1 Department of Clinical Neuroscience, Institute of Neuroscience and Physiology, Sahlgrenska Academy at Gothenburg University, 41390 Gothenburg, Sweden
2 Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, PA 15213, USA
Abstract
Epilepsy, a chronic neurological disorder characterized by recurrent seizures, affects a significant portion of the global population, with drug-resistant epilepsy (DRE) presenting a major treatment challenge. Insular epilepsy, originating from this complex region, exhibits a broad range of symptoms, making diagnosis particularly difficult. Advanced imaging techniques and invasive procedures like stereoelectroencephalography (SEEG) are often crucial for accurately localizing the epileptogenic zone. Surgical resection remains the primary treatment for DRE, with recent advancements in microsurgical techniques and neuroimaging improving outcomes. Additionally, minimally invasive approaches like laser interstitial thermal therapy (LITT) and radiofrequency thermocoagulation (RFTC) offer promising alternatives.
Keywords
- insular epilepsy
- diagnostic techniques
- surgical treatment
- SEEG
Epilepsy is a chronic disorder of the brain characterized by recurrent, unprovoked seizures, affecting an estimated 0.5%–1% of the global population. Approximately 32% of epilepsy patients suffer from drug-resistant-epilepsy (DRE), marked by persistent seizures despite optimal anti-seizure medication regimens [1]. Seizure episodes, post-ictal recovery challenges, and the adverse effects of anti-seizure medications on cognitive function, mood, and sleep, significantly impact the quality of life for those patients. These adverse effects also represent a major obstacle to achieving effective treatment [2]. Additionally, depression and anxiety are notably more prevalent among individuals with epilepsy, affecting almost one-third of those diagnosed [3].
The epileptogenic zone (EZ), the region of the brain where seizures are organized, can vary in anatomical locations, with the temporal lobe being the most common source. In contrast, the EZ originating from the insula, leading to insular epilepsy, is less common. Insular epilepsy poses significant challenges in both diagnosis and surgical treatment, often resulting in higher perisurgical morbidity compared to other types of epilepsy [4, 5, 6].
