Academic Editor: Rocco Salvatore Calabrò
Migraine is a prevalent heterogeneous neurological disorder, enumerated as the eighth most disabling neurological disorder by the World Health Organization. The growing advancement in technology and investigation of various facets of cerebral metabolism in migraine has shed light to metabolic mechanisms in migraine pathophysiology. A growing number of clinical research postulates migraine as a reaction to oxidative stress levels that go beyond antioxidant capacity or cerebral energy deficiency. This has become an extremely attractive subject area and over the past years there has also been a sustained research activity in using ketone bodies (KB) as a novel potential migraine prophylaxis. Not much epidemiological research has been conducted to exhibit the efficacy of ketone bodies in abnormal metabolism in migraine pathophysiology. Therefore, a better understanding of ketone bodies in metabolic migraine may provide novel therapeutic opportunities. The goal of this review is to assess present understanding on potential migraine triggers, as well as how ketogenic interventions support metabolic disability in migraines and address the therapeutic importance of ketones in migraine treatment, accenting clinical studies (including neuroimaging and therapeutic studies). This review is intended to demonstrate existing literature on the effects of ketone bodies on metabolic migraine traits to guide the readership through current concepts and foster a perspective for future research.
Headache affects a large population in the world and the estimated prevalence among adults of current headache disorder is about 50% [1]. This makes headache disorders become the most prevalent neurological conditions, yet a minority of people with headache disorders is diagnosed appropriately by health-care provider [1]. The impact of headache disorders on society is still an underrepresented public health issue, yet it is an exponentially fast-growing research subject because of the globally increasing disability rate caused by headache [2]. Nevertheless, among those estimated 50% of the adults aged 18–65 years in the whole world population, who have experienced headache in the last year, over 30% have reported migraine [1]. Migraine is a common and debilitating primary headache disorder and is regarded as the second most common neurological disorder, that diminishes quality of life [3]. It is characterized by mostly unilateral throbbing head pain accompanied with neurological symptoms including hypersensitivity to light, sound and smell, nausea, and a variety of autonomic, cognitive, emotional, and motor disturbances [4]. An estimate of 17% of woman, 8% of men in Europe and
Migraine evolves through the interplay of a genetically predisposed human in a suboptimal environment, hence considered as a multigenic and multifactorial disease [1]. According to IHS [1], the features of migraine are recurrent moderate to severe, typically throbbing, and unilateral headache attacks that generally last between 4 to 72 hours. These burdensome headache attacks are aggravated by any kind of physical activity and accompanied by either photo-, phono-, or osmophobia, nausea or a combination of these. Migraines occur usually with neurological symptoms during a premonitory phase, which precedes the headache by up to 12 hours and a postdrome phase, which is followed by the dragging migraine headache and can last hours or days [7].
Headache carries a history of nearly 600 years and the present conception of migraine was recognized at the very start of the 17th century [13]. In the early days, migraine used to be seen as a solely vascular disorder, in view with vasodilation as the largest cause to migraine headache [7]. Nevertheless, this assumption has meanwhile been refuted [7]. Today it is believed to be a neurogenic and possibly even a neuro-metabolic disorder [10]. The metabolic aspects of migraine were considered only relatively recently [1]. The activation and sensitization of the trigeminal pain pathway is understood as the current origin of the migraine headache, where afferents tightly innervate the meninges and its associated blood vessels [14, 15, 16]. The brain stem and diencephalic nuclei have been observed to control the trigeminovascular system, which involves efferent neurons providing vascular networks and afferent neurons that in turn send information to the trigeminal nucleus caudalis [17, 18, 19]. Given that, headache is considered as vasodilation and meningeal inflammation caused by the activation of these networks [13]. The exact process of how and where exactly it initiates is still unknown [12]. Clinically, the process can be subdivided in four stages: Premonitory, Aura, Headache and Post-dromal. These phases usually occur in sequences, but may overlap also [12]. Migraine occurs in women three times more often than in men, especially during the reproductive years of women [20]. The cause of this gender difference may be linked to the different sexual hormones produced in male and females, such as progesterone and estrogens that play an essential role in the disease [21]. For instance, migraines can be triggered during fluctuations in estrogen levels, and thus higher incidences of migraines can be observed around the time of menses, perimenopause and menopause [20]. Moreover, hormonal headaches during pregnancy seem to happen less often, but increase after delivery. Therefore, hormone replacement therapy and/or oral contraceptives may lead to migraine attacks [20]. Nevertheless, the significant gender difference in frequency, prevalence, and intensity of migraine cannot be explained by hormonal causes only, and thus other biological and behavioural differences should be taken into consideration [20].
Nutrition has changed greatly during the past 10,000 years and furthermore, the agricultural revolution guaranteed the existence of one macronutrient up until the present day: carbohydrate [22]. One study demonstrated that based on genotype, especially mitochondrial (mt) DNA-haplotype, certain diets consisting of excessive carbohydrates, may cause a decreased mitochondrial function and higher levels of oxidative stress [23]. Hence depending on the specific mitochondrial DNA (mtDNA) variations one has, a person may metabolise carbohydrate differently, which could affect a variety of diseases such as migraine [23]. Interestingly, in the past, the correlation between metabolism and migraine has been examined before where migraine was referred as a “hypoglycaemic headache” [24]. Nevertheless, most studies in migraine focused on its vascular origin until Willem Amery restored the concept again how metabolism plays a role in the pathogenesis of migraine [25].
