Introduction: Robotic partial nephrectomy (RPN) is a relatively safe nephron sparing surgery (NSS) approach for the treatment of small renal masses (cT1). However, a major perioperative complication is extensive bleeding and blood loss necessitating blood transfusion. This complication is most challenging during the intraoperative setting and requires proper tumor bed closure. Recently several biological tissue adhesives have been tested to decrease intraoperative bleeding. A novel adhesive, Starsil® Hemostat is a plant-derived polysaccharide that can be applied directly to a bleeding wound to achieve hemostasis. The aim of our current study was to perform an evaluation of the safety and efficacy of this novel hemostat in patients undergoing RPN. Methods: This prospective single arm study included twenty patients with T1a-T1b renal masses who underwent RPN between the years 2017-2018. Renal masses were classified according to size, exophytic/endophytic properties and anatomic location into low, moderate and high complexity cases as described by the R.E.N.A.L nephrometry score. Starsil® Hemostat was applied by a feeding tube through a laparoscopic port after tumor excision. Perioperative data were collected, including blood loss during surgery, blood product transfusion rates, short and long-term adverse events and surgeon satisfaction using a visual analog scale (VAS 1-10). Results: Twenty RPN surgeries were completed using the Starsil® Hemostat. The average age was 61.8 ± 14.3. Average maximal tumor diameter was 3.8 cm (range 1.5-5.7). The calculated mean R.E.N.A.L nephrometry score was 8.4 (range 5-12). Mean blood loss during surgery was 346 mL (range 50-1400 mL). Mean surgeon satisfaction (VAS 1-10) with bleeding control was 8.3, when recorded 24 hours post operation. In 17/20 procedures (85%), bleeding control was good (VAS 9-10) and only 2 patients required blood transfusion. None of the patients developed an allergic reaction. No adverse events related to the adhesive product were noted in the post-surgical follow up period. Conclusion: Tumor bed closure during NSS with the adhesive STARSIL® Hemostat is safe, feasible and easy to use. It has the potential to reduce blood loss and transfusion rate in patients undergoing RPN.
With the advance in technology and increased use of abdominal imaging, the incidence of small renal masses has significantly increased in the past decade [1,2].
The management of small renal masses varies and includes: surgical excision either by partial or radical nephrectomy, focal ablative therapies or mainly in elder or comorbid patients active surveillance [3]. According to recent American Urological Association guidelines for treating small renal masses (cT1), the oncological outcome in terms of disease specific survival following partial nephrectomy (PN) equals that of a radical approach. However, nephron sparing surgery (NSS) presents with improved overall survival attributed to improved kidney function and decreased metabolic or cardiovascular complications [4,5]. Robotic partial nephrectomy (RPN) is a relatively safe NSS approach. However, a major perioperative complication is extensive bleeding and blood loss necessitating blood transfusion [6]. This complication became less common since the era of laparoscopic surgery [7], possibly due to the effect of pneumoperitoneum and increased intra-abdominal pressure on renal vessels. However, RPN can still cause decreased hematocrit and increased need for packed red blood cell (RBC) transfusion which might poorly affect oncological outcomes as seen in colorectal cancer [8]. After removing the renal mass, renorrhaphy (tumor bed closure) is performed with absorbable sutures aimed to stop dissected parenchymal blood vessels from bleeding. In the current study we introduce a novel hemostat adhesive to control renal bleeding during RPN. The aim of the study was to perform an in vivo evaluation of safety and efficacy of this novel hemostat in RPN.
The current study was a prospective single center single arm trial involving 20 patients who underwent RPN using Starsil® hemostat for small renal masses. Patients were operated between the years 2017-2018. After receiving approval from the local Institutional Review Board (IRB: 0241-16 RMB), all patients gave full consent to participate in this study.
Adult patients with T1a-T1b renal masses undergoing RPN. No known bleeding disorder or treatment with blood thinners during the procedure.
Prior to surgery, renal masses were divided in accordance to R.E.N.A.L nephrometry score into low, moderate and high complexity cases determined by tumor size, exophytic/endophytic properties and anatomic location [9].