The insula, a crucial cortical region involved in the regulation of autonomic control and cardiorespiratory functions, has been implicated in an increased risk of Sudden Unexpected Death in Epilepsy (SUDEP) [7, 8, 9, 10]. SUDEP represents a severe and distressing outcome for individuals with epilepsy, and it is recognized as the leading cause of premature mortality among this patient population. The incidence of SUDEP is significant, affecting approximately 17% of all epilepsy patients, and up to 50% among those with chronic refractory epilepsy, where seizures remain resistant to optimal medical treatment [11].
The objective of this review is to summarize the extensive literature on insular epilepsy, with a focus on its anatomy, function, diagnostic studies, and surgical treatment, to aid researchers and clinicians in better understanding and managing of this complex condition.
The insula, often referred to as the fifth lobe of the brain, is concealed beneath the lateral sulcus and is covered by the opercula of the frontal, temporal, and parietal lobes. This region is also hidden behind a complex network of dense arterial and venous vasculature and is primarily supplied by the M2 segment of the middle cerebral artery [12]. The insula is multiconnected, receiving and sending information to both ipsilateral and contralateral neuron networks.
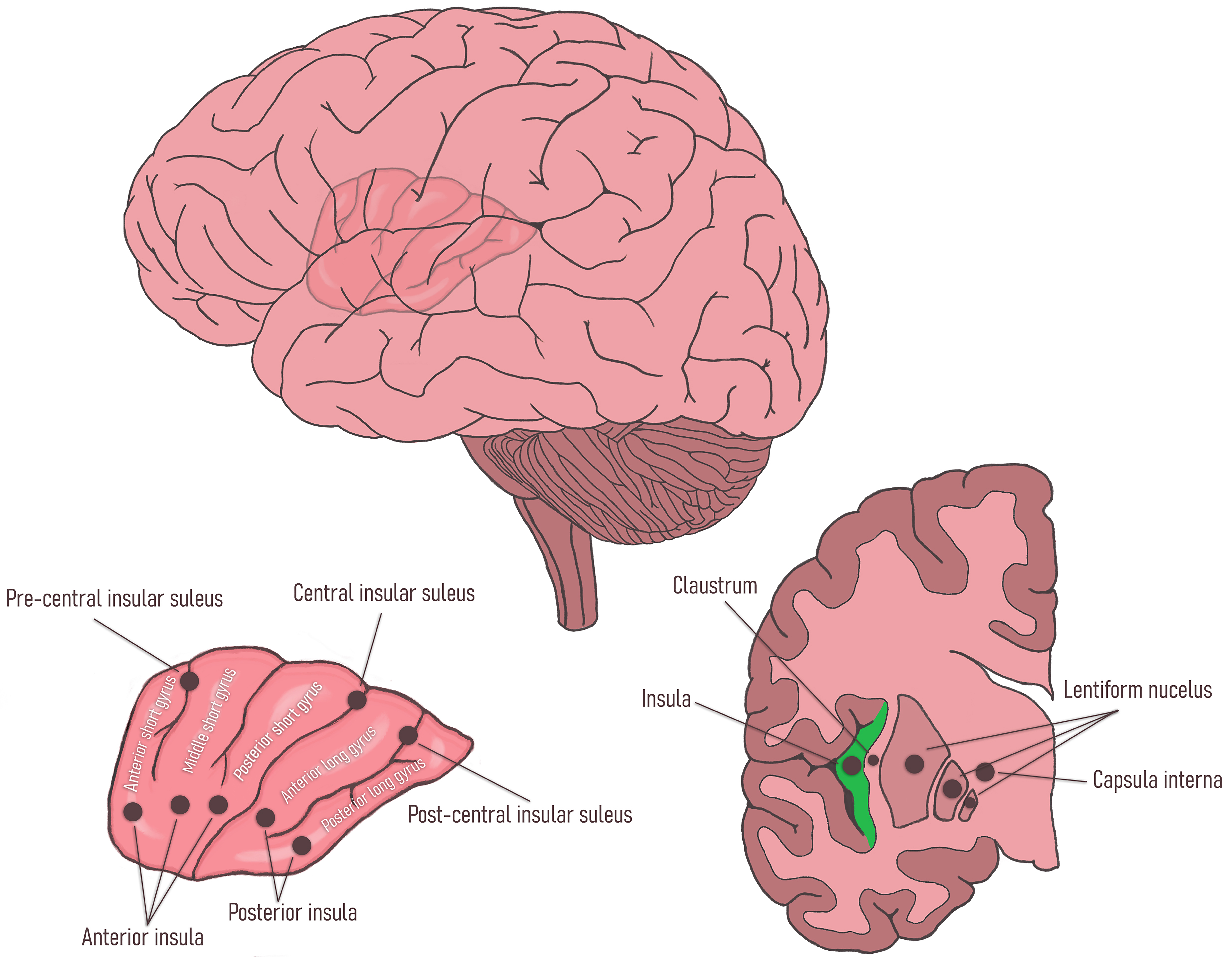
Anatomically, the insula is divided by the insular sulcus into two segments, with its cytoarchitecture comprising seven distinct subdivisions. The anterior insula contains three short gyri (anterior, middle, and posterior), while the posterior insula includes two long gyri (anterior and posterior) (Fig. 1). These anatomical features underpin the insula’s involvement in a myriad of complex functions, including sensory processing, autonomic control, emotional regulation, and cognitive tasks [13, 14].
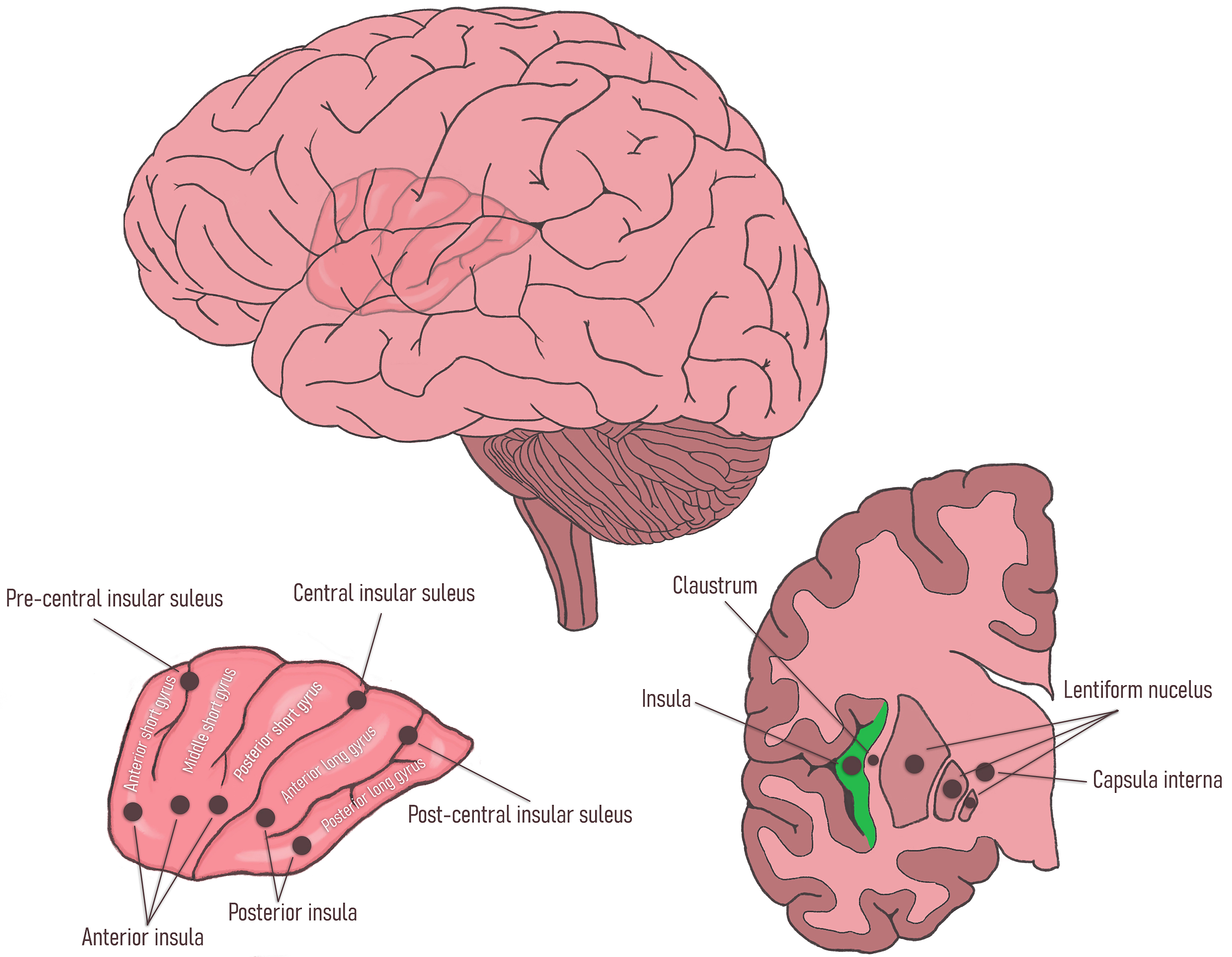 Fig. 1.
