Background and aim: Microscopic hematuria (MH) is a common finding in urinalysis, existing in up to 30% of evaluated patients. Due to the relatively high rate of malignancy reported in patients with MH, a full urologi-cal evaluation to detect urothelial cancer is advocated by the American Urological Association (AUA) and the European Association of Urology (EAU) in woman with asymptomatic microhema-turia, once infection or urolithiasis were rule out. In contrast to the strict guidelines, our personal experience shows a very low rate of malignancies in women with asymptomatic microhematuria. Hence, the aim of our current study was to assess the rate of urothelial malignancies found during evaluation of asymptomatic women with recurrent MH in a pilot study. Methods: In the current retrospective study we retrieved the records of all women with asymptomatic MH who underwent an elective cystoscopy in our outpatient clinic during the years 2010-2015. We ex-amined the impact of their age, smoking status, upper tract imaging (sonography or CT-Urography), urine cytology results on cystoscopy results and pathological outcome. Results: 165 consecutive patients were included in the study: 1 had abnormal imaging, 2 women had abnor-mal urine cytology (atypia), and 2 had abnormal cystoscopy; 5 women were younger than 35, and all had a completely normal workup. None of the patients were diagnosed with urothelial cancer. Conclusions: A full urological investigation had a low yield in our cohort of women with asymptomatic microhe-maturia, and therefore may be unnecessary, especially in younger women.
Asymptomatic microhematuria (AMH) is defined as ≥ 3 red blood cells (RBC) per high powered field (HPF) on a properly collected urinary specimen in the absence of an obvious benign cause. A clinically significant microhematuria (MH) is regarded as a predictor of urothelial carcinoma (UC), and as such, a full checkup including urine cytology, imaging studies and cystoscopy is recommended for all patients with MH aged > 35 years and for those aged < 35 years with risk factors [1]. The risk of urologic malignancy is increased in men, persons older than 35 years, and persons with a history of smoking. In general, urothelial cancer is prevalent in patients over the age of 60 and very rare under the age of 40 especially in non-smoking women.
In recent years, a growing number of studies suggests a review of this common practice especially in regards to asymptomatic women, citing differences in prevalence and etiology of MH [2]. The patient’s gender highly influences the differential diagnosis, and the risk of urinary tract malignancy (bladder, ureter, and kidney) is significantly lower in women than in men [3]. The main issue with the current guidelines is the seemingly unnecessary workup that results in a low benefit-cost ratio. Recent studies examining the cost-effectiveness of common diagnostic approaches for the evaluation of AMH conclude that the appropriateness of current clinical practices should be evaluated and potentially alter current guidelines [4]. To assess this issue, gender-specific studies, which take into account the various epidemiologic differences, are needed for refining the guidelines. In recent years, several countries have revised their local guidelines and updated their clinical practices accordingly, resulting in fewer patients undergoing a complete investigation. It has been shown that a minority of patients with malignancies or non-invasive tumors would have been missed based on the revised hematuria referral pathways [5].
In the present study, using our own data, we aimed to investigate the predictive value of the recommended workup on asymptomatic women diagnosed with AMH.
We retrospectively retrieved the records of all women who were referred to an elective cystoscopy in our outpatient clinic due to AMH during the years 2010-2015. Inclusion criteria were female gender, MH per definition [at least 3 consecutive urinalysis with microscopic evaluation showing MH (> 3 RBC/HPF during 1 year or more], a negative urine culture, results of 3 consecutive urine cytology specimens, upper urinary tract imaging-either computerized tomography (CT) or sonography, with no evidence of stone disease in upper tract imaging.
