Academic Editor: Rafael Franco
Objective: This study aimed to conduct proteomic analysis of the
sphincter in a neurogenic bladder caused by T10 spinal cord injury. The
differentially expressed proteins (DEPs) of the sphincters
(internal urethral sphincter) in the
neurogenic bladders (NBs) of rats after complete transection of the T10 spinal
cord segment were screened using tandem mass tag (TMT)-based quantitative
labeling, and their biological information was analyzed. Methods: Twelve
adult Sprague Dawley rats out of 40 were randomly assigned to the blank group
(n = 12), while the remaining 28 were placed in the T10 spinal cord
injury model via modified Hassan Shaker spinal cord transection; 12 of these rats
were then randomly selected as the model group. The rats in both groups underwent
urodynamics detection and hematoxylin and eosin (H&E) staining. The proteins
expressed in the bladder sphincter were detected using TMT-based quantitative
proteomics. DEPs were defined as proteins with fold change
A neurogenic bladder (NB) is a common complication of spinal cord injury [1]. Bladder dysfunction caused by complete injury of the T10 and above spinal cord segment is often characterized by simultaneous non-inhibitory contraction of the detrusor, bladder sphincter (internal urethral sphincter) and/or external urethral sphincter, resulting in increased internal bladder pressure [2], it manifests as urine retention, which may cause renal reflux and, over time, renal damage, eventually leading to renal failure in severe cases [3].
Previous research has revealed that the bladder sphincter, external urethral sphincter, and urethral mucosa each account for a third of micturition control [4].
The bladder sphincter is located at the bladder neck and is doubly innervated by
sympathetic (T11-L2) and parasympathetic (S2-S4) nerves, which have the function
of controlling urination. Under normal conditions, parasympathetic excitation of
the bladder during urination and inhibition of the sympathetic nerves cause
contraction of the detrusor muscle and diastole of the bladder sphincter,
producing urination. After an injury above the mid-thoracic spinal cord, the
sympathetic and parasympathetic regions of the spinal cord, which innervate the
bladder sphincter, lose control of the higher centers and become synergistically
impaired, with continued sympathetic excitation and increased release of
norepinephrine in the bladder sphincter, causing continuous contraction of the
bladder sphincter, resulting in increased pressure at the urethral outlet during
urination and impaired urination [5, 6]. Therefore, the idea of clinical
treatment for neurogenic bladder with synergistic dysfunction of the
detrusor-vesical sphincter (bladder neck) should be to suppress overactivity of
the bladder sphincter. Current studies have focused on the bladder forced urinary
muscle and the external urethral sphincter, and relatively little research has
been done on the bladder sphincter. Clinical treatment of bladder neck
dysfunction by cystotomy [7] and
Bioinformatics analysis focuses on exploring potential therapeutic targets; it can evaluate the pathological mechanism of a disease from multiple angles [9]. Tandem mass tag (TMT)-based quantitative labeling technology can comprehensively detect the proteins expressed in tissues, and this technology also has high sensitivity and high-depth proteome coverage for the application of proteomics [10].
In the present study, a T10 spinal cord injury model was prepared by modifying the Hassan Shaker spinal cord transection method [11]. Differentially expressed proteins (DEPs) in the bladder sphincter were then detected using TMT-based quantitative labeling, and bioinformatics analysis was conducted on these DEPs to identify new biomarkers and targets to guide the treatment of NB.
A total of 40 healthy adult female specific pathogen-free rats, weighing 250–280 g, were obtained from the Animal Center Laboratory of Hunan University of Traditional Chinese Medicine (certificate no.: 1107271911006889). The rats were given one week of adaptive feeding under standard conditions (temperature 24–26 °C, humidity 50–70%) in cages in the Animal Center Laboratory of Hunan University of Traditional Chinese Medicine. They were then randomly divided into two groups: a blank group (n = 12) and a model group (n = 28). The rats in the model group underwent T10 spinal cord injury via Hassan Shaker spinal cord transection, with a total of 25 surviving after spinal cord shock, 13 of which developed urinary retention. A total of 12 of the rats were selected by random number table and included in the final model group.