Fig. 1.
Anatomy of the insula.
The functions of the insula are less studied than most brain regions due to many factors, including its concealed location, extensive neuronal connections, and the rarity of isolated lesions [15]. A meta-analysis examining 1768 neuroimaging experiments identified four principal functions involving the insula: socio-emotional function (anterior-ventral region), sensorimotor function (mid-posterior); olfactory-gustatory (central), and cognitive functions (anterior-dorsal) [16].
• Interoception and Autonomic Control: The insula receives visceral stimuli from across the body, supporting its role as a pivotal hub of interoception and a regulator of autonomic control [16].
• Somatic Processing and Pain Mediation: The insula is activated by both ipsilateral and contralateral stimulation and houses the thermosensory cortex [16].
• Auditory and Vestibular Processing: The insula is involved in auditory processing and potentially vestibular function processing, though the latter is less explored [16].
• Emotional Experience: The insula plays a key role in facilitating emotional experiences by processing interoceptive and visceral signals [16].
• Empathy: The insula is involved in empathy, enabling the perception, comprehension, and experience of others’ emotions through interoception, self-awareness, social cognition, and sensorimotor activities [16].
• Risk-Decision-Making: The insula supports risk-decision-making by balancing rational analysis and emotional inputs, integrating cognitive and emotional information [16].
• Speech Production: The insula contributes to speech production, although to a lesser degree than Broca’s area [16, 17].
• Salience Network: The insula serves as the center of the salience network, detecting unusual stimuli across sensory modalities and coordinating relevant cognitive responses. It facilitates the switch from the ‘default mode network’ to the ‘central executive network’. Dysfunctions in this network are implicated in several clinical conditions, including autism spectrum disorder, frontotemporal dementia, and schizophrenia [18, 19].
Notably, there is functional asymmetry in the insula, with the left insula typically more involved in speech production and the right insula more crucial for salience network operations [18, 19].
The general characteristics of insular epilepsy are derived from its intrinsic functions and its interconnectivity with other brain regions. In most cases, the ictal discharge originating from the insula propagate to other brain areas, most frequently to the fronto-mesial region of the frontal lobe, leading to motor manifestations [20]. Identifying the insula as the origin of seizures is challenging due to this propagation. However, more specific semiology indicative of insular epilepsy includes a combination of somatosensory, visceral, or motor symptoms at the onset of the seizure [21].
• Somatosensory Manifestations: These include tingling or low-intensity paresthesias, laryngeal discomfort, throat constriction, or limb paresthesias, typically involving intra- or perioral areas and large cutaneous areas such as a limb or hemi-body. Bilateral and symmetrical symptoms, or those involving hot/cold sensations, reliably indicate the insular region [19, 20].
• Visceral Manifestations: These include nausea and epigastric or abdominal sensations, similar to mesial temporal lobe epilepsy, pointing to an anterior insular ictal onset [20, 21].
• Motor Manifestations: These often involve pedal-like motion, trunk rotation, or facial dyskinesia, predominantly occurring at night and resembling frontal lobe epilepsy. Auras in the form of somatosensory, visceral, taste, and/or auditory sensations preceding motor-manifestations strongly point to an insular onset, although they are often unreported due to being nocturnal [22].
• Auditory and Olfactory Hallucinations: These are rare but strongly indicate an insular onset [15, 19, 21].
• Speech Disorders: These range from complete speech interruption to decreased fluency [15, 19, 21].
A study on functional mapping and electric stimulation of the insula, aimed at mimicking symptoms of insular epilepsy onset, found that 61% of all evoked symptoms to be somatosensory, with paresthesias being the most common, followed by thermal sensations. Painful sensations, though rare, were highly specific to the insula and secondary somatosensory cortex, never elicited by stimulation of the primary somatosensory cortex or other cortical areas. Visceral symptoms accounted for 15% of insular stimulation, including constriction sensations, nausea, salivation, facial blush, dyspnea, urge to urinate, and sweaty hands. Less common symptoms included vestibular sensations, auditory hallucinations, speech impairment, and gustatory and olfactory sensations [23].
Diagnosing insular epilepsy and its EZ is complex but critical to ensure targeted and effective intervention. The symptomatogenic zone is often not located in the insula itself; for example, motor symptoms, the most common presentation, typically reflect the transfer of seizure activity to frontal regions [24]. This necessitates the use of advanced diagnostic tools to accurately evaluate patients preoperatively.
Diagnostic investigations can be broadly categorized into non-invasive and invasive studies. Non-invasive studies include electroencephalography (EEG), magnetic resonance imaging (MRI), magnetoencephalography (MEG), interictal positron emission tomography-computed tomography (PET-CT), and genetic testing. Invasive studies primarily involve the stereoelectroencephalography (SEEG) method using intracranial/intracortical electrodes.
• EEG: Detection of ictal or interictal epileptiform discharges in insular epilepsy is challenging due to the insula’s deep location. Detected signals often reflect seizure spread to adjacent neocortical areas, typically perisylvian. EEG signals may be absent, diffuse, multifocal, or misleadingly localized or lateralized. However, EEG can provide clues to a possible lateralized epileptogenic region within the temporo-perisylvian-insular network [19].
• MRI: MRI can strongly support a diagnosis of insular epilepsy if it reveals an insular lesion [25].
• MEG: MEG, which measures magnetic fields of neural activity in real-time, is particularly useful for diagnosing insular epilepsy, especially when neuronal clusters are present. MEG has been shown to be sensitive even when other non-invasive studies are negative, and it often aligns well with SEEG findings [4, 26, 27].