All procedures were performed by a single surgeon. Patients were placed in a flank position with the robot docked over the ipsilateral side. A standard three arm DaVinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) via transperitoneal approach was used for all cases. Pneumoperitoneum at 15 mmHg was achieved using the "Hasson" technique [10]. A 30 degree down camera lens was used throughout the operation. After colon reflection, the kidney was mobilized and the hilum was identified for the placement of a laparoscopic bulldog on the renal artery. All tumors were resected with monopolar scissors. The resection site base was not routinely cauterized. One Starsil® Hemostat application bottle consisting of 5 grams of powder was used for each procedure. The powder was applied with a feeding tube through a laparoscopic assistant port (Figs. 1 and 2). Renorrhaphy was then performed using 3-0 vicryl sutures with Lapra-TY® clips for deep cortical and blood vessels closure. We used 1-0 vicryl sutures with 10 mm Hem-o-lock® and Lapra-TY® for external cortical closure. The robotic needle driver was used to slide the Hem-o-lock® and Lapra-TY II® clips on the opposite end down the suture to a desired tension. Thereafter, the clamps were removed and the kidney was inspected for hemostasis. Hemostasis was rated as adequate if no excessive bleeding or oozing was observed.
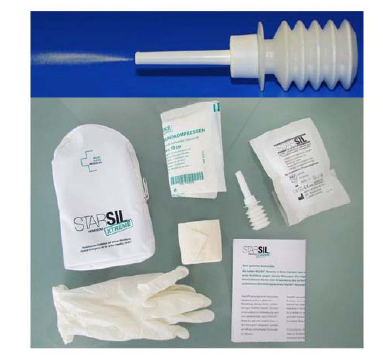 Figure 1.
Figure 1.Starsil® Hemostat kit before insertion during robotic partial nephrectomy.
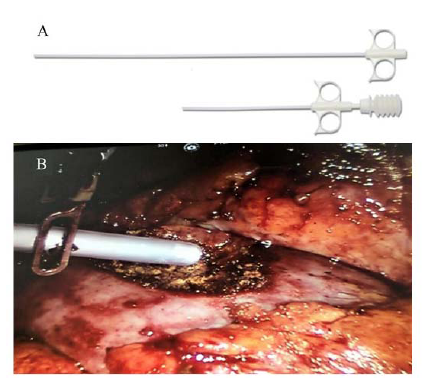 Figure 2.
Figure 2.A) Starsil UniTip - spray applicators that allow accurate delivery of the Starsil® Hemostat during robotic partial nephrectomy. B) Administration of Starsil® Hemostat during robotic partial nephrectomy: Robotic arm directing the feeding tube into the tumor bed before the assistant is applying the hemostat.
We collected data on blood loss during surgery, blood product transfusion rates, short and long-term adverse events (within 24 hours post-operation; at time of discharge from the hospital and at follow-up within 3-6 months) and patient comfort (pain level and overall satisfaction). Surgeon satisfaction with hemostasis was assessed using a visual analog scale (VAS 1-10; where a score of 1 was very bad and 10 was excellent satisfaction of hemostasis). We recorded surgeons’ response within 24 hours after surgery, preferably immediately after the operation.
Starsil® (HEMOSTAT GMBH Beckelmannsweg10m D-46342 Velen, Germany) is a sterile, white powder manufactured from a plant-based polysaccharide that can be applied directly to a bleeding wound to achieve hemostasis. The hemostatic effect is obtained by carboxymethyl starch particles that separate serum from the cellular constituents such as platelets and erythrocytes. This process acts to: (1). Concentrate these blood solids with the hemostat to form a gel like matrix or "plug" that acts as a temporary mechanical barrier. (2). Accelerate the physiologic clotting cascade. Complete degradation and absorption of the powder by endogenous α-amylase is achieved within approximately 2 days.
Twenty RPN procedures were performed using Starsil® Hemostat for bleeding control assistance. Patient and renal tumor characteristics were collected (Table 1). Average blood loss was 346 mL (50-1400 mL). Mean surgeon satisfaction with bleeding control was 8.3 on average when recorded 24 hours post-operation (VAS, Table 2). In 17/20 procedures (85%), bleeding control was adequate (VAS score 9-10). In one case (5%), additional stitches were required after removal of the arterial clamp. Surgeon satisfaction from the hemostat was scored low (VAS < 5) in only three cases (15%).
| Robotic partial nephrectomy (n) | 20 |
| Age (years, average) | 61.8 (range 32-84) |
| Average tumor diameter (cm) | 3.8 (range 1.5-5.7) |
| R.E.N.A.L nephrometry score (average) | 8.4 (range 5-12) |
| Follow up date |
VAS (Average) |
| 24 hours post-operation | |
| Patient comfort (satisfaction) | 8.2 |
| Doctor's satisfaction with hemostasis | 8.3 |
| Discharge day | |
| Patient comfort (satisfaction) | 9.6 |
| Doctor's satisfaction with hemostasis | 9.7 |
| 3-6 months post-operation | |
| Patient comfort (satisfaction) | 9.3 |
| Doctor's satisfaction with hemostasis | 9.4 |
VAS; Visual Analogue Score 1-10.
