al., 2011), immune rejection (Lalit et al., 2014), risk of embolization (Furlani et al., 2009), and low survival (Zaim et al., 2012) have hindered their clinical application. Recent data suggest that the cardiac protective function of stem cells is mainly achieved through paracrine mechanisms (mainly mediated by exosomes) rather than directional differentiation via migration to injury sites (Hashimoto et al., 2018). It is long recognized that exosomes have good stability, biocompatibility, high efficiency, low immunogenicity, and no teratogenicity or embolism risk (Beretti et al., 2018; International Stem Cell Initiative, 2018; Lamichhane et al., 2015; Mentkowski et al., 2018). Hence, they may represent an alternative to stem cell transplantation by circumventing their limitations (Ren, 2018, 2019). Such, exosomes-based cell-free therapy opens a new chapter in the treatment of cardiovascular diseases.
A large number of studies have shown that exosomes carry proteins, lipids, RNA and microRNAs, participating in intercellular communication. (Zhang et al., 2019), promoting angiogenesis (Ju et al., 2018), conferring anti-apoptotic effects (Pan et al., 2019a), reducing infarct size (Li et al., 2019b), and improving cardiac function (Zhu et al., 2018). Their roles in antigen presentation, inflammatory response, and immune regulation (Chan et al., 2019) have aroused significant research interest. It is well known that inflammation is involved in the occurrence and development of cardiovascular diseases (Horckmans et al., 2017). Mononuclear/macrophage, T lymphocyte, B lymphocyte, and inflammatory factors play roles within this process. (Ren, 2018), and as such represent targets of cardiovascular disease treatment.
Myocardial infarction (MI) is the most serious type of cardiovascular disease, which is characterized by the sudden interruption of coronary blood flow, leading to hypoxia and ischemic necrosis of the supplied myocardial cells. Upon myocardial infarction, massive cardiomyocyte death triggers a strong inflammatory response, which constitutes a vital process of cardiac injury, repair, and remodeling (Frangogiannis, 2014; Pan et al., 2019b; Prabhu and Frangogiannis, 2016). Overwhelming and increasing evidence has shown that exosomes from different tissues inhibit inflammatory responses through different molecular mechanisms, play an immune regulatory role, and reduce in myocardial infarction. Shao et al. (2017) found that mesenchymal stem cell-derived exosomes (MSC-Exos) alleviated the inflammatory response and improved cardiac function by decreasing the infiltration of inflammatory cells in the infarct area via down-regulating the expression of CD68. In order to enhance their cardiaoprotective effect, exosomes can be pretreated by hypoxia preconditioning (Luo et al., 2019), gene programming (Wang et al., 2018), or drug intervention (Huang et al., 2019). Pan et al. (2019a) showed that exosomes derived from miR-146a-modified adipose-derived stem cells ADSC-Exos inhibit the release of interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), as well as reducing the local inflammatory response, improving the local microenvironment, and attenuating acute myocardial infarction-induced myocardial damage via the down-regulation of early growth response factor 1 (EGR1). In the same study, Pan et al. also demonstrated that suppression of EGR1 expression reversed AMI or hypoxia-induced TLR4/NFκB signal activation, which prevented cardiomyocyte apoptosis, alleviated cardiac fibrosis, reduced myocardial infarct size, and improved cardiac function. Huang et al. (2019) demonstrated that exosomes obtained from ATV-pretreated MSCs promoted angiogenesis and inhibited the elevation of IL-6 and TNF-α in the peri-infarct region. In addition, MSCATV-Exos resulted in improved recovery of cardiac function, further reduction in infarct size and reduced cardiomyocyte apoptosis. Mechanistically, the authors identified that MSCATV-Exos are rich in lncRNA H19; in addition regulate the expression of miR-675 and activate pro-angiogenic factors VEGF and ICAM-1, thereby improving vascular endothelial function following myocardial infarction. Liu et al. (2018) reported that adipose-derived stromal cells (ADSC)-derived miR-93-5p-containing exosomes conferred a greater protective effect, reducing autophagy, inhibiting apoptosis, reducing the expression of IL-6, IL-1β, and TNF-α, and mitigating myocardial injury. These beneficial effects centred upon the up-regulation of the expression of TLR4 through targeting of the downstream gene Atg7, as well as the inhibition of the inflammatory response, the reduction of apoptosis of cardiomyocytes, and the improvement of the local microenvironment.