• Interictal PET-CT and Single-photon emission-computed tomography (SPECT): These tools measure brain metabolism using specific markers and are well-established for diagnosing temporal lobe epilepsy. However, they have less specificity for insular epilepsy due to the insula’s vast interconnectivity, making it difficult to distinguish from frontal or temporal lobe epilepsy [20, 25].
• Video-EEG (VEEG): VEEG combines EEG with video recording to provide insights into the onset, progression, and cessation of seizures and any associated physical manifestations. Indicators of insular epilepsy on VEEG include ictal discharges on EEG followed by delayed hypermotor manifestations, expressions of pain, or hand movements to the throat [20, 28].
• Genetic Testing: Genetic testing can be valuable in MRI-negative insular dysplasias. Mutations in the CHRNB2 and CHRNA4 genes have been identified in cases of sleep-related hypermotor seizures, while DEPDC5 gene mutations have been associated with familial focal epilepsy syndrome [29, 30].
Due to the low specificity of non-invasive tools for precisely locating the EZ in the insula, invasive studies are often necessary when insular epilepsy is suspected, particularly when MRI findings are negative. SEEG is an invasive method involving the insertion of electrodes into the brain to record electrical activity and pinpoint the origin of epileptic seizures. With accurate vascular mapping, SEEG is a safe and effective diagnostic tool, although a strong hypothesis of insular epilepsy is crucial for its success [20, 28].
There are two main surgical trajectories for SEEG implantation: orthogonal and oblique. The choice of approach depends on the hypothesized EZ location and surgeon preference.
• Orthogonal Approach: This involves inserting electrodes perpendicularly to the sagittal plane. It is more commonly used but requires traversing the dense vascular network of the middle cerebral artery, posing a higher risk of vascular complications without detailed vascular studies [12].
• Oblique Approach: This approach samples information from the frontal and temporal opercula and provides good coverage of the mediolateral insula. It is preferred when there is no hypothesis of opercular EZ involvement and when frontal or parietal lobe involvement is suspected. The oblique approach allows entry to the insula through a relatively non-eloquent corridor but necessitates longer electrodes, potentially reducing placement accuracy [22, 31, 32, 33].
In a large case series involving 135 patients undergoing SEEG, 303 electrodes were placed, with at least one electrode contacting the insular cortex. Among these, 96 patients exhibited semiology indicative of insular epilepsy, while 39 had SEEG based on non-invasive test findings. The analysis revealed that in 78% of cases, the initial hypothesis of insular involvement was refuted, while 17% were confirmed to have an insular seizure focus. Additionally, 4% showed seizure propagation to the insula from another focus, and in 2% of cases, the seizure origin remained undetermined. Notably, there were no intracerebral hemorrhages, although there were complications including one medical complication, a subdural hematoma, and meningitis leading to an intracerebral abscess [33].
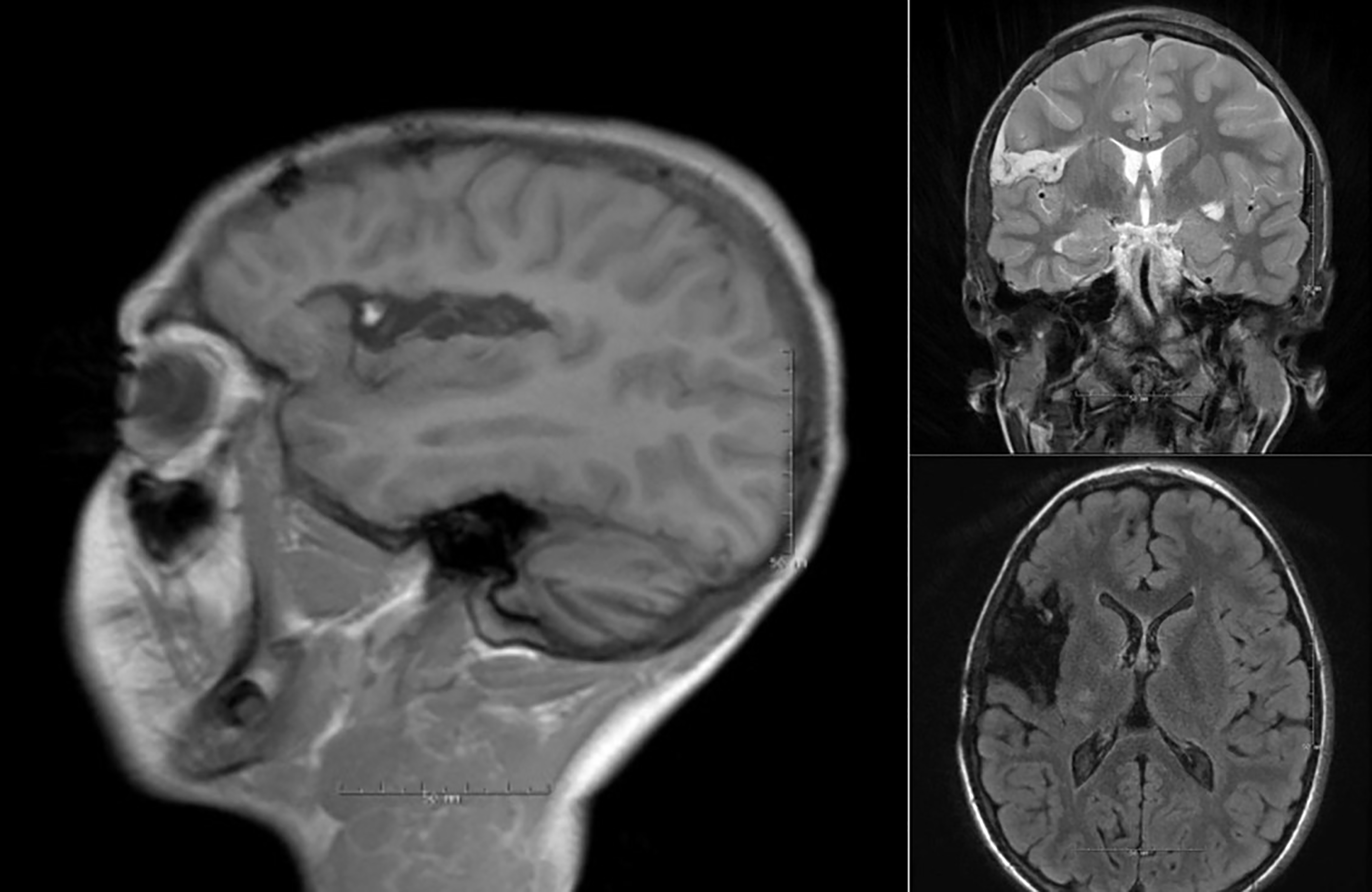
Surgical resection remains the first-line treatment for patients with DRE (Fig. 2). Initial attempts at insular resection in the 1950s and 1960s were abandoned due to high morbidity and low rates of seizure freedom [34, 35]. However, advancements in microsurgical techniques and neuroimaging have since led to improved outcomes and lower morbidity rates.
