Academic Editors: Sergio Bagnato and Gernot Riedel
Medical well-regarded policy recommendations for patients with disorders of consciousness (DoC) are almost exclusively relied on behavioural examination and evaluation of higher-order cognition, and largely disregard the patients’ self. This is so because practically establishing the presence of self-awareness or Selfhood is even more challenging than evaluating the presence of consciousness. At the same time, establishing the potential (actual physical possibility) of Selfhood in DoC patients is crucialy important from clinical, ethical, and moral standpoints because Selfhood is the most central and private evidence of being an independent and free agent that unites intention, embodiment, executive functions, attention, general intelligence, emotions and other components within the intra-subjective frame (first-person givenness). The importance of Selfhood is supported further by the observation that rebooting of self-awareness is the first step to recovery after brain damage. It seems that complex experiential Selfhood can be plausibly conceptualized within the Operational Architectonics (OA) of brain-mind functioning and reliably measured by quantitative electroencephalogram (qEEG) operational synchrony.
Selfhood is the most central and private core of being an independent and free agent that feels directly and immediately present as the center of a phenomenal multimodal perceptual reality (a sensed “center of gravity”; for an overview, see [1]), thus having the phenomenal first-person perspective [2, 3, 4, 5, 6]. Regadless the current debate [7, 8], it is generally accepted that this implicit full-fledged first-person mode of givenness [9, 10, 11] is present most of the time in neurotypical humans, not only during wakefulness but also during sleep with dreams [12] and functionally underpins all types of conscious experience. This is characterized as the “mineness” of consciousness [13], where a conscious being implicitly knows that it is the very being it is (so-called “de se constraint” of consciousness [14]).
Sustained impairment of (self-)consciousness obtained after brain damage constitutes a devastating condition that “requires a better understanding of the corresponding physiopathology in order to cure patients and to take care of them optimally” (p. 1 in Ref. [15]). Such impairments are summed up under the generic expression of “Disorders of Consciousness” (DoC) used in clinical practice to characterise patients who have regained the wakefulness-sleep cycle from a period of being comatose but have lost the ability to demonstrate overt behaviours and who are usually in one of the following states [16, 17]: (i) vegetative state (VS) [18] recently dubbed unresponsive wakefulness syndrome (UWS) [19] and defined as a “clinical condition of complete unawareness of the self and the environment” (p. 1499 in Ref. [20]), (ii) minimally conscious state (MCS) [21] (now includes MCS- and MCS+ [22]), which is “a condition of severely altered consciousness in which minimal but definite behavioral evidence of self or environmental awareness is demonstrated” (p. 350–351 in Ref. [21]), and (iii) mostly conscious but cognitively impaired patients after emerging from a MCS (EMCS and related states) [23] and are in a confusional state [24, 25].
When dealing with patients with DoC, clinicians primarily attempt to answer the following questions: is the patient conscious? if not conscious, will that condition change? and if it can, how may recovery of consciousness be aided? and ultimately, what the patient’s quality of life will be in the future? Therefore, a clear distinguishing of (self-)consciousness is essential. Medical well-regarded policy recommendations for patients with DoC are almost exclusively relied on behavioural examination and evaluation of higher-order cognition, and largely disregard the patients’ self. This is so because to practically establish the presence of self-awareness or Selfhood is even more challenging than evaluate the presence of consciousness. Indeed, the diagnostic tools/instruments available for clinicians (as for example, the Coma Recovery Scale-Revised [26]) are intended to assist them recognizing overt behavioral responses to auditory and visual stimuli, which are indirect indicators of self and environmental awareness. They are of no help (regardless of how meticulously they are administered) in the specific situation where the patient may be conscious but show no behavioral signs of it at all. And, in fact, the findings published in 2006 [27] demonstrated just this case. Since then, multiple subsequent studies utilizing magnetic resonance imaging (MRI) or quantitative electroencephalography (qEEG) confirmed this and showed that a significant percentage of patients (~20%) diagnosed as VS/UWS worldwide appeared not to be vegetative at all, but simply physically non-responsive with retained awareness, the covert signs of which could be revealed by functional MRI or qEEG (for a meta-analysis see [28]). Further, even on the occasions where self-awareness is concerned, the target of the clinical evaluation is solely on higher-order cognition, not the self with its inherent first-person phenomenology and the sense of agency [29]. At the same time, establishing the potential (actual physical possibility) of Selfhood in DoC patients is important clinically, ethically significant and morally salient because, as we have observed above, Selfhood is intrinsically valuable as the core of human nature and personhood and, as such, it has moral status by providing the phenomenological experience of “life worth living” [30].