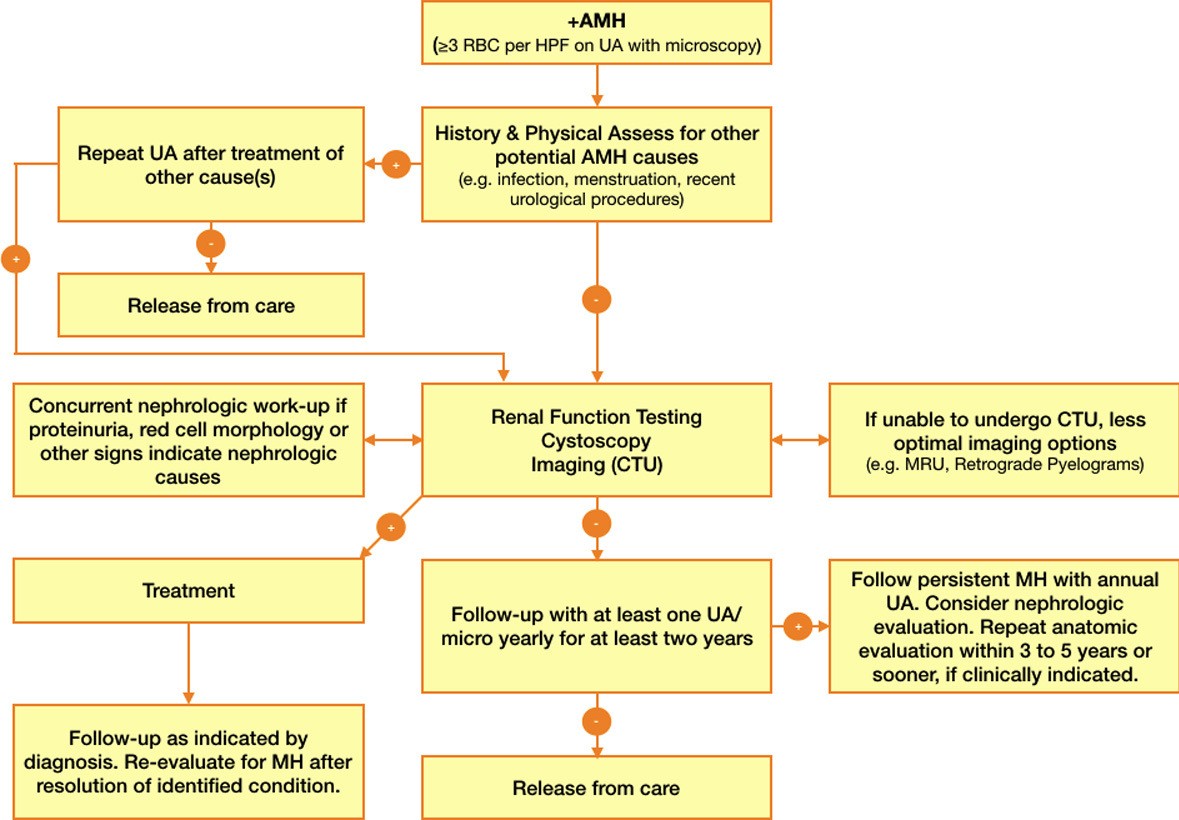 Fig. 1.
Fig. 1.A summary diagram showing the scheme of the complete workup.
The variables that were examined for this cohort were age, smoking status, upper tract imaging (sonography or CT-Urography), urine cytology results and cystoscopy results.
The study cohort comprised of 165 consecutive women diagnosed with AMH and had all the inclusion criteria. The median age was 58 years and women had negative urine cultures. 117 had urinary tract sonographies, all of which were normal. 86 of the patients had a CT-Urography (CTU), of which only 1 was abnormal, demonstrating thickening of the bladder wall. This patient was 55 years old, her smoking status was unknown, and had normal cytology and cystoscopy.
| Total pts. no. | 165 | ||
|---|---|---|---|
| Age median (range) | 58 (30-78) | ||
| Positive | Negative | Unknown | |
| Smoking status no. (%) | 23 (14%) | 40 (24%) | 102 (62%) |
| Sonography no. (%) | 117 (71%) | 0 | 48 (29%) |
| CTUno. (%) | 85 (52%) | 1 (0.6%) | 79 (48%) |
| Urine cytology no. (%) | 154 (93%) | 2 (1%) | 9 (5%) |
| Cystoscopy no. (%) | 157 (95%) | 2 (1%) | 6 (4%) |
Out of 159 patients who had urine cytology results, 2 were abnormal (i.e. atypia). In one of these patients, the repeat cytology was normal, and in the other patient a consecutive workup which included a split-cytological study was normal.
In the cohort, 2 patients aged 51 and 67, displayed an abnormal cystoscopy; both had normal urine cytology specimens and normal upper tract imaging. Neither was known to be a smoker. Both underwent a consecutive transurethral resection of bladder tumor (TUR-BT), and in both cases there was no evidence of malignancy in the pathological specimens. 5 patients (3%) were below the age of 35, 2 of which were smokers. All patients in this age group had normal imaging, cytology and cystoscopy results.
The median and mean age for any positive test result (CT, Culture, Cytology, Cystoscopy) was 55.
None of the participants in our study was diagnosed with urothe-lial carcinoma.