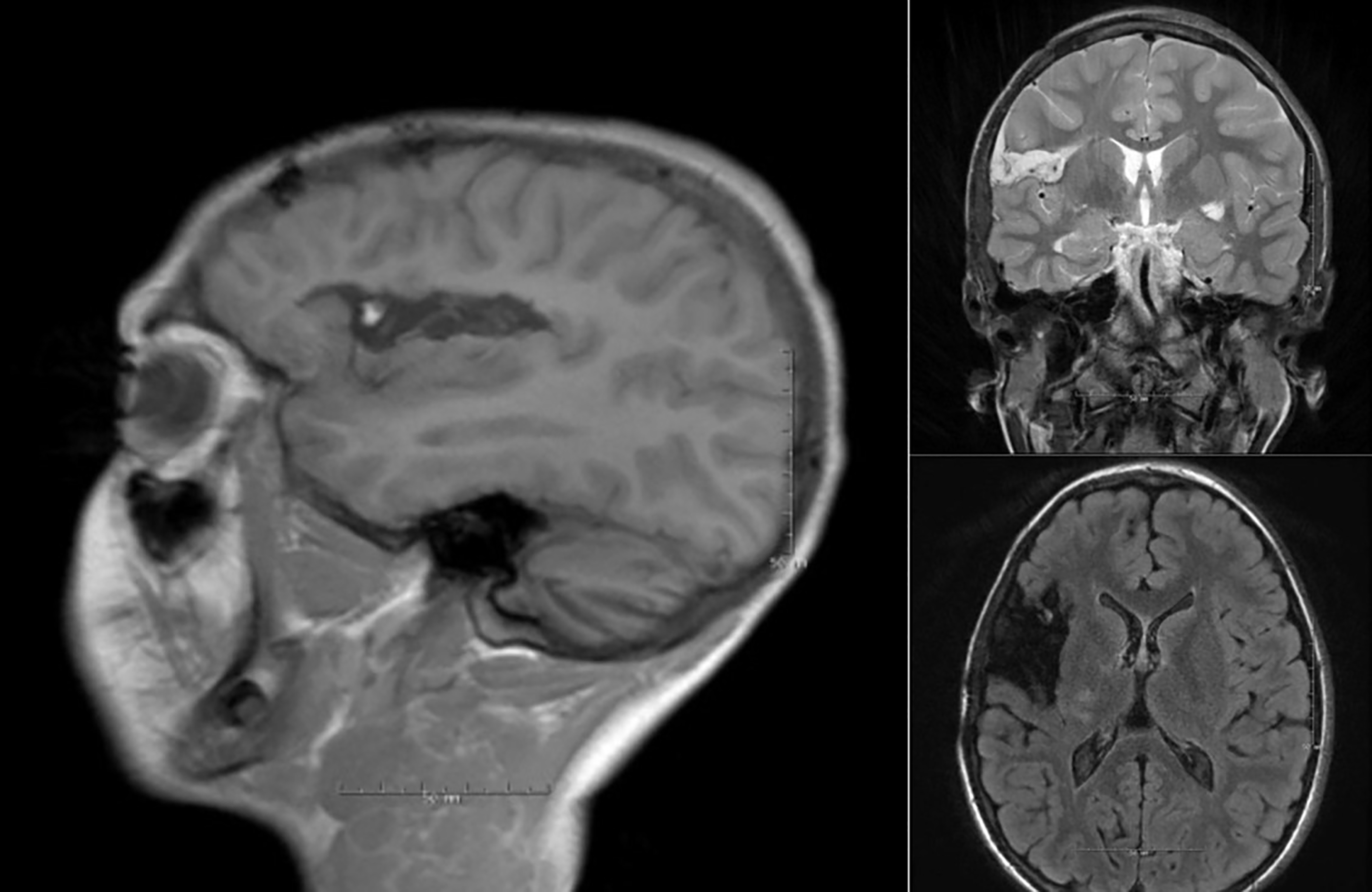 Fig. 2.
Fig. 2.
Post operative MRI after dorsal insula resection due to medically refractory epilepsy. The resection resulted in complete arrest of the epileptic activity. MRI, magnetic resonance imaging.
