†These authors contributed equally.
Academic Editors: Nicola Montemurro, Andrea Stoccoro and Giovanni Grasso
Background: To investigate the predictive accuracy of three-dimension
(3D) time-of-flight (TOF) MR angiography (MRA) and 3D Fast Imaging Employing
Steady-state Acquisition (FIESTA) techniques in assessing neurovascular
compression (NVC) with specific vessels in patients with primary trigeminal
neuralgia (TN). Methods: Patients with single-site primary TN undergoing
microvascular decompression (MVD) were retrospectively recruited. All patients
had available preoperative magnetic resonance imaging (MRI) scans. A quantitative
NVC scoring system was applied to assess the severity of NVC on MRI. The
radiological findings were correlated with the intraoperative result to determine
the diagnostic accuracy of MRI techniques. Besides, the radiological indicator of
MVD was determined. Results: Seventy-three TN patients were recruited.
Thirty-three patient had bilateral NVC but with unilateral neuralgia. The average
NVC score of the asymptomatic side was significantly lower than that of the
symptomatic side (1.6 vs. 6.7; p
Trigeminal neuralgia (TN) is an exemplary condition of neuropathic facial pain, characterized as short-lasting episodes of unilateral electric shock-like pain with abrupt onset and termination [1, 2]. Patients usually have characteristic paroxysmal pain and concomitant continuous pain in 50% of patients [2].It has affected 4.3–27 per 100,000 people per year worldwide with high prevalence among women and increased with age [3]. In terms of pathophysiology, TN have two different clinical entities, primary or secondary TN, which is caused by other neurological diseases, such as multiple sclerosis and cerebral tumors [3].
Approximately 30%–70% of primary TN patients are found to have compressed trigeminal nerves caused by surrounding vessels in the cerebellopontine angle cistern, which is well-known as neurovascular compression (NVC) [4, 5, 6]. Microvascular decompression (MVD) is the common and effective surgical procedure to relieve NVC-related pain by decompressing NVC [7, 8]. MVD is often offered to patients if their pain is not sufficiently released medically or if the medical treatment is poorly tolerated, which should be informed at an early stage [9]. However, NVC can also often occur in subjects without clinical relevance and not causing symptoms [10, 11, 12, 13]. Therefore, the correlation of NVC and clinical-significant TN is important regard making the surgical decision of MVD.
Previous studies have reported that the degree and location with respect to the root entry zone (REZ) of NVC were associated with TN and surgical outcomes of MVD [8, 13, 14, 15, 16]. Thus, the preoperative assessment of NVC is more important beyond its existence only. The anatomical trigeminal nerve change (indentation, displacement, or distortion), NVC at REZ and NVC at distal area are all correlated with TN [15, 17]. Furthermore, other research group has argued that only severe NVC with arteries at REZ can cause TN [18]. Taken together, the relationship of degree and location of NVC to TN has been unaddressed.
Therefore, this study aims to investigate the association of NVC severity and TN by introducing a quantitative assessment system [19] and establish the radiological indicator of MVD. Furthermore, the diagnostic accuracy of MRI correlated to surgical findings will be determined. High-resolution three-dimensional (3D) time-of-flight (TOF) MR angiography (MRA) and 3D Fast Imaging Employing Steady-state Acquisition (FIESTA) with the superb advantages in depicting the anatomical structures of nerves and vessels [20, 21] were performed to assess NVC in this study.
Consecutive patients who underwent MVD for single-site primary typical TN at Huzhou Central Hospital and Sir Run Run Shaw Hospital (SRRSH) from January 2018 to December 2020 were retrospectively included into the study. TN was assessed and diagnosed by specialized neurologists based on the International Classification of Headache Disorders [22]. As part of the TN work-up, all patients did MRI scans within one week before surgery to exclude secondary TN and assess NVC. All images were retrieved and reassessed by well-trained neuroradiologists who were blinded to the side of the pain by utilizing a quantitative NVC scoring system [19]. Bilateral imaging findings were linked to clinical symptoms and surgical results to evaluate the predictive value of MRI in assessing TN and detecting NVC confirmed by surgery.