Despite migraine being known as a very heterogeneous disorder, two major subtypes of migraine are differentiated: (1) migraine without aura and (2) migraine with aura.
As formulated in the International Classification of Headache Disorders (ICHD-3), migraine without aura (MO), also known as common migraine or hemicrania simplex, is a recurrent headache disorder manifesting in attacks lasting 4 to 72 hours [1]. At least five attacks are required to distinguish a MO from symptomatic migraine-like attacks. The typical characteristics of this headache are unilateral location, moderate or severe intensity, pulsating quality, aggravation by routine physical activity, and association with nausea and/or phonophobia and photophobia [1]. Bilateral migraine headache can be found more often in children and adolescent aged under 18 years than in adults. Unilateral pain generally develops in late adolescence or early adult life.
According to ICHD-3 [1], one third of migraineurs suffer from migraine with aura (MA). MA is usually known by the term “classic migraine”. MA is a syndrome that is featured by recurrent attacks of reversible focal neurological symptoms, such as visual, sensory, speech and language, motor, brainstem, and retinal symptoms, that generally develop gradually over 5 to 20 minutes and last for less than 60 minutes. Sharing the same criteria as MO, it is followed by headache [1]. Many migraine patients who suffer from MA also experience attacks without aura [1]. Visual aura is considered as the most frequent type of aura, arising in over 90% of migraine patients with MA.
This symptom frequently is observed as a fortification spectrum, such as a zigzag pattern close to the fixation point that continuously spreads from right to left and undertakes a convex shape with an angular sparkling edge, remaining absolute or inconstant degrees of relative scotoma in its wake [1]. In youth, less characteristic bilateral visual symptoms happen that can indicate an aura. A visual aura rating scale with high specificity and sensitivity has already been established and validated [26]. Sensory disturbances are the next common type of aura, arising in the shape of pins and needles moving slowly from the point of origin and influencing a significant part of one side of the body or face. In its wake, patients might experience numbness [1]. Speech disturbances, mostly aphasic, appear less frequent among the migraine patients. Interestingly, if multiple aura symptoms exist, they happen in succession such as starting with visual, then sensory and finally aphasic. Nonetheless, the reverse and different orders have been recorded as well. Most aura symptoms last around one hour, but motor symptoms often last longer [1].
Currently, it is still discussed whether MA and MO belong to the same spectrum of illness or are two distinct disorders [26]. An overview of the diagnosis of MA and MO is given in Table 1 (Ref. [1, 13]) based on the standardized headache identification tool by the International Classification of Headache Disorders (ICHD). It is worth mentioning, that the diagnosis of headache disorders is established mainly on the clinical manifestations [13].
| Migraine without aura | Migraine with aura |
| -Minimum of five headaches within 4–72 h | -Minimum of five headaches, among which at least two episodes must be accompanied by an aura |
| -Pulsation | -The headache should begin with, or be within 60 min of, the aura |
| -Unilateral location | The aura must consist of |
| -reversible dysphasic speech | |
| -Intense pain | -unilateral sensory |
| -homonymous visual symptoms | |
| -Exacerbation of headache with routine activities | |
|
|
|
| Adapted from [13]. | |
When trying to understand the pathogenesis of migraines, looking at trigger factors seems very helpful [9]. Therefore, it has been suggested that modifications of lifestyle might prevent migraine, which in turn would reduce the burden to migraine patients, and health-related costs [27]. Dieting and fasting, exercising and other physical activities, stress level and coping skills, health-related behaviours, sleeping and resting habits, smoking and drinking, medications and drugs are all part of lifestyle factors [27]. A study with 1207 patients has shown that a migraine attack is often associated with different internal and external triggers, for instance: hormonal imbalance, sensory overload, stress, sleep disorders and skipping meals [28]. Furthermore, a systematic review conducted by Peroutka [29] showed that migraine triggers such as weather changes, dehydration, physical exercise (including sexual activity), and alcohol are also very common [10]. The distinction between trigger factors and premonitory symptoms of migraine attacks is not always evident, as some premonitory symptoms might be misunderstood [30]. Examples for premonitory symptoms are fatigue or the consumption of calorie dense foods like chocolate [31]. In addition, these metabolism-related triggers are directly associated to energy homeostasis, but also noteworthy is, that majority of the triggers have a shared denominator, which is oxidative stress [32]. A few common triggers in correlation to oxidative stress will be addressed in detail in the following paragraphs.