The AUA recommendations for evaluating a clinically significant MH regarding bladder cancer, include performing urine cytology, cystoscopy and imaging studies for all patients with microscopic hematuria aged > 35 years and for those aged < 35 years with risk factors [1] This workup is demanding for the patient and may lead to severe side effects related to the exposure to radiation, the administration of contrast agents, and the invasiveness nature of cystoscopy, which may lead to complications such as urinary tract infection, hematuria, dysuria and injury to the bladder or urethra [6].
Our clinical evaluation suggests a review of these guidelines regarding women, and indeed our study showed that only 2 out of 165 female patients evaluated for AMH, had atypical urothelial cells in the urine. However, urothelial malignancy was not documented at all; all other 163 women had a negative evaluation.
A limitation of our study is the fact that 79 of the patients only had a sonography for upper tract imaging. However, 86 patients (52%) had CT-U, of which only 1 was abnormal, and demonstrated thickening of the bladder wall. Despite this, the majority of women had available cystoscopy results, which is the gold standard for the diagnosis of urothelial cancer.
Our study suggests that a complete evaluation for any woman with MH is possibly unnecessary as only 2 patients had atypical cells in the urine; however, the data available in this cohort are insufficient to differentiate these patients from the entire group.
In our study, all of the women aged below 35 had a completely normal evaluation, regardless of smoking status. This may suggest that the need for a complete workup in women aged less than 35 may be unnecessary, even in those with risk factors.
A complete investigation for MH includes invasive examination (cystoscopy) and requires exposure to high levels of radiation. Considering the low yield of a complete workup, especially in the younger age group, the possible complications and adverse events of the workup may outweigh the benefits of this complete evaluation.
MH is a common finding in women, especially of child-bearing age. This, in part, is due to the relative difficulty to obtain a non-contaminated specimen. The immediate contamination concern, in a MH finding in women, is that of uterine bleeding or vaginal atrophy. This may prompt a pelvic exam to rule out a gynecological cause for MH, even in post-menopausal women [2, 7, 8]. A catheterized urine sample is suggested as the only reliable option to obtain a specimen free of external contamination from the natural flora on vulva and labia [9]. Both procedures may count as intrusive for asymptomatic women.
The overall estimated prevalence of AMH is 13-14% in the general population of men aged > 35 and similar figures for > 55-year-old women [10]. Assuming a proper specimen was obtained, the most common causes of MH in women are cystitis and urogenital calculi. This is not the case with men, and studies show many epidemiologic differences in etiologies of MH between genders [2, 11, 12]. This leads to the main claim, that although the prevalence of MH is relatively similar, the incidence of bladder cancer in women is much lower. This claim aims to contradict MH as a predictive factor for bladder cancer in women. It is generally accepted that the incidence of bladder cancer is 4-fold higher in men than in women. The incidence of bladder cancer in younger women with MH is very close to zero. While the cut-off age for low-risk women is somewhat variable, older age seems to correlate with higher risk in every study [2, 10, 13-15] Nevertheless, it is important to point out the opposite point-of-view, where the prevalence of MH in women that were already diagnosed with bladder cancer is estimated at 17.8% [16].
While urine cytology remains the gold standard for initial bladder cancer screening, it is not without problems regarding low risk patients such as asymptomatic women with MH, due to its low sensitivity in identifying low-grade urothelial cancers, which are the majority of cases in AMH [17].
As for the continuation of the MH investigation, published reports suggest that cystoscopy has a low yield (< 1%) in “low-risk” patients with MH. Therefore, it may be appropriate to defer cystoscopy for these “low-risk” patients. Furthermore, the importance of limiting the use of CTU for evaluating MH is emphasized for ’radiation sensitive’ populations, such as pregnant women and children, or with patients with known conditions (such as calculus disease), in spite of its best testing characteristics [18].
Significant variation exists among current guidelines for AMH with respect to who should be evaluated and in what manner [19]. Moreover, there is evidence that guidelines are not adhered to, and this reflects the necessity for introducing selection criteria and maybe variable levels of investigation for MH depending on the individual patient [20].
While current guidelines recommend a complete evaluation for women with MH aged 35 and above, or below 35 with risk factors, our study suggests that a full workup has a low yield and its possible complications, especially in younger women, hence possibly outweighing its benefits.
Currently there are no available alternative investigation options in clinical practice. Further research may reveal additional predictors to a positive evaluation, allowing for better patient selection.
We are making efforts to increase the study cohort in order to identify women with urothelial cancer in an attempt to possibly identify a unique parameter that would differentiate them from the patients without malignancy.
Thanks to all the peer reviewers and editors for their opinions and suggestions.
The authors declare no competing interests.