Patients at Huzhou Central Hospital underwent a preoperative MRI exam by using a
3.0-T MR scanner Discovery 750 (General Electric, Boston MA, USA), and patients at Sir Run Run Shaw Hospital
were scanned by 1.5-T GE Signa Excite and GE 3.0-T Signa HD (General Electric, Boston MA, USA). 3D-FIESTA and 3D-TOF
were acquired with the following parameters: time of repeat (TR) 5.0 ms, time of
echo (TE) 2.3 ms, matrix size 256
The cross-sectional image was analyzed at GE advanced workstation version 4.6 (General Electric, Boston MA, USA). 3D-TOF MRA sequences were reconstructed to get the coronal and oblique sagittal images by performing the Reformat module with a slice thickness of 1.0 mm. The relationship between the vessel and the trigeminal nerve was then evaluated in three different planes. 3D maximal intensity projection (MIP) was used to reconstruct TOF images to assess the course and origin of the conflict vessel. Two neuroradiologists with ten-year working experience who were blinded to the side of pain and surgical results reassessed NVC by using a NVC scoring system was adapted from Chen et al.’s [19] study. The location of NVC was measured as the shortest distance between the trigger point to REZ.
The system has four degrees of neurovascular contact assigned with score 0–3. Score 0 refers no relationship between the nerve and the vessel, or the relationship is difficult to be evaluated. Score 1 is marked by vessel crossing or touching the nerve without any root deformity and celebrospinal fluid. A significant indentation on the trigeminal nerve root caused by the compression of the offending vessel could be defined as Score 2. An existence of a distortion and/or a displacement of the compressed root, compared with the asymptomatic side should be identified as Score 3, the most severe one. Besides, the overall NVC score is calculated in the sum of three scores assessed separately from axial, oblique sagittal, and coronal planes. The assessment illustration is seen in Fig. 1.
 Fig. 1.
Fig. 1.NVC score assessment illustration. Upper panel: 3D-FIESTA with axial (A), oblique sagittal (B), and coronal (C) images of a 68-year-old female: the NVC score of (A), (B), and (C) were 1, 1 and 1, respectively. The overall NVC score of this patient was 3. Lowe panel: 3D-FIESTA with axial (D), oblique sagittal (E), and coronal (F) images of a 60-year-old female: the NVC score of (A), (B), and (C) were 3, 3 and 3, respectively. The overall NVC score of this patient was 9; yellow arrow: trigeminal nerve; red arrow: offending vessel (NVC, neurovascular compression; FIESTA, Fast Imaging Employing Steady-state Acquisition).
Patients with symptomatic TN and MRI-confirmed NVC were referred to undergo MVD. Patients who were suitable for general anesthesia and posterior cranial fossa exploration were proceeded with MVD. A retrosigmoid incision was performed in all patients. The arachnoid was cleared to expose the cerebellopontine angle and its containing structures. After opening the cistern and then draining the cerebrospinal fluid, the trigeminal nerve was exposed. The surrounding arachnoid was cleared under the endoscope to visualize the offending vessels, which were separated from the nerve with a folded Teflon patch afterward. The nerves without any NVC were confirmed before closing the dura mater. The drainage tube was inserted if necessary. Patients were followed up in one month, three months and six months after surgery.