Two cases of high complexity R.E.N.A.L score tumors were noted. These patients required blood transfusion during or after the operation: One patient (R.E.N.A.L. 9, hilar tumor) lost 1000 mL of blood during the operation and received 2 units of packed RBCs. After the operation, he suffered from a urinary leak that was treated with insertion of a double J ureteral stent. Another patient (R.E.N.A.L. 10) was converted to a robotic radical nephrectomy due to proximity of the tumor to the main renal artery. These events were classified as non-product related.
None of the patients developed an allergic reaction to the hemostatic adhesive powder. All patients were discharged in good health (average hospital stay was 4.8 days). No adverse events related to the product were noted in the post-surgical follow up period of up to 6 months. No cases of arteriovenous fistula or pseudoaneurysm were noted. None of the patients needed conversion to open surgery, reoperation or selective angio-embolization for late bleeding. Review of the pathology specimens from the operations, demonstrated 16 cases of clear cell renal cell cancer (RCC) and 4 cases of oncocytoma.
Minimally invasive NSS is the gold standard operative procedure for small renal masses due to complete local resection of the renal tumor while preserving maximal possible functioning parenchyma [11]. Tumor bed closure is performed by approximating the transected margins with various suture techniques, sometimes together with hemostatic substances [12]. Acquiring satisfactory bleeding control during RPN is ultimately achieved by experienced urologic surgeons, and although severe, hemorrhage after partial nephrectomy is a rare event [13], maximizing hemostasis is still needed, especially in case of complex tumors that are either very endophytic, large or in close proximity to the renal hilum.
Hemostasis control and tumor bed closure are considered the most difficult steps during a laparoscopic partial nephrectomy mostly due to the relatively prolonged warm ischemia time. To overcome this challenge, several tissue sealants and adhesives were developed to aid or replace suturing the renal parenchyma [14,15].
Brandão et al., compared the use of sutures versus biological glue for the closure of porcine renal parenchyma during laparoscopic partial nephrectomies. They found that the use of Bioglue® (CryoLife Inc.), a surgical adhesive that contains purified bovine serum albumin (BSA) and glutaraldehyde, had a significantly lower warm ischemia time without compromising bleeding control [16]. Based on these results, Hidas et al., compared the clinical outcomes of open partial nephrectomy (OPN) using Bioglue® versus traditional suturing technique for tumor bed closure. The use of Bioglue® reduced the estimated mean blood loss (P = 0.001) and blood transfusion rates (P = 0.014) [17].
Numerous hemostatic agents are nowadays in clinical use for laparoscopic partial nephrectomy operations [18-20] and a meta-analysis by Galanakis et al., demonstrated that most adhesive agents show good results for complete bleeding control or facilitation of hemostasis [21].
In our current study we have elected to use Starsil® Hemostat, a plant-derived hemostatic powder that is commonly used for treatment of sternal bleeding after median sternotomy [22]. According to Hemotec Medical GmbH, the use of Starsil makes it possible to achieve bleeding control in diverse cardiac operations such as coronary-arterial bypass graft (CABG), implantation of pacemaker or an implantable cardioverter defibrillator (ICD), replacement of ascending aorta for repair of aortic aneurysm, aortic valve replacement and even heart transplantations [23].
According to the product manufacturing information, one of the main advantages of Starsil® Hemostat is that unlike xenograft products such as bovine derived, this is a plant-derived product with diminished chance for an allergic reaction or other adverse effects. Another advantage is that this is an off-the-shelf product and there is no need for storage at low temperatures prior to application. We also found it to be very easy to use in the laparoscopic setting by the robotic assistant.
In the current study, we described for the first time the use of Starsil® Hemostat in genitourinary operations. In our trial, it was found to be safe, easy to use and adjunctive measure for facilitating hemostasis in robotic partial nephrectomy operations. Surgeon satisfaction with hemostasis was high in most cases.
Our study had several limitations: first, in each RPN, we elected to use only one unit of Starsil® Hemostat for bleeding control. The option of using a second unit in case of unsatisfactory bleeding control, was not part of this safety study. Second, the current study was a single arm trial with no control arm. Third, all RPNs were performed by a single surgeon who rated satisfaction of the product. In the future, we hope to perform a multi-center placebo-controlled trial so that the safety and efficacy of Starsil® Hemostat will be fully evaluated.
Tumor bed closure during NSS with the STARSIL® Hemostat adhesive is safe, feasible and easy to use. It has the potential to reduce blood loss and transfusion rate in patients undergoing RPN.
We thank all the co-authors that participated in the study.
There is no conflict of interest, real or perceived.