In this opinion paper, we propose that Selfhood of patients with DoC should be considered seriously because it could have important theoretical ramifications for the ongoing debate about the nature of Selfhood, as well as potential medical and ethical/social/legal implications for the management of patients with DoC [29], particularly with regard to life-sustaining treatment [31]. Indeed, the knowledge of the state of self-awareness beyond clinical assessment in patients with DoC “is absolutely imperative for making a substituted judgement and can be extremely helpful when there is a need to establish or even justify particular treatment options” (p. 217 in Ref. [32]). Additionally, the clarity about the physical actual potentiality of having self-awareness can affect how the patient’s family members behave toward the patient: they might, for example, feel more motivated and compelled to address their noncommunicative relative directly if signs of Selfhood could be demonstrated using some neuroimaging technology even when clinical signs are absent (see also [33]).
Similar to the recent (2020) guidelines of the European Academy of Neurology that recommend that when there is discrepancy between the level of consciousness detected by different paradigms and instruments, a patient should be diagnosed with the “highest level of consciousness” suggested by any of the approaches [34], the same logic should be applied regarding the state of Selfhood establishment. Though until a while ago the reliable assessment of Selfhood was not possible clinically in patients with DoC, recent developments in the neurophysiological understanding of consciousness [35, 36] open such a possibility [37, 38]. According to current theoretical understanding, consciousness is an emergent phenomenon of coherent but dynamic interaction among operations produced by multiple, relatively large, long-lived and stable, but transient neuronal assemblies in the form of spatiotemporal patterns within the brain’s electromagnetic field nested hierarchical architecture [35, 36] (see also [39, 40]). This brain’s nested electromagnetic architecture, referred to as “operational”, constitutes the core of the Operational Architectonics (OA) framework of brain–mind functioning [41]. In brief, the OA theory states the following (for a detailed description, see [35, 36]): The brain generates a non-random, dynamic, and highly structured extracellular electromagnetic field in spatial–temporal domains and over a wide frequency range [39, 40, 42]. This field exists within the brain’s internal physical space-time, and the most efficient method for capturing it is a qEEG measurement [43], where the relatively stable segments in the local electroencephalography (EEG) signals are the neurophysiological substrates of single operations (standing electromagnetic fields) produced by different individual neuronal assemblies. According to the OA theory, these single operations present different qualia or aspects of the whole object/scene/concept. At the same time, the wholeness of the consciously perceived or imagined is originated as a result of synchronized operations (electromagnetic fields) of many brain functional and transient neuronal assemblies in the form of dynamic and ever increasing spatial–temporal patterns termed Operational Modules – OMs (every OM is composed of a set of brain areas that are tightly functionally connected to one another within a set; for extensive discussion, see [35, 36]). The recombination of the operations produced by neuronal assemblies in new configurations (OMs) opens the possibility of presenting phenomenally an almost infinite number and complexity of various qualities, patterns, and objects, including the intricate experiential Selfhood [12].