Recent meta-analyses have highlighted the outcomes of open resection for insular epilepsy. According to Kerezoudis et al. [5], a study involving 204 patients across 19 studies reported a median age of 23 years. Common clinical features included secondary generalization and painful paresthesia. A significant portion of patients showed positive findings on MRI, PET, and MEG, with 76% undergoing SEEG. Pathological findings varied, with cortical dysplasia being the most frequent. Twelve months post-surgery, 64.4% of patients achieved seizure freedom according to Engel I or International League Against Epilepsy (ILAE) 1+2 classifications. However, complication rates were notable, with transient deficits in 33.9% and permanent deficits in 9.8% of cases, primarily affecting motor functions of extremities and facial muscles. A positive MRI was a significant predictor of favorable seizure outcomes. Despite these insights, the study’s major limitation was the absence of a meta-analysis statistical model to account for between-study variability, a fundamental component for a reliable meta-analysis [5].
Another meta-analysis by Obaid et al. [4] explored predictors of successful seizure outcomes across traditional resective surgeries and less invasive methods like MR-guided laser interstitial thermal therapy (MRgLITT) and radiofrequency thermocoagulation (RFTC). This analysis included patients with an average age of 9 years, most of whom experienced early motor symptoms and a high daily seizure rate before surgery. SEEG was used in 85% of cases. MEG showed the highest diagnostic concordance with SEEG at 73%. The seizure freedom rate was 67%. However, 43% of patients experienced complications, with 34% being transient and 8% permanent, predominantly affecting motor functions. Factors such as young age, use of SEEG, and minimally invasive methods like MRgLITT and RFTC were linked to higher seizure recurrence. Additionally, complications were more frequent following resections involving the posterior insula and frontal operculum, particularly on the dominant side of the brain. The worse seizure outcomes associated with MRgLITT and RFTC may partly be due to these interventions being used when traditional surgery is contraindicated or in non-curative patients [4].
Permanent motor deficits after insular resection are possibly due to damage to the lenticulostriate arteries or the small caliber perforating arteries originating from the middle cerebral artery (MCA), leading to symptomatic infarcts in the ipsilateral caudal corona radiata, which carry motor and sensory fibers [28, 36]. A study on the vasculature around the superior limiting sulcus (SLS) of the insula in 20 cadavers found that 87% of arteries passing through the insula to the brain parenchyma and reaching the corona radiata were located within a 5 mm zone at the peak of the superior limiting sulcus. The study suggests using the SLS as a landmark to limit ischemia in the corona radiata during surgery [37]. The same author published a technical case report of three patients, none of whom developed motor deficits after preserving the superior posterior insula while still achieving significant seizure reduction [38].
In conclusion, insular resection appears to have a similar seizure freedom rate to temporal lobe resection for mesial temporal lobe epilepsy but with a higher risk profile, particularly for resections involving the posterior insula.
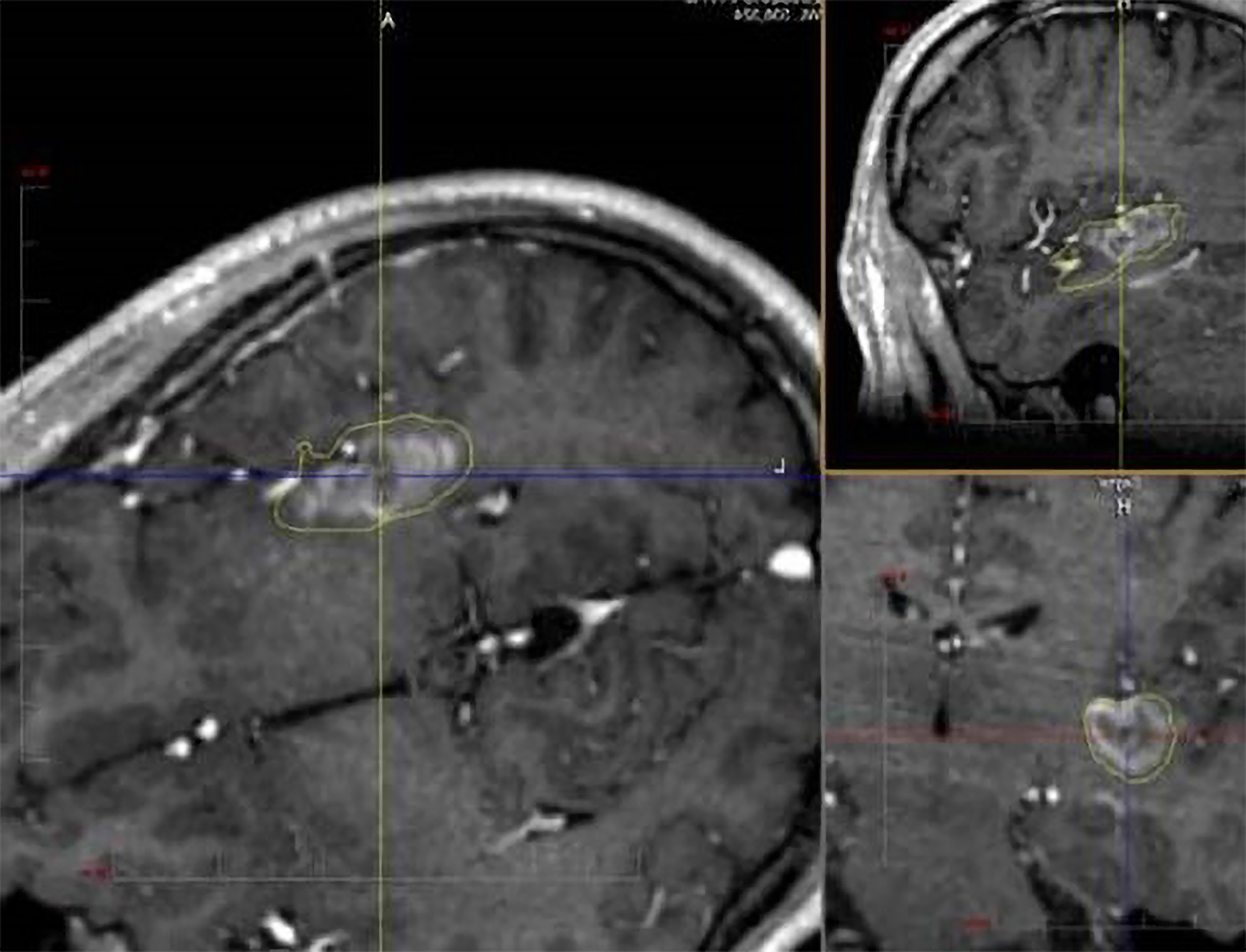
Another surgical approach to insular epilepsy is laser interstitial thermal therapy (LITT) (Fig. 3), first utilized for temporal lobe epilepsy in 2014 [39]. LITT is a minimally invasive procedure where a laser diode is inserted through a small cranial opening. The laser’s light is absorbed by the surrounding tissue, increasing heat and resulting in thermal ablation [40].