The data was presented as mean
Seventy-three patients with single-side typical TN undergoing MVD from two
hospitals were enrolled into the study, and 32.9% (24/73) were males. The mean
age of this cohort was 60
| Characteristic | (N = 73) | Value |
| Age at surgery, yrs | 60 | |
| Gender, n (%) | ||
| Male | 24 (32.9%) | |
| Female | 49 (61.7%) | |
| Side of symptom, n (%) | ||
| Left | 34 (46.6%) | |
| Right | 39 (53.4%) | |
| Distribution of pain, n (%) | ||
| V1 only | 2 (2.7%) | |
| V2 only | 36 (49.3%) | |
| V3 only | 12 (16.4%) | |
| V1 and V2 | 5 (6.8%) | |
| V2 and V3 | 16 (21.9%) | |
| V1–V3 | 2 (2.7%) | |
| Preoperative duration of symptom, yrs | 4.8 | |
| Previous medication treatment, n (%) | ||
| Carbamazepine | 59 (80.8%) | |
| Phenytoin | 3 (4.1%) | |
| Gabapentin | 9 (12.3%) | |
| Oxcarbazepine | 13 (17.8%) | |
| Tramadol | 1 (1.4%) | |
| Previous treatment, n (%) | ||
| Block | 3 (4.1%) | |
| Radiofrequency ablation | 6 (8.2%) | |
| Endotoxin injection | 2 (2.7%) | |
| Acupuncture | 4 (5.5%) | |
Sixty-four MRI cases were performed at Sir Run Run Shaw Hospital (1.5T: 45 cases
and 3.0T: 19 cases), and nine cases were recruited from Huzhou Central Hospital.
The intra-observer and inter-observer variability of NVC measurement for the
symptomatic side showed strong agreement with ICC of 0.95 (p
There were 33 patients having bilateral MRI NVC but with unilateral neuralgia.
The conflicting vessels were superior cerebellar artery (SCA), petrosal vein
(PV), and SCA + PV, accounting for 57.6%, 33.3% and 9.1%, respectively. For
the asymptomatic side, the average NVC score was significantly lower than that of
the symptomatic side (1.6 vs. 6.7; p
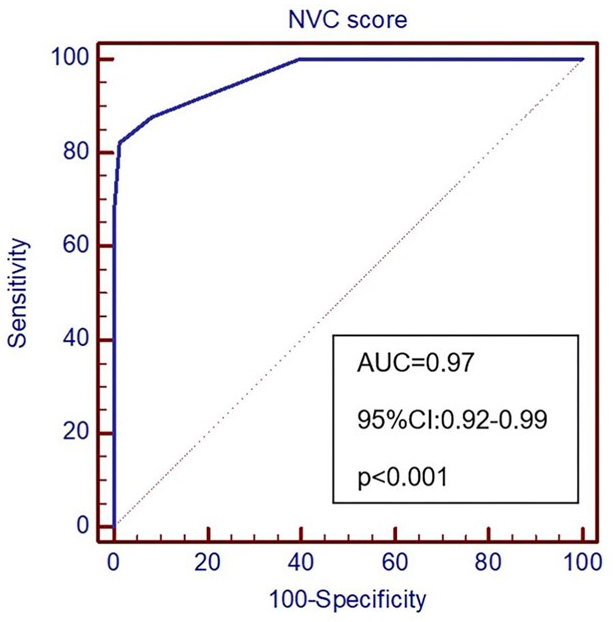 Fig. 2.
Fig. 2.ROC curve of TN prediction. NVC, Neurovascular Compression; AUC, Area Under Curve.
| Cut-off value of NVC score | Sensitivity (95% CI) | Specificity (95% CI) |
| 100.0 (95.1–100.0) | 60.3 (48.1–71.5) | |
| 87.7 (77.9–94.2) | 91.8 (83.0–96.9) | |
| 82.2 (71.5–90.2) | 98.6 (92.6–100.0) | |
| 67.1 (55.1–77.7) | (95.1–100.0) | |
| AUC, Area under curve; 95% CI, 95% confidence interval; NVC, Neurovascular compression. * refers to the optimal cut-off value in predicting trifacial neuralgia. | ||
The mean distance to REZ of the symptomatic side was 1.04
| NVC distance to REZ | Symptomatic side (N) | Asymptomatic side (N) |
| 47 | 1 | |
| 1–2 mm | 7 | 2 |
| 2–3 mm | 5 | 4 |
| 3–4 mm | 3 | 3 |
| 4–5 mm | 4 | 4 |
| 3 | 19 | |
| NVC, Neurovascular compression; REZ, Root Entry Zone. | ||
For surgical results, 72 of 73 patients had a clear-cut NVC involving at least one of the neighbouring vessels. 68.5% of patients had a single conflicting vessel, and superior cerebellar artery (SCA) was the predominate vessel (46.6%). More than a third of patients had two conflicting vessels, and SCA with PV was the most common NVC pattern when involving multiple vessels. At surgery, four patients were found to have unknown venules or arterioles compressing trigeminal nerve, which were undetectable on MRI. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of MRI to detect NVC were 95.8%, 100%, 100% and 25%, respectively. All the patients had pain relieved after surgery and did not have recurrence during follow-up.