Recently, within the OA framework, the neurophysiological three-dimensional construct model of the complex experiential Selfhood was introduced (for a detailed description and empirical data, see [1]). This triad model of Selfhood accounts for the phenomenological distinctions between three major aspects of Selfhood, namely phenomenal first-person agency (referred to as “Self”), embodiment (referred to as “Me”), and reflection/narration (referred to as “I”) (all three are commensurate with one another [44, 45]), and thus reflects the multifarious nature of self-awareness [46, 47]. These three aspects work together to create a unified sense of Selfhood [1, 12]. Neurophysiologically, these three aspects of Selfhood correspond to three specific OMs within the so-called brain self-referential network (SRN) [1], also intermittently referred to as the default mode network [12, 48, 49, 50, 51, 52, 53]. A set of nine EEG operationally synchronized cortical areas is used to estimate the synchrony strength within the three OMs (anterior OM: F3-Fz-F4 EEG locations; left posterior OM: T5-P3-O1 EEG locations; and right posterior OM: T6-P4-O2 EEG locations) that have been established previously to belong to SRN and each related to specific functions that can be subsumed by the names “Self”, “Me”, and “I” based on the current empirical evidence from psychiatric and neurological conditions (for a further detail including the causal relationship, see [1]). Lately, it has been confirmed experimentally that the three OMs of brain SRN and related to them three phenomenological aspects of Selfhood are causally linked [1]. More specifically, experienced meditators mentally manipulated (either by increasing/up-regulating or decreasing/down-regulating) every component of the Selfhood triad (“Self” or witnessing observer, “Me” or representational-emotional agency, and “I” or reflective agency) in a randomised, independent, and controlled manner while the dynamics of functional integrity of the three SRN OMs were measured by qEEG. Mental upregulation of the expression of Self, Me, or I was found to result in a significant increase in the functional integrity (indexed by qEEG operational synchrony measure [54, 55]) of the corresponding SRN OMs, whereas conversely, down-regulation of the Self/Me/I phenomenological sense had resulted in a significant decrease in the functional integrity of the respective SRN OMs [1]. Importantly, the participants’ self-reported alterations in the phenomenological experiences during up- or down-regulation of Selfhood components and phenomenological factors estimated by a set of standardized questionnaires were consistent and significantly correlated with the observed changes in the functional integrity of the SRN OMs [1].
By the same token, it has been documented that the functional integrity changes (indexed by qEEG operational synchrony measure) in the triad of SRN OMs predictably follow the changes in the phenomenology of self during different neuropsychopathologies like, for example, depression [56], post-traumatic stress disorder [57], or brain damage [58] and also during different altered states of Selfhood [59]. It is important to keep in mind that SRN does not exist in isolation and interacts with other brain networks. Particularly, the fronto-parietal network’s non-competitive state is crucial because if its activity is dominated, it will have an impact on awareness [38].
Such approach where (i) neurophysiological third-person data (e.g., EEG) is integrated with first-person phenomenological data (e.g., subjective experience and reports) and (ii) they mutually inform one another, is called Neurophenomenology [60, 61, 62, 63]. In this context, if a causal link between objective and subjective accounts is established, it may offer an attractive possibility to assume the state of one account (phenomenal) by measuring the other one (neuro). This is especially important for cases of nonresponsive patients, who cannot provide subjective reports.
The following characterization is largely taken from our previous publication [1], as it is a standard description.
As specified by the triad model of Selfhood, the anterior OM of the SRN is associated with the phenomenal first-person perspective and the phenomenal sense of agency [1], where agency is treated as (i) the “sense of ownership” of thoughts, perceptions, and actions relevant to Selfhood [2, 5, 11, 64, 65] and (ii) the sense of the implicit first-person mode of givenness that undergoes the subjective experience [9, 10, 11]. It is labeled the “witnessing observer” or simply the “Self” in the narrowest sense [1]—as the phenomenal non-conceptual core in the act of knowing itself [5]. When the “Self”-component is up-regulated, the subject experiences an “… increased sense of being an epistemic agent that possessess increased self-concept clarity, established a self-representational kind of knowledge for the body, as well as epistemic self-control, all of which together are sufficient for creating a phenomenological first-person perspective …. Further, … this phenomenology also contributes to a sense that one has the capacity for selective, top-down attentional control, and also knows that it (oneself) possesses this capacity—thus having the phenomenal ownership” (p. 18 in Ref. [1]). On the contrary, while the “Self”-component is down-regulated, the subject reports that there is “no-one who thinks or observes” and, therefore, the experience appears to be “empty” [1]. In the extreme case, it presupposes “… a complete absence of any form of phenomenal Selfhood, even the minimal form of spatial-temporal frame of reference—unextended point capable of epistemic self-control, as well as the absence of intentional content, complete emptiness, a void” (p. 19–20 in Ref. [1]).