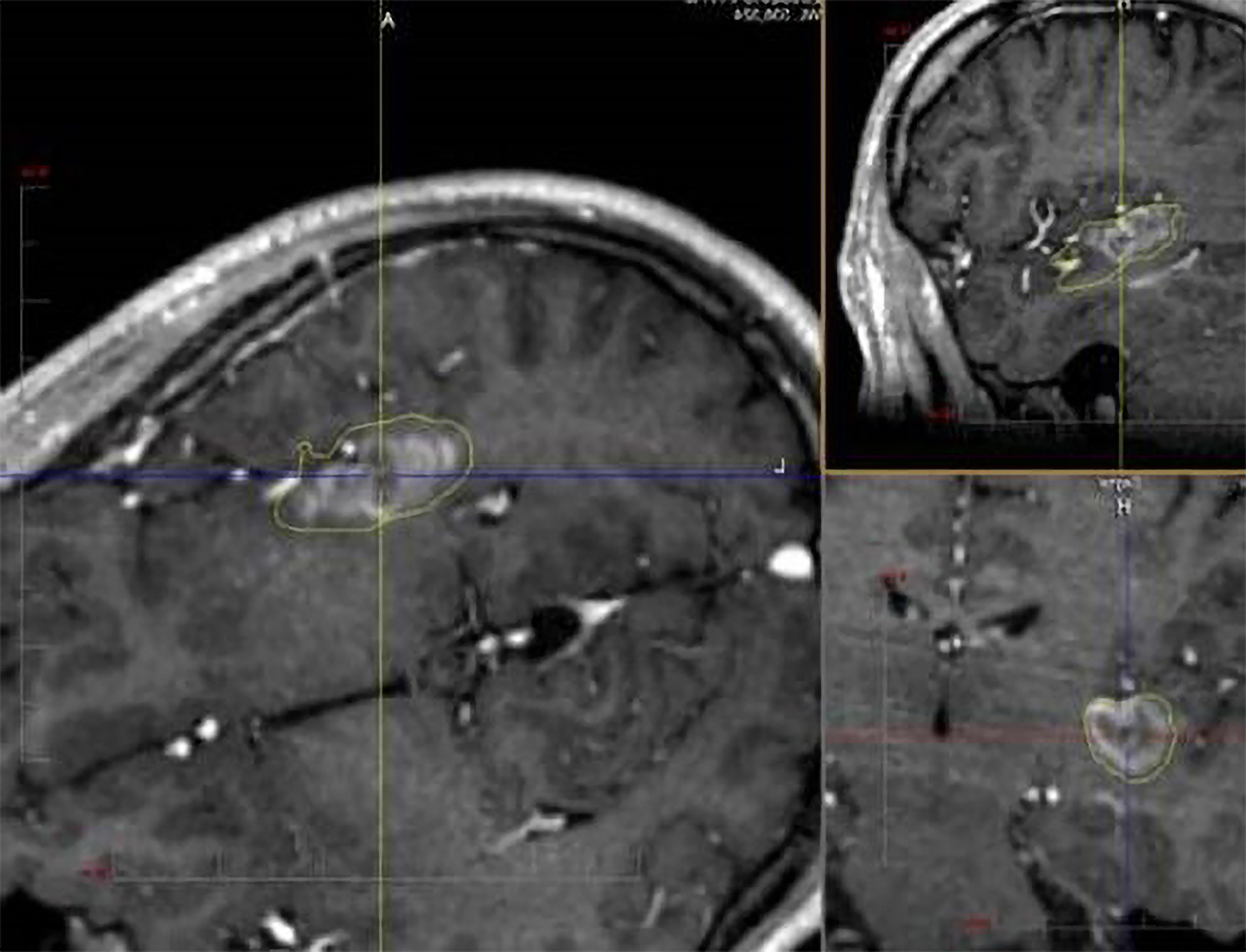 Fig. 3.
Fig. 3.
SEEG-guided laser ablation of the posterior insula in a patient with medically refractory epilepsy. The procedure was well tolerated and results in seizure freedom. SEEG, stereoelectroencephalography.
Several cohort studies have evaluated the effectiveness of MRgLITT in various patient groups, including those with non-lesional MRIs and pediatric cases. Across these studies, combined seizure freedom rates were as follows: 52% achieved Engel I, 14% achieved Engel II, 23% reached Engel III, and 11% were categorized as Engel IV. Transient complication rates were 36%, encompassing conditions such as paresis, mild facial droop, dysphagia, expressive language dysfunction, supplemental motor area-like syndromes, and one significant case of intracerebral hemorrhage leading to transient aphasia and weakness. Notably, no permanent complications were reported [41, 42, 43, 44].