Some researchers believe that migraine is primarily a disorder of sensory processing depending on how migraineurs react to stimuli [18]. However, next to the premonitory symptoms, visual, auditory and olfactory triggers are also very well-known migraine triggers [33]. Moreover, intense stimulation is also known to be associated with higher oxidative stress [34]. Although, the direction of whether sensory trigger factors happen to be the attack generators or arising from stimulus hypersensitivity on account of the premonitory phase stays inconclusive. Few good examples from our daily lives like cigarette smoke, perfume or odorant inhalation have been proved to intensify markers of oxidative stress in healthy subjects after exposure [35, 36, 37]. While a large percentage of the population uses the phone or computer on a daily basis, bright light such as blue light is also a very common migraine trigger and likewise, higher oxidative stress in the retina has been found [38]. Loud noises also appear to increase oxidative stress [39].
While extreme aerobic exercise and physical effort is often considered as a migraine trigger, hence it might also constitute a candidate to experimentally trigger migraine [28, 40], low levels of aerobic exercise, on the other hand can help preventing migraine [41]. Mental stress is also a migraine trigger [42]. Both physical stress and severe psychological stress produce oxidative stress in the central nervous system [43, 44]. Findings have observed how chronic stress in mice can cause damage to the structure of brain mitochondria due to excessive oxidative stress [45], which is also associated with changes in energy metabolism [46].
Circadian disruptions caused by suboptimal sleeping patterns are often a major factor for brain dysfunction as well as affecting other body systems, such as metabolism [47, 48]. A study showed that nurses with different work schedules (day work, two-shift rotation, night work, three-shift rotation) have a greater prevalence of chronic headache, medication overuse headache as well as migraine compared to those who have a regular work schedule [49]. Hence, it can be argued that sleep deprivation uses up metabolic reserves. Other studies have also shown that sleep deprivation causes the glycogen stores in animal models to become drained, whereas on the contrary oxidative stress and inflammation are elevated [50, 51]. Another study conducted in healthy humans has shown how one night of sleep deprivation was already reducing glutathione, cysteine, ATP and homocysteine levels significantly [52]. Together, these results indicate how suboptimal sleep patterns can increase migraine attacks via different metabolic pathways [47, 49, 50, 52].
Fasting as well as skipping meals are not only amongst the most often cited triggers [29, 33, 53, 54], but also a popular way to experimentally evoke migraine attacks in affected patients [9, 55]. In a study conducted by [55], 12 migraine patients fasted for 19 hours and 9 of the patients had a migraine attack. Additionally, an intriguing observation during Ramadan, where Muslims fast every day from dawn to sunset, showed that migraine attacks happened more often [56]. Another experiment on rats found that repeated cerebral hypoglycaemia results in damaged oxidative phosphorylation distinugished by a lower mitochondrial membrane potential and ATP levels [57].
As expected, diet is a core factor for a healthy lifestyle [27]. For this reason, managing an optimal diet plays a significant role in promoting health in the society [27]. On the other hand, because of the complexity of migraine, as a multidimensional disease, and also the difficulty of establishing studies to investigate how dietary factors can influence migraine [58], inconsistency prevails in the literature, ranging from a limited significance of dietary modification for migraine to some promising effects. Dietetic intervention by using very high-fat, low-carbohydrate ketogenic diet has been proposed conceptually in respect to a possible investment to non-pharmaceutical interventions for migraine patients [59]. Moreover, the efficacy of ketogenic diet has been observed within only one week [60]. Nevertheless, it should be considered that improvements might happen later in some patients, and if there are no response to the ketogenic diet within 3 months, a higher ketogenic ratio should be taken into account [60]. Given that, about 50% of the patients who sustain a low-carbohydrate ketogenic diet for over 12 months, benefit from its efficacy persistence even after stopping [60]. Clinical studies [61, 62] in which migraine patients had to report their common headache instigators have shed some light on the prevalence of dietary triggers. The reported dietary triggers were caffeine (14%), alcohol (29% to 35%), and monosodium glutamate (MSG) (12%). Especially high amount of MSG are known to be migraine triggers [13]. A common solution to this issue is an elimination diet [13], by discovering dietetic ingredients that possibly trigger migraines and then eliminating these specific ingredients from one’s usual diet [13]. This personalized approach constitutes a step in the right direction considering the drastic change in food consumption that may trigger headache [13]. It is also worth remarking that migraine triggers vary from person to person. Foods that trigger migraines vary among people with different immunological responses [63]. It is therefore worth stressing the degree of effort to determine specific foods that could cause migraine. On top of all, genetic factors should also be considered as predisposing factors. In this scenario, certain individuals are vulnerable to different food ingredients while others are vulnerable to some sort of drinks [10, 13]. For this reason, evading these potential triggers may support to prevent migraine.
Adenosine triphosphate (ATP) is the universal primary carrier of energy in cells, also known as the principal donor of free energy in tissue and is majorly produced in the brain by oxidative phosphorylation of ADP in mitochondria. As shown in Fig. 1 (Ref. [64]), this energy storage molecule is linked to creatine kinase reactions which releases phosphate (Pi) emerging from phosphocreatine (PCr) to ADP to then re-synthesise ATP from ADP in the so-called ATP-PCR system [64]. During metabolic stress, the ATP-PCR system enables rapid mobilization of restricted high-energy phosphates to restore ATP.