The right posterior OM of the SRN is linked with the experience of self as an entity normally localized (through interoceptive and exteroceptive sensory processing) within bodily boundaries, as well as related to it emotional states and autobiographical emotional memories [1]. It is labeled “representational-emotional agency” or simply “Me” [1]. The distinctive feature of the Me-module is that, in contrast to a phenomenal first-person perspective, here only a purely geometrical first-person perspective is present that originates from within the body representation, thus designating an egocentric spatiotemporal self-model [5]. Importantly, the body is viewed in this context as a “vehicle” that enables one to be a self in the world, rather than as just one more (among many) physical objects [66, 67, 68, 69, 70, 71, 72]. The up-regulation of “Me”-component is markedly correlated with the feeling of hyperembodiment, allowing a globalized form of self-identification with the body as a whole (the “material me”), thus laying the foundation for a basic, minimal and pre-reflective aspect of self with related emotional states [1]. During down-regulation of the “Me”-component subjects experience disembodiment, bodilessness, or out-of-body experience (“the experience of the body is identified with limitless space”); they have diminished or absent automatic and immediate sense of physical agency, first-order experiential sense of ownership (that it is me who owns the body), as well as body self-location, body image and body schema, all accompanied by a subjective suspention of time and space (for more discussions, see [1]).
The left posterior OM of the SRN is associated with the experience of thinking about and reflecting upon oneself, including momentary narrative thoughts and inner speech, as well as the reinterpretation of self-related episodic and semantic memory events—autobiographical story telling [1]. It is labeled “reflective agency” or simply “I” [1]. Crucially, such narrative self-reflection relies on the capability for language [9, 73, 74] and lays the foundation for the sense of invariance of Selfhood over time [75, 76, 77]. The up-regulated “I”-component is “… associated with activation of autobiographical memories, comprising of episodic and semantic memories that consist of either concrete and specific items/episodes of personal information that are closely related to events situated in the past or semantic personal information such as general knowledge of personal facts, but also general (repeated and extended) events” (p. 17 in Ref. [1]). Further, there is increased self-reflection and thinking about one’s own narrative self, which presupposes a clearer self-concept expressed in a better understanding of one’s own states, traits and dispositions [1]. Such narratives normally involve a high level of cognition. During down-regulation of “I”-component, subjects report that “the inner commentator is quiet and the contents of experience could freely change and flow without a story”; in such state “… disconnected thoughts just popped-up “in and out” in the absence of any explicit subjective sense of presence, past or future, thus indicating disruption in narration and self-reflection that together are a prerequisite for the cognitive self that persists across experiences” (p. 18 in Ref. [1]).
Summarising, these three Selfhood aspects are complementary rather than opposed to one another [44, 45, 59], whereas the integration of dynamics of three SRN OMs that support the triad of Selfhood aspects enables nonreductive intertwining of these aspects (witnessing observer, representational-emotional agency, and reflective agency), resulting in a coherent substantiation of the singular, complex phenomenal pattern—a Selfhood [1, 12].
Utilising this empirically-grounded triad model of Selfhood in the assessment of patients with DoC may help shed light on whether and which patients have full or minimal self-awareness, and which (or all) aspects of Selfhood are present, diminished or absent. Further, keeping with a demonstrated causal link between three aspects of Selfhood and three SRN modules (measured by qEEG) [1], clinicians (and relatives) may get insight into the phenomenal experience of a given patient. This knowledge may give at least some hints as to whether the patient enjoys the moral status of the kind and degree that is sufficient for personhood (in other words be a subject of a life) or only to support some aspects of phenomenal self-experience, as for example, embodiment (pleasure and pain). According to Levy [78] to have a full moral status is to have an interest in life, conceive itself as persisting in time, and be able to have future-oriented desires, thus having a motivation to continue living. As a first approximation, one may speculate that at least the “Self” and “I” aspects of Selfhood should be present to have this kind of moral status.
While systematic and rigorous research using the triad model of Selfhood in patients with DoC is largely lacking, some preliminary results are starting to emerge.