 Figure 2.
Figure 2.Intercellular communication.
Exosomes from different sources exhibit either pro- or anti-inflammatory effects in atherosclerosis (Yanan et al., 2019). Exosomes from different sources exhibit either pro- or anti-inflammatory effects in atherosclerosis (Yanan et al., 2019). Atherosclerosis is a chronic inflammatory disease which lipid deposits cause damage to the walls of blood vessels. Macrophages are the main inflammatory factors in atherosclerotic plaques and play a key role in the pathophysiological process (Koelwyn et al., 2018; Raggi et al., 2018; Zysset et al., 2016). Macrophages, one of the principal components of the innate immune system, are originated from the yolk sac (tissue-resident macrophages) or from monocytes (circulating macrophages) derived from bone marrow. They participate in inflammatory responses and tissue homeostasis through phagocytosis and antigen expression (Epelman et al., 2014). Numerous studies have shown that macrophages have different functional properties in different microenvironments, exhibiting significant heterogeneity (Cho et al., 2014). Macrophages can be divided into two major categories according to their phenotype and secreted cytokines, namely classically activated macrophages (M1 type) and alternatively activated macrophages (M2 type). M1 macrophages have the strong antigen-presenting ability. They secrete a large number of pro-inflammatory factors such as TNF-a, IL-6 and other pro-inflammatory effects, and amplify the inflammatory response. M2 macrophages secrete inhibitory cytokines such as TGF-β and IL-10, which down-regulate the immune response to effectively control inflammation as well as participating in tissue repair (Biswas et al., 2012). Li et al. (2019a) demonstrated that mesenchymal stem cell-derived exosomes reversed the progression of atherosclerosis by inducing the transformation of macrophages from M1 to M2 type via the up-regulation of miR-let7. These results were then confirmed by in vitro study. The mechanism study found that the miR-let7/IGF2BP1/PTEN pathway inhibited the infiltration of macrophages within the plaque, and that the miR-let7/HMGA2/NF-kB pathway promoted the transformation of macrophages into the M2-type, ameliorating atherosclerosis. In addition to macrophages, dendritic cells and helper T lymphocytes are also involved in the development and progression of atherosclerosis. Another study (Gao et al., 2016) have found that dendritic cell-derived exosomes promote the progression of atherosclerosis. Dendritic cells can secrete a large number of pro-inflammatory cytokines, including IL-6, interleukin-12 (IL-12), and TNF-α, which induce an inflammatory responses in endothelial cells. Exosomes secreted by these dendritic cells have the same immune effects, namely the activation of endothelial cells through the NF-KB signaling pathway, causing the release of adhesion molecules (VCAM-1, ICAM-1, and E-selectin), thereby, inducing atherosclerosis.
Reperfusion therapy is currently the most effective method to reduce acute myocardial ischemic injury and decrease myocardial infarct size. However, the process of reperfusion may induce further myocardial injury, and as such is termed myocardial reperfusion injury. The ischemia/reperfusion (I/R) process triggers the cardiac inflammatory cascade, the intensity and duration of which are closely related to myocardial injury and scar formation. Most research to date has identified macrophages as playing an important role in this process (de Couto et al., 2015; Frantz and Nahrendorf, 2014). M1 macrophages are known to produce a pro-inflammatory environment that removes damaged myocardial cell debris. M2 macrophages not only secrete anti-inflammatory cytokines, but also secrete a mixture of growth factors that play a key role in wound healing and scar formation. Zhao et al. (2019) studied the immunomodulatory effects of mesenchymal stem cell-derived exosomes in a mouse model of ischemia-reperfusion injury. In this study, MSC-Exos attenuated myocardial ischemia-reperfusion injury, reduced inflammation, decreased myocardial infarct size, and improved cardiac function via the shuttling of miR-182 which acts by modifying the polarization status of macrophages. Mechanistically, it was determined that miR-182 regulated the polarity of macrophages through the TLR4/NF-kB/PI3K/Akt signaling pathway. This study provided new insights for the MSC-Exos as potential therapeutic tools for myocardial I/R injury.