 Fig. 1.
Fig. 1.
Demonstration of the ATP-PCR System in Brain of Familial Hemiplegic Migraine (FHM) adapted from [64].
As phosphorus magnetic resonance spectroscopy (P-MRS) offers information about metabolites that play key roles in tissue energy metabolism, including the high-energy phosphates ATP and PCr—a reservoir for ATP generation through the creatine kinase reaction. Hence, P-MRS studies have surprisingly supported theories of mitochondrial dysfunction in migraine [65, 66]. P-MRS can reveal an deterioration of the mitochondrial oxidative phosphorylation in individuals with migraine [10]. In fact, most consistent findings in migraine research were developed with the P-MRS technique suggesting an interictal disturbed brain energy metabolism [65]. PCr content, suggestive of free cellular energy, is significantly lower at rest in the brain of both migraine with and without aura patients [64]. Interestingly, decreased PCr was also observed during migraine attacks in patients with aura [67]. P-MRS findings in migraineurs show a significant disparity between enhanced brain metabolism and reducion in free cellular energy supply, hence hypothesized as a biochemical substrate for headache attack [64]. This arrangement of impaired mitochondrial respiration with low PCr, high Pi and high ADP found in mitochondrial cytopathies indicates how migraine might also possess related aspects of pathology [64, 68]. Additionally, the reduction of brain high-energy phosphates in regular state measurements indicates an asymmetry between ATP generation and ATP usage in migraine patients, and also imply that the brain of a migraineur is threatened to handle certain metabolic stress [65].
As mentioned above, the human brain needs a compelling amount of energy supply for standard functions of the brain and comprises about one fifth of the human body’s entire energy usage at rest, in spite of the fact that it only embodies ~2% of the entire body weight [11, 69]. For this reason, majority of the brain’s energy intake is resulting from glucose oxidation and is for the most part utilized to support synaptic transmission [70, 71]. The brain is especially subjected on energy supplies from the circulation due to restricted glycogen reservoirs and therefore is especially susceptible to their scarcity [10]. Given that a restricted amount of glycogen is available in the brain, a sufficient and uninterrupted reservoir of fuel is a condition for basic cellular functions in the brain [72]. For this reason, different clinical symptomatologies are recorded in pathological states especially during times when brain metabolism is challenged, such as glucose transporter type 1 (GLUT-1) deficiency leading to disabled cerebral glucose uptake, which in turn clinical symptoms may emerge as seizures, movement disturbances, cognitive impairments [73]. Furthermore, it is also worth mentioning that migraine takes part in the clinical characteristics of GLUT-1 [74, 75]. Other than that, hypoglycaemia has been associated with migraine headache for nearly 100 years [10, 55]. Comparisons between migraine-associated symptoms and symptoms of hypoglycaemia report considerably similarities including symptoms such as dizziness, pale skin, cold hands and feet, binge eating and/or sugar cravings, yawning, nausea, low blood pressure, shaking, cognitive difficulties, tiredness, fatigue, visual dysfunction and slurred speech [10, 76]. Interestingly, hypoglycaemia is correlated to age and gender, especially during the productive years of life where various life events require high glucose expenditure [77]. For instance, diabetic women during pregnancy are at a higher risk to develop complications such as experiencing migraines [77]. All of these shared symptoms are suggesting inadequate storage of glucose to the brain or by release of catecholamines due to sympathetic activation [10]. Recent 18-fluorodeoxyglucose positron emission tomography (FDG-PET) studies have become very useful for examining energy dynamics in the brain and showed several areas of altered glucose metabolism in MA and MO [78, 79]. In consequence, there has been an increased interest in exploring the metabolism of glucose in migraine pathology in recent years. FDG-PET imaging exhibits the magnitude of localised cerebral glucose metabolism by using a radiotracer-labelled glucose analogue [64]. Another study observed during interictal periods in episodic migraine patients substantial glucose hypometabolism in various areas associated in central pain processing, in comparison to controls [80]. Considering that, recurrent migraine attacks over time may result in progressive decline in glucose metabolism of central pain processes [80]. In a study where resting cerebral glucose uptake by using 18-fluorodeoxyglucose PET and visual cortical activation with visual evoked potentials were compared to, they were able to show that visual neuronal activation surpassed glucose uptake in visual areas in 90% of patients with interictal migraines without aura, but only in 15% of the healthy controls [78]. Hence, around half of glucose absorbed in the brain goes to the astrocytes, where energy is stored in the central nervous system (CNS). Likewise, findings in functional neuroimaging studies (functional MRI and PET) discovered significant increased metabolism in healthy controls in comparison to migraine patients [78]. These results support the hypothesis that energy supply is significantly lower in migraine patients, meaning that the argument of a disproportion between glucose metabolism and neural activity is supported and could be a keystone of migraine pathophysiology [10, 78]. Also worth mentioning, GLUT1 deficiency syndrome (GLUT1DS) is a distinct metabolic disorder that comes from impaired glucose transport into the central nervous system [81]. Another study suggests that GLUT1DS may also provoke migraines [79].