In the healthy neurotypical persons there is a rather high level of functional integrity within Self-, Me- and I- modules of brain SRN (Fig. 1A) [12]. Interestingly, these levels of integrity are rather stable during rest and also while individuals are confronted by specific task-demanding activities, thus stressing that relative stability of Selfhood in everyday normo-typical conditions [12] is needed to support the first-person perspective taking and an experience of agency (see also [2, 5, 9, 10, 11, 65]).
 Fig. 1.
Fig. 1.A schematic illustration of findings on the dynamics of operational modules (OMs: Self, Me, I) of the brain self-referential network (SRN) in patients with disorders of consciousness (DoC). (A) Relationship between the SRN functional integrity and self-consciousness espression. (B) Dynamics of functional integrety within three OMs (Self, Me, I) in patients with VS/UWS. The horizontal dotted line represents OMs’ functional integrity in healthy fully self-conscious subjects (NORM) and is taken as a reference. (C) The functional integrity of Self-OM (Self-component of Selfhood) as a function of future self-consciousness recovery in relation to the stochastic level (dotted horizonal line). Below this stochastic level/threshold any measurement is random. VS/UWS, vegetative state/unresponsive wakefulness syndrome; MCS, minimally conscious state; E-MCS, patients who emerged from a MCS.
Such levels of Self-, Me-, and I- modules’ functional integrity within brain SRN are, however, profoundly altered in patients with DoC (as expected). For example, it was documented [79] that overall SRN functional integrity (comprising three OMs together) was at the stochastic level or absent in patients with VS/UWS, intermediate in patients with MCS, and the highest in healthy fully self-conscious individuals (Fig. 1A). The largely diminished level of SRN functional integrity during VS/UWS likely presents the lowest level of functionality that is already insufficient to support representational content, which has reference to self from the first-person perspective. Considering the previous study on the causal relationships between the triad SRN OMs and three aspects of Selfhood [1], the observed significant drop in the functional integrity of the SRN OMs triad (Fig. 1B) may signify a phenomenological state of selfless, bodiless and timeless presence, characterised by a lack of individual first-person perspective and an “emptying out” of all phenomenological contents, including thoughts. However, some cognitive operations performed without self- (or any other) consciousness are still present during such a condition, indicating the so-called return to the rigid stimulus-response behaviour of earlier phylogenetic animal lineages [80]. This is in line with previous studies showing that the functional integrity of SRN is absent or lowest in several conditions that are characterised by a lack of self-awareness: brain death—absent completely [81], coma—largely not detectable [82], and VS/UWS—severely disrupted [83]. During an MCS state, on the other hand, some level of SRN functional integrity may support an unstable or “flickering” sense of self that is neither fully balanced nor completely fragmented, similar to dreaming or being in an altered state of consciousness [84]. Indeed, patients with MCS have been shown to have altered states related to self, such as time distortion, thought acceleration, and various transcendental phenomena, like, for example, dissolution of the the body or out-of-body experience [85, 86].
Further, it was found [79] that the Self-module of SRN (in comparison with the Me- and I-modules) demonstrated the strongest decrease in functional integrity as a function of self-consciousness loss in patients with DoC (VS/UWS vs MCS) (Fig. 1B), thus stressing the principal role of the Self-module in self-referential processing [87], serving as the “witnessing observer”—the phenomenal non-conceptual core in the act of knowing itself (for a substantial discussion, see [1]). Keeping with this finding, the neurophenomenological study of the Selfhood triad in experienced meditators shows that “a significant functional disintegration of the [Self] SRN OM, was associated with a significant decrease or complete absence of intentional content, phenomenal spatiotemporal self-location and first-person perspective, as well a general ability to introspectively create indivisible units of experience (not even a single one), thus leading to a suspension of the subject’s inner narrative life” (p. 23 in Ref. [1]). Hence, the SRN Self-module can be considered as “the foundation upon which our ‘autobiographical’, ‘narrative’ and ‘social’ selves (represented by both posterior OMs) are built” (p. 47 in Ref. [87]); thus, it represents some kind of a hub that integrates motivational, emotional, sensory, motor, and mnemonic information, serving as the ‘observing self’ to maintain any conscious state regardless of the precise thought content. Interestingly, the central role of Self-module of SRN as a prognostic marker of self-consciousness recovery in patients with DoC was also demonstrated [87]: it was shown that those VS/UWS patients who recovered stable minimal or full self-consciousness later (up to six years post-injury) in the course of the disease exhibited stronger Self OM functional integrity (among three SRN OMs) already at an earlier stage (three months post-injury) compared to the patients who continued to stay in the persistent VS/UWS condition (Fig. 1C).