The concordance of MRI and surgical findings was further assessed, and the overall K was 0.632, indicating that MRI is a good approach for NVC detection before surgery. For subanalysis regarding vessel numbers, K of single conflicting vessel was 0.663, however, K of multiple vessels significantly decreased to 0.443. The overall surgical and MRI findings of NVC are summarized in Table 4.
| Offending vessel | Surgical findings | MRI findings of symptomatic side | MRI findings of asymptomatic side |
| PICA | 4 (5.5%) | 4 (5.5%) | - |
| SCA | 34 (46.6%) | 32 (43.8%) | 19 (57.6%) |
| AICA | 7 (9.6%) | 7 (9.6%) | - |
| VA | 2 (2.7%) | 1 (1.4%) | - |
| PV | 3 (4.1%) | 3 (4.1%) | 11 (33.3%) |
| AICA + PV | - | 1 (1.4%) | - |
| SCA + PV | 14 (19.2%) | 12 (16.4%) | 3 (9.1%) |
| SCA + AICA | 2 (2.7%) | 4 (5.5%) | - |
| SCA + AICA + PV | - | 1 (1.4%) | - |
| SCA + PICA | 1 (1.4%) | 1 (1.4%) | - |
| PICA + AICA | 1 (1.4%) | - | - |
| VA + AICA | - | 1 (1.4%) | - |
| Persistent trigeminal artery | - | 2 (2.7%) | - |
| Unknown venules | 2 (2.7%) | - | - |
| Unknown arteriole + PV | 2 (2.7%) | - | - |
| No NVC | 1 (1.4%) | 4 (5.5%) | - |
| NVC, Neurovascular compression; MRI, Magnetic Resonace Imaing; PICA, Posterior Inferior Cerebellar Artery; SCA, Superior Cerebellar Artery; AICA, Anterior Inferior Cerebellar Artery; VA, Vertebral Artery; PV, Petrosal Vein. | |||
This retrospective study assessed the relationship of NVC severity and location
to primary TN by applying 3D TOF MRA and 3D FIESTA on a cohort of 73 patients in
a quantitative manner. The main findings were summarized as follows: (1) NVC was
a frequent finding on the asymptomatic side, accounting for 45.3%. (2) The
overall NVC score
NVC without morphological changes—a simple contact commonly occurred in patients with facial pain or even without causing symptoms [4, 18, 23], which is considered as a normal neuroanatomic variant [3, 9]. However, these patients may have the tendency to develop into classical paroxysmal pain, which indicates the importance of follow-up for asymptomatic patients with observed NVC. To answer the question that when neurovascular contact turns into a conflict, several studies have reported the significant TN predictors, including thinning nerve, distortion of the course of nerve, and nerve indentation or displacement [4, 24].
The results from our study show the agreement with these studies but in a
quantitative manner. The NVC score of the symptomatic side was significantly
higher than that of asymptomatic side (6.7 vs. 1.6; p
NVC severity assessment, particularly morphological changes, is also important
for TN patients who are referred for MVD. Preoperative NVC assessment may help
identify patients who could obtain more benefits from MVD. Previous studies have
found that the severity degree of NVC was significantly associated with surgical
outcomes [25, 26]. NVC with morphological changes on preoperative MRI images,
where distortion, dislocation, and distension were observed, may predict the
excellent outcomes [27, 28]. However, the assessment methods are not well
standardized. Some studies only assessed dichotomously-with or without NVC [29, 30]. T. Satoh et al. [13] classified the NVC severity into four grades
according to the extent of the nerve circumference in contact with the vessel.