Before breaking down the biological and therapeutical effects of KBs, it is vital to comprehend the fundamental biological and physiological context of KBs. Ketone body metabolism is an essential matter in physiological homeostasis. Ketogenesis is a metabolic pathway that produces ketone bodies (KBs), which deliver an alternative form of energy for the body [82]. As shown in Fig. 2 (Ref. [12]), ketogenesis demands the attendance of at least three enzymes: (1) mitochondrial Acetoacetyl-CoA thiolase, (2) mitochondrial 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) synthase (HMGCS2), and (3) HMG-CoA lyase, which form the KB, acetoacetate. Given this, KBs are generated as a by-product of the fat metabolism process [83]. The exact synthesis of KBs in the liver will not be further addressed due to the scope of this review. Ketosis can be defined as the metabolic state of increased ketone bodies in the blood (
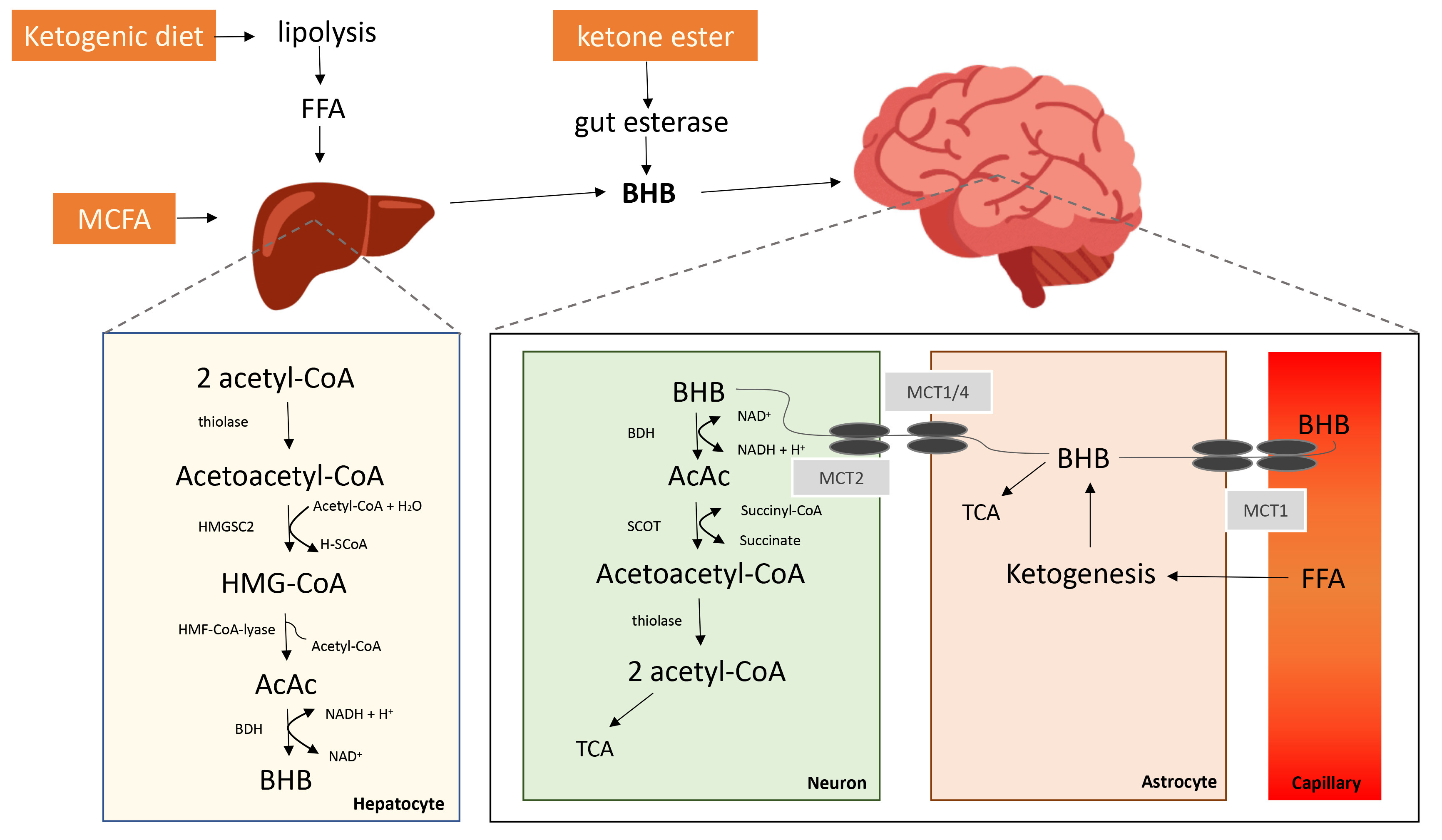 Fig. 2.
Fig. 2.
Potential Ketone Bodies Agents and its Pathways involved in Synthesis and Catabolism (adapted from [12]).