Dilated cardiomyopathy (DCM) is the most common complication of myocarditis. It is closely related to inflammation, usually presenting as the increase of IL-1, IL-6, TNF-α and endogenous immune cells (Givertz and Mann, 2013). Lankford et al. (2018) have shown that MSC-Exos significantly reduces left ventricular dilation in dilated cardiomyopathy, thereby improving cardiac function, decreasing the expression of inflammatory cytokines in peripheral blood, decreasing the number of pro-inflammatory M1 macrophages, and increasing the number of anti-inflammatory M2 macrophages. In terms of pathogenesis, MSC-Exos was found to created an anti-inflammatory microenvironment by regulating the balance of M1 and M2 macrophages via the JAK2/STAT6 pathway. This study shed new light upon the application of MSC-Exos as a potential therapeutic tool for the treatment of dilated cardiomyopathy.
Heart failure is a chronic inflammatory process whose severity is closely related to plasma or serum pro-inflammatory cytokines (van de Vrie et al., 2011). Ye et al. (2017) revealed that plasma-derived exosomes carry mtDNA in chronic heart failure patients, through activation of the TLR9/NF-κB pathway, thereby, promoting the secretion of inflammatory factors and aggravating inflammation in heart failure. The application of chloroquine inhibited the activity of the TLR9/NF-κB pathway, reduced the inflammatory response, and achieved the purpose of delaying heart failure, provided a new direction for the treatment of clinical heart failure. Studies were also conducted by Beg et al. (2017), in which it was shown that plasma exosome miR-146 levels were significantly increased in heart failure patients compared with controls group. Because miR-146 can reduce the inflammatory response, it could serve not only as a therapeutic target for heart failure, but also serve as a biomarker for the diagnosis, prognosis, and evaluation of treatment effect of heart failure.
Exosomes were first discovered in sheep reticulocytes in 1983, and were subsequently named in 1987 by Johnstone. In 2007, Valadi et al found them to be rich in biologically active molecules, as well as their capabilities in transmitting information and regulating target cells. Ongoing research, continues to support the notion that exosomes play a particularly prominent role in the cardiovascular field. Their protective effects in cardiovascular disease is reflected in their known roles in anti-apoptosis, anti-myocardial fibrosis, as well as in vascular regeneration and immune regulation. However, little is known about the functional and molecular mechanisms of exosomes with respect to specific inflammatory and immune cells in cardiovascular disease processes. How exosomes selectively express some signal molecules, and how they regulate the behavior of receptor cells remain unanswered questions requiring further study.
The field of exosome study holds other challenges. First, the purity of exosome preparation is not satisfactory. There is currently no prevailing standard methodology for the separation of exosomes, and as such their purity within the experimental setting is difficult to establish. Second, with current methods, it is difficult to generate large quantities of exosomes. Isolation by ultracentrifugation takes a long time and requires a large quantity of cell supernatant and biological fluid, still yielding only a limited number of exosomes. Third, exosomes remain inadequate for clinical application, pending rigorous scientific validation of their source, safety, dosage, route of administration, and biological availability.
In conclusion, the immunomodulation of exosomes shows great advantages and potential in heart injury, repair, and remodeling, opening up new avenues for the diagnosis and treatment of cardiovascular diseases.
Thank numerous individuals participated in this study.
The authors declare no competing interest.
