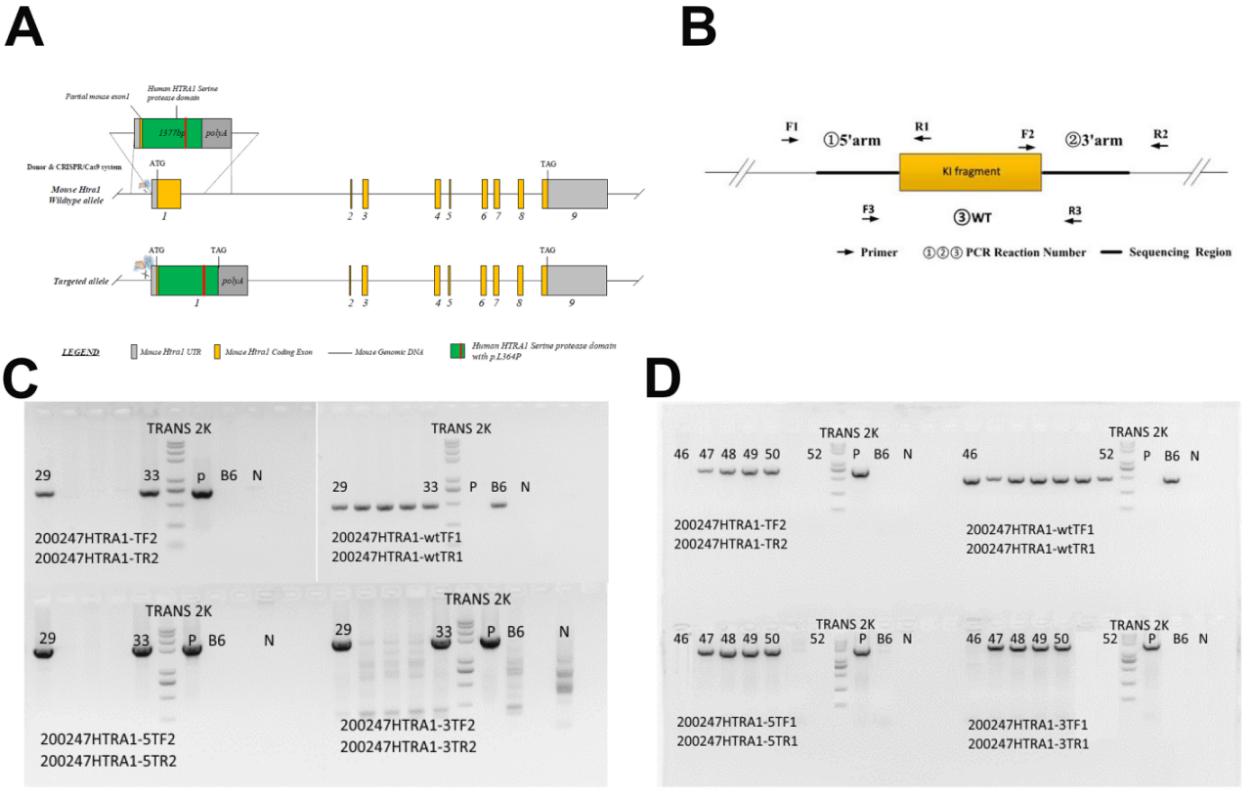
Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy is a rare form of inherited cerebral small vessel disease associated with mutations in the high-temperature requirement serine peptidase A1 gene. As of now, only about 50 cases have been reported. In 2012, our group reported a family with a novel mutant of the high-temperature requirement serine peptidase A1 gene in China for the first time. To further explore the molecular pathogenesis of cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy, a recombination mouse model expressed human high-temperature requirement serine peptidase A1 gene mutant identified by our group was generated using the Donor & Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 system and termed the Mut-high-temperature requirement serine peptidase A1 geneL364P mouse model. Results show that Mut-high-temperature requirement serine peptidase A1 geneL364P mice present similar pathological characteristics to patients with cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy, suggesting that the Mut-high-temperature requirement serine peptidase A1 geneL364P mouse model was generated successfully. Moreover, apoptosis was induced in mouse brain vascular smooth muscle cells derived from Mut-high-temperature requirement serine peptidase A1 geneL364P mice. In summary, the cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy mouse model described in this study will be beneficial to demonstrate the pathological mechanism of cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy and provide new therapeutic targets for clinical treatment.