Apart from fasting, dietary ketogenic supplements and ketogenic diets have the potential to enhance the circulatory pool of KBs [12]. In the recent years exogenous
Common migraine triggers are causing an imbalance of oxidative stress levels through unfavourably influencing energy metabolism or mitochondrial functioning [9, 29, 33]. A protective mechanism of KBs can be demonstrated in the improvement of mitochondrial function because oxidation in the brain decreases as ketone body levels increase [12]. Therefore ketone bodies are also used as protective molecules against refractory epilepsy [114]. It has been proven that
While inflammation is a set response with protective tissues against disease, infection, or injury, involvement of neurogenic inflammation in migraine headache stays inconclusive, and not that migraine is not classified as an inflammatory disease. Various studies have demonstrated that pro-inflammatory peptides or a “sterile neurogenic inflammation” can be exhibited in migraine pain [9, 123, 124]. First and foremost, molecules linked with migraine headache both in animal and human studies have shown the involvement of calcitonin gene related peptide (CGRP), nitric oxide (NO), substance P, vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP) [9, 125, 126]. Cytokines are referred to as pain mediators in neurovascular inflammation, and they could have an effect on the generation of migraine pain [125]. Moreover, cytokines are essential mediators of the inflammatory pathway and are associated with migraine pathogenesis [125]. Similarly, observations of several studies hint that peripheral and central levels of cytokines and immunological changes are present in migraineurs [127, 128, 129] though it should be noted that it is still unclear what might be the triggering factor that activates the inflammatory cascade leading to a migraine attack. A potential reasoning would be that a genetic predisposition exists to have an abnormal inflammatory response to exogenous stimuli (such as high-carb diet or other environmental factors) and could represent a trigger for headache attacks [125]. In fact, twin studies and recent findings based on genetic mutations causing hemiplegic familial migraine hint that genetic background play a significant role in migraine pathogenesis [130]. Finally, many studies on migraine patients have focused on peripheral and central levels of cytokines and the role of inflammation in migraine pathogenesis, despite that data are controversial. Having this mentioned, reduction in inflammation can be seen in prolonged fasting and also caloric restrictions [131, 132]. In another combined animal and cellular study using exogenous KBs has discovered the anti-inflammatory protection of the NLRP3 inflammasome [133]. Thus, NLRP3 inflammasome is prevalent in especially immune and inflammatory cells following activation by inflammatory stimuli [134]. Furthermore, it has been shown that in response to urate crystals, ATP and lipotoxic fatty acids, KBs inhibits activation of the NLRP3 inflammasome [133]. Given the findings above, a decrease in inflammation and pain can be recorded in rats who underwent a ketogenic diet [135, 136]. Finally, the capability of underlying mechanisms of KD efficacy to support mitochondrial energy metabolism and counteract neural inflammation was also demonstrated in an observational study on migraine patients [137].
As mentioned above, the brain is exceptionally relied on energy sources from the circulation that can transcend through the blood-brain barrier and is in particular susceptible to their shortage due to its restricted glycogen sources and high energy demands [9]. Further research has shown that intracellular glucose levels are increased in the presence of
Cortical spreading depression (CSD) implies a slowly propagated wave of depolarization of the neurons and glial cells across the cerebral cortex followed by depression of cortical activity and has been suggested as a possible underlying pathophysiological mechanism in migraine aura. There is a strong correlation between metabolic factors and CSD susceptibility. A potential CSD trigger is hypoxia and above all, cerebral glucose availability regulates extrinsically induced CSD in both ways [148, 149, 150, 151]. In addition to that, hypoglycaemia greatly extends CSD length while hyperglycaemia also safeguards the tissue from CSD initiation [138]. During a short- and long-term treatment with a middle chain triglyceride enriched ketogenic diet, similar protective effects against CSD were observed in an experimental animal study, where the brain was provided with another energy substrate to glucose [152].
Rises in ketone body availability to the CNS in humans lead to substantial changes in cerebral fuel metabolism [12]. Further results have demonstrated that in healthy adults, an in vivo infusion of
| Ketogenesis | ATP level | Glucose level | CSD | Oxidative stress | Inflammation | Migraine | |
| Healthy Brain | Occasionally* | Optimal | Optimal | No | No | No | No |
| BHB in Healthy Brain | Yes | Optimal | Optimal | No | No | No | No |
| Migraine Brain | No | Suboptimal | Suboptimal | Yes | Yes | Yes | Yes |
| BHB in Migraine Brain | Yes | Better** | Suboptimal | Less | Less | Less | Less |
| Note: In this figure, an overall comparison of healthy brain and a migraine brain with/or without Beta-Hydroxybutyrate ( * e.g., during fasting/skipping meals. **if enough |
|||||||
Certainly, the aim of intervention for migraine is to diminish the duration and severity of the migraine attack [157]. Other objectives crucial for improvement comprise restoring functioning ability, reducing oxidative stress, increasing antioxidants, and encouraging comprehensive handling with no or little side effects [43, 158]. An interesting finding is, that the most common preventive measure reported for migraine is diet [13]. Diet is a significant element and modifiable aspect of lifestyle, thus dietary behaviours that might act as a trigger for migraine can be strategically identified and implemented to help prevent migraine attacks [159]. More recently, the underlying metabolic and endocrine mechanisms triggering migraine have begun to unfold. Therefore it is of critical importance to consider the benefits from supplying an alternative energy substrate for the human brain with restricted energy stocks [160]. Henceforward, for patients with compromised energy metabolism, an alternative source of fuel for the brain through ketogenic diet and/or use of exogenous ketogenic substances, such as medium-chain triglycerides or exogenous ketone body salts can have beneficial effects and hence supports the role of abnormal metabolism in migraine [137, 161].