Cell and tissue total protein extraction kit (Kangchen Bio-tech, Shanghai, China); KC™ chemiluminescence kit (Kangchen Bio-tech, Shanghai, China); bicinchoninic acid (BCA) protein quantitative kit (Kangchen Bio-tech, Shanghai, China); 3% pentobarbital sodium (Merck KGaA, San Jose, CA, USA); penicillin sodium (North China Pharmaceutical, Shijiazhuang, Hebei, China); MP150-WSW multichannel physiological signal recorder (Biopac, Goleta, CA, USA); WZ-50C6 dual-channel micro injection pump (factory No.: 150202698, Smiths Medical Instrument, Jiaxing, Zhejiang, China); automatic biochemical analyzer (Toshiba, Tokyo, Japan).
Two hours before modeling, 200,000 units of penicillin sodium were injected intraperitoneally to prevent inflammation. Intraperitoneal anesthesia was then administered with 3% pentobarbital sodium (50 mg/kg). The rats were placed prone and fixed on a rat plate, with the 13th thoracic vertebra as the bone marker. Skin preparation and disinfection were undertaken at the 8th–9th thoracic vertebrae, after which a longitudinal incision of 2–3 cm was made and the subcutaneous tissue was cut. The bilateral erector spinalis and paraspinous muscles were bluntly separated to expose the spinous processes of T8 and T9 and the adjacent vertebral arch. The T8 lamina and bilateral pedicle were removed with a micro bone remover in order to expose the spinal cord. The spinal cord was then drawn out with a dental hook and transected with a no. 11 surgical blade; multiple transections were made to ensure complete severing of the spinal cord. The muscles were sutured, the incision and its surroundings were disinfected with 5% complex iodine, and the skin was sutured.
After the operation, the rats were placed on an electric blanket at a constant temperature until they regained consciousness, after which each rat was placed in a separate cage. Penicillin sodium (200,000 U/animal) was injected intraperitoneally in the morning and evening one week after the operation. Credé manipulation was used to assist urination in the morning, at noon, and in the evening every day. In the case of bedsores and self-mutilation, disinfection was undertaken with iodophor, and penicillin powder was sprinkled on the corresponding parts.
The rats in the blank group were routinely fed until the urodynamics test, and no other treatment was given during the period.
The rats in the T10 spinal injury model were assessed for hind-limb motor function and bladder-voiding function. For hind-limb motor function, the inability to move the hind limbs when walking, dragging on the front limbs, and the Basso, Beattie and Bresnahan (BBB) score [12] of 0 points were noted; for bladder-voiding function, urinary retention, bladder distention, and inability to autonomously urinate after the bladder shock period were noted. If these two conditions were met at the same time, the modeling was considered successful.
Urodynamics was examined on the 18th day after modeling, after which the rats were euthanized through decapitation. The bladder sphincter was then removed and divided into two parts: one part was used for hematoxylin and eosin (H&E) staining, and the other was stored at –80 °C for TMT-based proteomics detection.
On the 18th day after modeling, urodynamic examination was performed in the blank group and the model group. A bladder perfusion test was performed after anesthesia, during which an F3 catheter was inserted into the top of the bladder and placed horizontally with the bladder; the MP150 host pressure baseline was set to zero. The catheter, MP150-WSW 16-channel physiological recorder, and WZ-50C6 microinjection pump were connected through a three-way pipe. The microinjection pump was turned on and the perfusion rate was set to 6 mL/h at a temperature of 25–35 °C. Changes in the bladder pressure curve and urine leakage, including urine overflow from the urethral orifice, when urine overflow occurred for the first time, and leak-point pressure (LPP) at the time of first urine overflow, were then observed and recorded. The maximum cystometric capacity (MCC) was taken as the volume of fluid filled from the beginning of perfusion of normal saline to the leakage of urine from the urethral orifice. Perfusion continued until a stable waveform appeared.
The right atrial appendage was cut after intraperitoneal injection of 3% pentobarbital sodium for anesthesia, and the left ventricle was rapidly perfused with normal saline until the outflow fluid became clear, after which the ventricle was fixed with 4% paraformaldehyde. The bladder neck was removed and fixed with 4% paraformaldehyde for 48 hours, after which it was rinsed, gradually dehydrated with ethanol, treated with xylene until it became transparent, soaked in wax, embedded, sliced into sections, stained, sealed with neutral gum, and observed under a light microscope.