NVC with the vessel contacting the trigeminal nerve covering
More recent studies have further developed the NVC evaluation system, particularly focusing on the morphological changes of the trigeminal nerve. Tone Bruvik Heinskou’s group has graded the NVC contact as simple contact, displacement (displacement or distortion), and atrophy with a reduced volume of the nerve [27]. The scoring system used in the current study also included the morphological changes. Although it has been reported before [19], we have adapted by taking the sum of NVC values from axial, oblique, and oblique sagittal planes to achieve more comprehensive and detailed morphological assessment of NVC. The prognostic value of this updated 3D NVC scoring system should be investigated in the future study.
The study also evaluated the diagnostic value of 3D TOF MRA and FIESTA in detecting NVC correlated with surgical findings. The combined use of two sequences offers a more promising diagnose of NVC with sensitivity of 95.8% and specificity of 100% than most of other studies. Based on different imaging protocols by using only 3D TOF MRA [30, 31, 32], and a combination of 3D T2-weighted and TOF MRA [13, 29, 33], or 3D T2-weighted, 3D TOF MRA and 3D T1-Gad [34, 35], the sensitivity and the specificity varied, respectively, from 67% to 100% and from 50% to 100% [10]. Our study supports the use of a combination of 3D T2-weighted and TOF MRA in the evaluation of NVC in patients with primary TN, especially if gadolinium is contraindicated for patients.
Although many studies have assessed the overall diagnostic capacity of MRI, the vessel-level diagnostic accuracy has been less investigated. This study further envaulted the consistency of MRI and surgical findings measured by Cohen’s kappa, and K value was higher than 0.6 suggesting a good reliability of MRI to predict origins of offending vessels. The exact origins (i.e., SCA, AICA, PV) were accurately predicted in 72.6% of patients, and the misdiagnose was mainly due to multiple offending vessels or unknown small vessels. The K value of multiple-vessel prediction was only 0.4 shown in the present study. Brînzeu et al. [34] also demonstrated the diagnostic accuracy of MRI in predicting offending vessels showing similar overall K value. The present study differs in that subanalysis of multiple vessels were performed, whereas the study by Brînzeu et al. [34] investigated the predictive value per single artery or vein [34]. The prediction of a single offending vessel was more accurate in Andrei’s group (K: 0.8 vs. 0.6), which may attribute to the difference between imaging protocols. Although MRI with 3D T2-weighted and TOF MRA sequences shows an overall good ability to predict specific vessels, the prediction of multiple vessels or small vessels needs to be improved by applying more advanced imaging techniques.
This study has several limitations. First, it was a retrospective study and the clinical data retrieving work had the deficiency. Second, patients were recruited from two centres using different MRI scanners which may bias the results. However, the intra-observer and inter-observer variability was performed in the present study showing a good agreement regarding NVC measurements. Thirdly, the site of NVC along the nerve and compression at the trigeminal root entry zone were not assessed in the present study. Fourthly, the study may need a more reliable control group for analysis such as pain-free subjects rather than patients with single pain-free side. Subjects with NVC not causing any-site TN with MRI findings should be analysed in the future prospective study. Lastly, the sample size of this study was relatively small, and the finding is warranted to be confirmed in a larger-scale prospective study in the future. Furthermore, the more detailed prognostic value of the adapted NVC scoring system is worthy of investigation if linking to surgical MVD outcomes of patients with primary TN.
A combination of 3D TOF MRA and FIESTA offers an excellent diagnostic
performance of NVC in patients with primary TN and shows an overall good ability
to predict specific offending vessels. NVC score
JS, MH and WZ designed the research study. MH and WZ performed the research and analyzed data. WS and HZ provided help and advice on data analysis. MH and WZ wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The study has been approved by local ethical Institution Review Board (IRB) of Huzhou Central Hospital and Sir Run Run Shaw Hospital with the IRB number (20181108-01; Huzhou) and (20200423-43; SRRSH) . The patient consent form was waived which was approved by IRB.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research was funded by Science and Technology Bureau Program of Huzhou (No. 2018GYB56).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