“Ketogenic diet” refers to its correlation of increased KBs and acetoacetate (AcAc) in blood and urine due to mimicked fasting by forcing fatty acid breakdown by reducing the intake of carbohydrates [162]. Low carbohydrate diets or ketogenic diets (KD) typically restrict carbohydrates to less than 20 grams per day [96]. Few days into a ketogenic diet, the reserves of glycogen are drained and KBs are generated to maintain energy production within the mitochondria. Immense reduction in migraine frequency (up to 80%) have been noticed after one month on ketogenic diet [137]. Interestingly, ketogenic diets have been primarily utilized to fight obesity in individuals and to treat epilepsy [82, 163, 164]. Furthermore, due to altered tissue excitability in migraine, ketogenic diet might benefit brain metabolism restoration and excitability in migraine pathophysiology [82]. A well-designed study recorded significant improvements through a 3-month ketogenic diet in patients with medication overuse headache [165]. Moreover, ketogenic diet is more advantageous to patients with migraine compared with a low-calorie diet [13]. Furthermore, a low-calorie diet is not recommended for migraine patients [137] and migraine improvement has been reported in those on a ketogenic diet and continued improvement was reported for two months after the ketogenic diet was stopped [102, 166]. In another study where they tried to examine the efficacy of ketogenic diets in migraine patients observed a significant decline in attack frequency as well as the amount of days with headaches during the initial month of ketogenesis [137]. Likewise, additional studies by the same authors reported differences in cortical excitability, and decreased attack incidences and duration after one single month on diet [167]. Further findings also suggest the critical role of KBs particularly in the cerebral cortex, and not subcortically [168]. A key strength of KBs is its independence from GLUT1, as well as ketosis has a variety of other effects that are potentially valuable in understanding migraine pathophysiology, such as increased anti-oxidant capacity, mitochondrial biogenesis, upregulation of GLUT1 and ketone body transporters, increased GABA but inhibition of glutamate transport and, therefore, reduced excitatory synaptic transmission and inflammation [9].
As mentioned above, KBs are a productive alternative energy source for the human brain during low glucose reserves, restoration in brain energy homeostasis is expected after a sufficient elevation in KBs, and therefore carry the ability to attenuate some of the abnormalities in glucose metabolism as well as glucose transport discovered in migraine patients. The role of KBs is especially relevant for the management of hypoglycaemia/hypometabolism, as well as for glucose transport [64]. Thus, KBs play a crucial role in the improvement of cerebral metabolism. On top of all, since KBs have the potential to positively impact other pathways and are considered to be involved in migraine pathophysiology, such as cerebral excitability, oxidative stress, CSD, inflammation mitochondrial functioning, hypoglycaemia/hypometabolism and the gut microbiome, KBs may also be considered as potential signalling molecules [120]. Given this, an elevation of KBs through exogenous nutraceuticals has been hypothesized to possibly impact all of the foregoing migraine pathophysiological mechanisms, and might support a comparatively side-effect free solution for a proportion of the migraine population [9]. For instance, high doses of riboflavin (400 mg/day) may improve mitochondrial metabolism in migraine patients with a mutation in mtDNA [169]. The effects of the
The present review has some limitations. Firstly, due to insufficient amount of literature on using KBs for migraine prevention, more extensive studies are needed to validate its efficacy and effects. Secondly, due to poor methodologies and weak study designs of existing research, including lack of control groups and small sample sizes, further studies with larger population groups are required to investigate the impact of KBs for migraine treatment. Thirdly, no inferences can be made on gender and age regarding metabolic mechanisms in migraine patients due to limited literature, hence future studies should aim to conduct a well-designed and mechanism-driven longitudinal research to investigate this further as outcomes are generally affected by diverse factors such as age, genetics, gender and environmental factors. Fourthly, to our knowledge, there are no existing literature on the impact of diurnal variations on using exogeneous KBs in migraine prevention, hence should be further investigated since disruptions in circadian rhythms cause impairment in metabolism [1]. Given that, there are limitations associated with the variety of how different mechanisms might lead to the migraine phenotype and hence future research should seek to substantiate these concepts and their potential application for patients. In addition, further clinical research is needed to validate the hypothesized pathophysiological effects of KBs in migraine patients. Finally, other prophylactic medications of migraine were not considered in this review which deserves to be further explored and put into comparison. Future studies should also consider applying KBs in migraine prevention for more extended periods. Moreover, further efforts are required to minimize patient burden during a ketogenic diet, and to improve palatability of the diet would also have to be discussed. Regardless of the limited amount of data, the findings delineated in this review have demonstrated the potential therapeutic implications of KBs. This poses