The nature of the dynamical interactions among the three OMs and the corresponding three phenomenological aspects of Selfhood was examined in a six-year longitudinal study of a single patient’s self-consciousness recovery (from a MCS to full self-consciousness and almost complete functional independence) after the extremely severe traumatic brain injury [58]. The analysis revealed (Fig. 2) a gradual restoration of the functional integrity within each of the three OMs of the brain SRN that are responsible for first-person agency (or “Self”), representational-emotional agency (or “Me”), and reflective agency (or “I”). Such mutual dynamics in the Self-Me-I components and corresponding to them OMs were (i) not monotonous and rather unique for each component/OM (some recovered quicker than others), and (ii) closely paralleled by and significantly correlated with findings from clinical examinations and tests, such as the Level of Cognitive Functioning scale (LCF) and Functional Independence Measure (FIM) (Fig. 2) [58, 88].
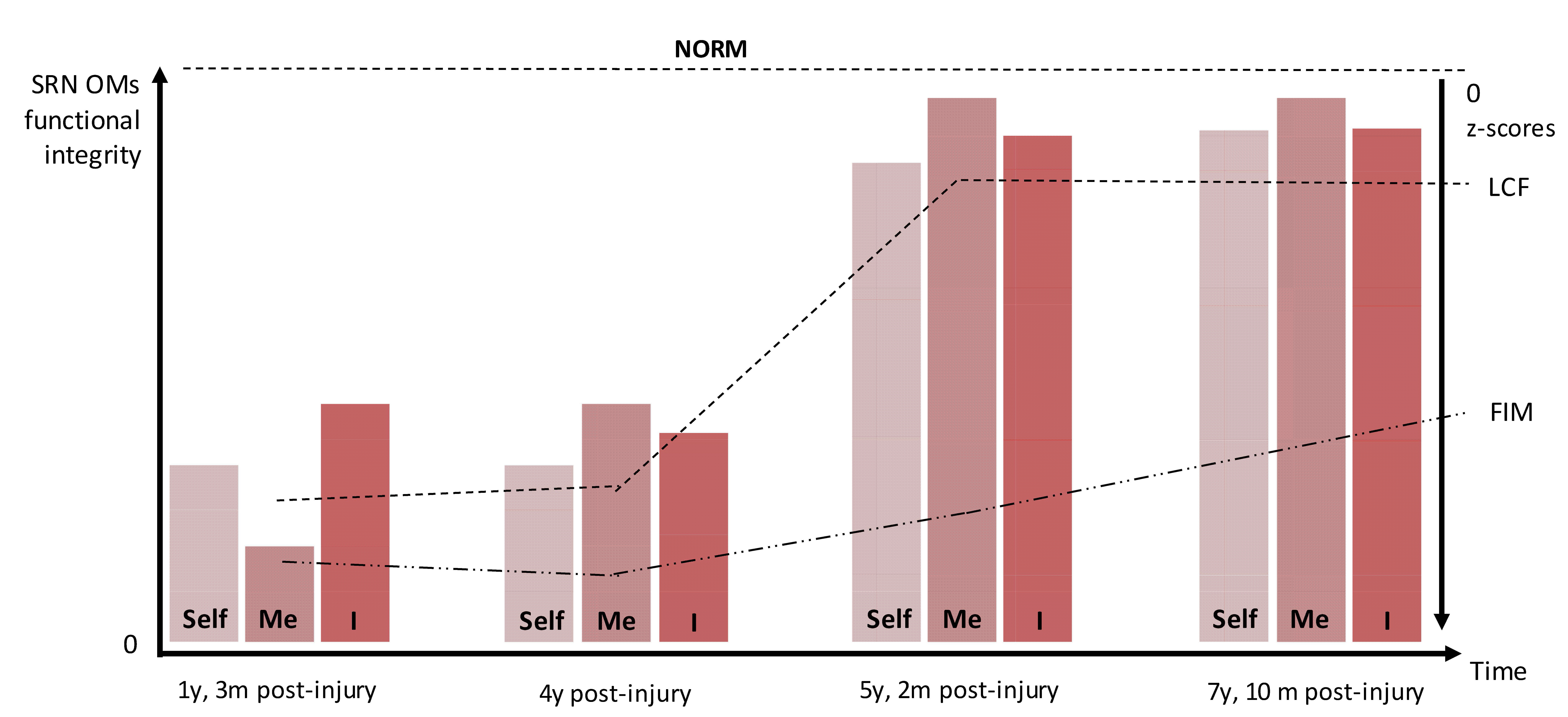 Fig. 2.