RFTC is a minimally invasive surgical
technique in which SEEG electrodes are heated to create multiple small lesions
that can converge into larger lesions. This approach, using SEEG recordings to
guide the ablation, was first applied in 2004 [45]. Three studies have reported
outcomes after RFTC for insular epilepsy: two cohort studies and one meta-analysis [4, 46, 47],
with a transient complication rate of 40% and no permanent complications observed. The two
cohort studies also reported post-RFTC ablation volumes, achieving averages of 6.82
The surgical management of insular epilepsy has seen significant advancements, but several challenges remain. As our understanding of insular epilepsy deepens, future directions in surgical treatment must focus on refining techniques, improving patient selection, and integrating innovative technologies to enhance outcomes.
Future research should emphasize the development of more precise imaging modalities and mapping techniques to improve the localization of EZ within the insula. Advanced neuroimaging technologies, such as high-resolution functional MRI (fMRI) and diffusion tensor imaging (DTI), could offer improved spatial resolution and better delineation of the insular cortex and its connections. Enhanced imaging could facilitate more accurate preoperative planning, allowing for targeted resection and minimizing damage to adjacent functional areas.
Incorporating advanced electrophysiological techniques, such as high-density electrocorticography (ECoG) and novel neurostimulation modalities, could improve the precision of intraoperative mapping and functional localization. Real-time intraoperative monitoring with high-density ECoG could help identify critical areas within the insula and guide resection efforts, thereby reducing postoperative deficits. Additionally, the development of refined algorithms for SEEG analysis and interpretation may enhance the ability to pinpoint the exact origin of epileptic activity and tailor surgical interventions accordingly.
The evolution of minimally invasive surgical techniques, such as LITT and RFTC, presents a promising alternative to traditional open resection. Future research should focus on optimizing these techniques to improve efficacy and safety. Studies should investigate the long-term outcomes of minimally invasive approaches, particularly in patients with insular epilepsy who are not candidates for conventional resection. Additionally, innovations in catheter design and thermal imaging could enhance the precision and effectiveness of these procedures.
Personalized medicine is a crucial frontier in the surgical treatment of insular epilepsy. Future approaches should aim to tailor surgical strategies based on individual patient characteristics, including lesion morphology, seizure semiology, and comorbidities. Developing personalized surgical plans could involve integrating genetic, neuroimaging, and electrophysiological data to optimize treatment outcomes. Additionally, personalized approaches may include preoperative neuropsychological assessments to better understand and mitigate the potential cognitive and functional impacts of surgery.
While immediate seizure control is a primary goal, future research should also address the long-term outcomes and quality of life for patients undergoing surgery for insular epilepsy. Studies should focus on longitudinal follow-ups to assess the durability of seizure freedom, cognitive function, and overall quality of life. Understanding the long-term effects of various surgical interventions can guide future surgical planning and postoperative care, ensuring that interventions not only control seizures but also preserve or enhance quality of life.
Addressing the complexities of insular epilepsy requires a multidisciplinary approach. Future advancements will benefit from increased collaboration among neurosurgeons, neurologists, radiologists, neuropsychologists, and rehabilitation specialists. Collaborative efforts can facilitate the integration of diverse perspectives and expertise, leading to more comprehensive treatment plans and improved patient outcomes.
As surgical techniques evolve, ethical and socioeconomic considerations will play a critical role. Ensuring equitable access to advanced surgical treatments and addressing potential disparities in healthcare will be essential. Furthermore, ongoing discussions regarding the ethical implications of emerging technologies and interventions will help guide their implementation in clinical practice.
Insular epilepsy represents a complex clinical entity, characterized by a myriad of intricacies spanning neuroanatomy, diagnostic modalities, and therapeutic strategies. The insula, situated deep within the cerebral cortex, functions as a critical nexus for various sensory, motor, and cognitive processes, making its involvement in epileptogenesis particularly challenging to elucidate. Diagnostic efforts necessitate a comprehensive integration of neuroimaging techniques, electrophysiological assessments, and neuropsychological evaluations, each providing essential insights into the precise localization and underlying mechanisms of epileptic foci within the insular cortex.
The management of insular epilepsy requires a nuanced and multidisciplinary approach. Pharmacological treatments, neurostimulation modalities, and, when indicated, surgical interventions must be carefully coordinated to optimize patient outcomes. Surgical resection, often considered the definitive treatment, demands a careful balance between achieving seizure control and preserving critical neurological functions, highlighting the need for precise localization and thorough preoperative planning. Moreover, emerging techniques such as LITT and responsive neurostimulation (RNS) present promising alternatives for patients who are not candidates for conventional resective surgery.
In conclusion, the management of insular epilepsy exemplifies the convergence of advanced neuroscientific knowledge and clinical expertise. A deep understanding of neuroanatomical complexities, diagnostic challenges, and therapeutic options is essential for achieving favorable clinical outcomes. Through collaborative efforts and interdisciplinary approaches, the multifaceted nature of insular epilepsy can be more effectively addressed, leading to enhanced patient care and improved quality of life.
FRE designed the research study, performed the literature review, analysed the data and wrote the manuscript. JAGM designed the research study, reviewed and edited the manuscript. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We would like to express our sincere gratitude to Dr. Naoki Ikegaya for his invaluable assistance and insightful discussions that greatly enhanced our understanding of the insula. Declaration of AI and AI-assisted Technologies in the Writing Process: During the preparation of this work, the authors used Chat-GPT in order to check spell and grammar of all the text. After using this tool, the authors reviewed and edited the “1. Introduction” and “5. Surgical Treatment for Insular Epilepsy” sections as needed and took full responsibility for the content of the publication.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.