an area for future work. Hence, future research carrying out other ketogenic approaches should aim to reinforce the idea of a ketone-based metabolic progress in migraine patients. Having said that, this review has highlighted the possibility of future studies in elucidating particular mechanisms of glucose metabolism impairment in migraine. Despite that, the modulation of this metabolic pathway in migraine demands further exploration. Several dietary treatments have been proposed for migraine prevention and ketogenic diets that result in elevation of KBs, has demonstrated promising contribution in the prevention of migraines [172, 173]. Nonetheless, prospective research is required to confirm this hypothesis. Above all, it has to be remarked that migraine triggers such as poor sleep patterns, stress, hormonal changes, bright lights and diet-related triggers, including caffeine, hypo-hydration and skipping meals, vary from person to person [59]. Given the fact that, there are gender and age differences in the prevalence of migraine and hypoglycaemia, future research should conduct a longitudinal study to investigate these factors further. Considering the spectrum of potential triggers implies further complexity for developing effective intervention strategies. Consequently, future research should consider how intervention strategies can be personalised to the triggers that are specific to individuals. Additionally, recent research has observed that ketosis may have benefits in the treatment of other conditions such as neurodegenerative diseases like Alzheimer’s disease, Parkinson’s disease, multiple sclerosis [12, 39, 174, 175]. Consequently, neurological and neuropsychiatric diseases, for instance, epilepsy, autism, depression and affective disorders, anxiety, and traumatic brain injury might also benefit from a ketosis treatment [176, 177, 178, 179, 180]. Last but not least, ketosis might also open avenues in the treatment of metabolic disorders like cancer, glycogen storage disease, GLUT1 deficiency syndrome [105, 181, 182]. Additionally, more studies researching the effects of ketone bodies on (cerebral) metabolism, oxidative stress, inflammation would be of great interest. Expanding inclusion criteria to also incorporate those with severe headache, not only migraine, should be addressed in future research. Lastly, neuroimaging methods have excellent translational values and can be widely applied in future studies to identify dietary effects on brain functions in humans.
Migraine is a highly complex multifactorial neurological disease that differs in frequency, severity, and its impact on the quality of life. Furthermore, genetic factors must be considered when defining an individual’s susceptibility to migraine. Findings have shown that the migraine pathophysiology emphasizes the aspects of different triggers that may initiate a migraine attack or increase the frequency of the attacks. In this review, metabolic bases of migraine regarding the use of KBs were discussed. With migraine being such a multigenic and complex disease, diverse aspects of the most prevalent migraine triggers were mentioned that have a strong correlation to the pathophysiological mechanisms: (1) sensory trigger, (2) mental stress vs. physical stress, (3) sleep changes, (4) fasting/skipping meals, and (5) dietary factors. While migraine is also a prevalent disease, it is often under-diagnosed. On top of all, it is yet to be perceived adequately by certain migraine patients who are eligible for prophylactic care. In that case, these therapies could increase migraine patients’ quality of life and reduce the physical and functional disabilities of migraine. Furthermore, this work defines migraine as a complex and heterogeneous neurological disease, along with several common genetic polymorphisms and thus abnormal mechanisms resulting in the migraine phenotype. In this review, only two typical and important pathophysiological mechanisms have been discussed in detail and thus, also their possible exploitation through exogenous ketone bodies such as
AcAc, acetoacetate; AcetylCoA, acetyl coenzyme A; BBB, blood–brain barrier; BDH, beta-hydroxybutyrate dehydrogenase; CGRP, calcitonin gene-related peptide; CNS, central nervous system; FDG-PET, 18-fluorodeoxyglucose-PET; FFA, free fatty acids; FHM, familial hemiplegic migraine; GLUT-1, glucose transporter type 1; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; HMGCS2, 3-Hydroxy-3-methylglutaryl-CoA Synthase 2; IHS, International Headache Society; KB, ketone body; KD, ketogenic diet; MA, migraine with aura; MCFA, medium-chain fatty acids; MCT, monocarboxylate transporter; MO, migraine without aura; MRI, magnetic resonance imaging; NO, nitric oxide; PACAP, pituitary adenylate cyclase-activating polypeptide; PCr, phosphocreatine; PET, positron-emission tomography; P-MRS, phosphorus magnetic resonance spectroscopy; SCOT, succinyl-CoA,3-ketoacid Coenzyme A transferase; TCA, tricarboxylic acid cycle; VIP, vasoactive intestinal peptide; YLD, years lived with disability;
PH and PC conceived and designed the review; PH performed the literature analysis and overview; PH and PC discussed the results; PC aided in interpreting the results and worked on the manuscript. PH wrote the paper in consultation with PC; All authors read and approved the final manuscript.
Not applicable.
We would like to thank the reviewers for their helpful comments and efforts towards improving our manuscript.
This research received no external funding.
The authors declare no conflict of interest. PC is serving as one of the Editorial Board members of this journal. We declare that PC had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to RSC.