Fig. 2.A schematic illustration of the dynamics of functional integrety within three operational modules (OMs: Self, Me, I) of the brain self-referential network (SRN) and clinical assesments in the patient during 6 years of observation (For detailed results and discussion see [58]). NORM, healthy fully self-consious subjects; LCF, Level of Cognitive Functioning scale; FIM, Functional Independece Measure; y, year; m, month.
Summarising, this limited data establishes the potential basis for a neurophenomenological [60, 61, 62, 63] framework of further efforts to prospectively and longitudinally characterize dynamics in both brain SRN OMs functional integrity and their corresponding functions within Selfhood across a wide range of DoC states. Studies of ongoing recovery after brain injury utilizing knowledge about every separate aspect of complex Selfhood will likely also assist in the development of more targeted and thus more effective rehabilitation programs for such patients (see Ref. [89] for an example) and also monitor evolution over time in those patients who have recovered [58], with the goal of improving their well-being [88, 89]. Indeed, as previously discussed [90], recovered self-awareness appears to be a critical first step toward full consciousness and cognition recovery.
Our hope is that by contemplating the issues related to Selfhood in patients with DoC, researchers, clinicians and theorists alike will begin to grasp what it is like to be in such states from the first-person perspective and to appreciate the patients’ subjective realities even without being able to fully understand them. Given the critical importance of major ethical decisions (i.e., in particular, withdrawal of life-sustaining therapy) that are often made while dealing with patients in DoC, such patients would benefit from the brain assessment aiming to evaluate the level of functional integrity of SRN and its OMs, and thus infer which patients are at least minimally self-aware and which aspects of Selfhood dominate, regardless of whether these patients do not exhibit self-reflective abilities on additional behavioral/instrumental tests. Such an approach would be in agreement with the most recent (2020) guidelines of the European Academy of Neurology for the evaluation of levels of consciousness in patients with DoC [34] and also with the “practice guidance” recommendations developed by a joint effort of the American Academy of Neurology, the American Congress of Rehabilitation Medicine and the National Institute on Disability, Independent Living, and Rehabilitation Research [91]. Both of them recommend inclusion of neuroimaging-based measures in the assessment protocol of patients with DoC and characterisation of a patient with the highest level of consciousness revealed by any of the assessment measures involved during the multimodal evaluation process [84].
DoC, disorders of consciousness; FIM, Functional Independence Measure; LCF, Level of Cognitive Functioning scale; MCS, minimally conscious state; MRI, magnetic resonance imaging; OA, operational architectonics; OM, operational module; qEEG, quantitative electroencephalogram; SRN, self-referential network; UWS, unresponsive wakefulness syndrome; VS, vegetative state.
AnAF formulated the idea of the article. AlAF provided discussion on the topic and prepared all figures. Both authors researched references, wrote, reviewed/edited the manuscript. Both authors contributed to editorial changes in the manuscript. Both authors read and approved the final manuscript.
Not applicable.
The authors thank D. Skarin for English editing.
This research received no external funding.
The authors declare no conflict of interest. An.A.F and Al.A.F are the scientific co-founders of BM-Science that is involved in fundamental and applied neuroscience research, development of EEG-based brain analyses and well-being applications. Both authors hold senior researcher positions at BM-Science.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