The bladder sphincter was removed under 3% pentobarbital sodium anesthesia.
Each sample was added to 1000
Each sample was subjected to high-speed centrifugation for 10 minutes, after
which the supernatant was transferred to a new Eppendorf tube and the TMT was
balanced to room temperature before opening. A total of 41
A total of 2
The original data obtained after LC-MS/MS analysis were searched on MaxQuant
(version 1.6.1.0, Thermo Scientific, Massachusetts, CA, USA) and
subjected to TMT quantitative analysis; iBAQ non-standard quantification was
performed at the same time. The false discovery rates of polypeptide and protein
levels were
DEPs were defined as proteins with fold change (FC)
The gene symbol corresponding to the DEPs was imported into KOBAS 3.0 [13]
(http://kobas.cbi.pku.edu.cn/), and the species Rattus
norvegicus was chosen to undergo KEGG pathway enrichment analysis, p
Data were analyzed using statistical software SPSS 25.0 (International Business
Machines Corporation, New York, NY, USA). Normally distributed measurement data
were presented as mean
The general health of the rats in the blank group was good. In the rats in the model group, after the spinal cord shock period, the hind limbs were completely paralyzed, random movement disappeared, and the rats began dragging their hind limbs when walking. The rats also had urinary retention and bladder distension in the lower abdomen. The cages were slightly wet, and resistance at the urethral orifice could be felt during manual urination.
The LPP and MCC in the model group were significantly higher than in the blank
group (p
| Group | n | LPP (mmHg) | MCC (mL) |
| Blank group | 12 | 19.362 |
0.352 |
| Model group | 12 | 39.643 |
4.740 |
| Note: *, p | |||
HE staining showed that the blanks group had a clear hierarchical structure of bladder sphincter tissue, with neat and tight arrangement of mucosal epithelium without detachment (①), and the lamina propria was full of elastic fibers without inflammatory cell infiltration; smooth muscle fibers were arranged in an orderly manner. Compared with the blank group, the mucosal epithelial layer of the bladder sphincter in the model group was thick, and the mucosal epithelium was detached in some areas (①); the intrinsic layer was infiltrated by a large number of inflammatory cells (②), and the elastic fibers were reduced; the nuclei of smooth muscle cells in the muscle layer were enlarged (③), and the muscle fibers were thickened and disordered, as shown in Fig. 1.
 Fig. 1.
Fig. 1.Bladder sphincter injury by H&E staining. In the blank group, the tissue hierarchy of the bladder sphincter was clear, the mucosal epithelium was neatly and tightly arranged, and there was no shedding phenomenon (arrow ①). The lamina propria was full of elastic fibers without infiltration of inflammatory cells; smooth muscle fibers were arranged in an orderly manner. In the model group, the epithelial layer of the bladder sphincter mucosa was thicker, and the mucosal epithelium was sloughed off in some areas (arrow ①). A large number of inflammatory cells infiltrated in the lamina propria (arrow ②), and the elastic fibers decreased. The smooth muscle cell nucleus in the muscle layer is enlarged (arrow ③), the muscle fiber is hypertrophic, and the arrangement of the muscle fiber is disordered.
A total of 47,947 peptides and 6684 proteins were detected by TMT-based
quantitative proteomics, 6099 of which were quantifiable proteins. According to
the conditions of FC
 Fig. 2.
Fig. 2.Volcano diagram of 6099 proteins. Green represents down-regulated differentially expressed proteins (DEPs), red represents up-regulated DEPs, and gray represents proteins without differences of statistical significance.
GO functional annotation (including biological process [BP], cellular component [CC], and molecular function [MF]) was carried out using the Cytoscape BiNGO plug-in. With regard to BP, the DEPs were identified as being mainly involved in the cellular process, biological regulation, multicellular biological process, stress response, and negative regulation; with regard to CC, the DEPs were identified as being involved in the cytoplasmic region, extracellular region, plasma membrane, and vesicle of CC; and with regard to MF, the DEPs were identified as being involved in protein binding, structure, and molecular activity, receptor binding, calcium binding, enzyme site binding, and sugar binding proteins (see Fig. 3).
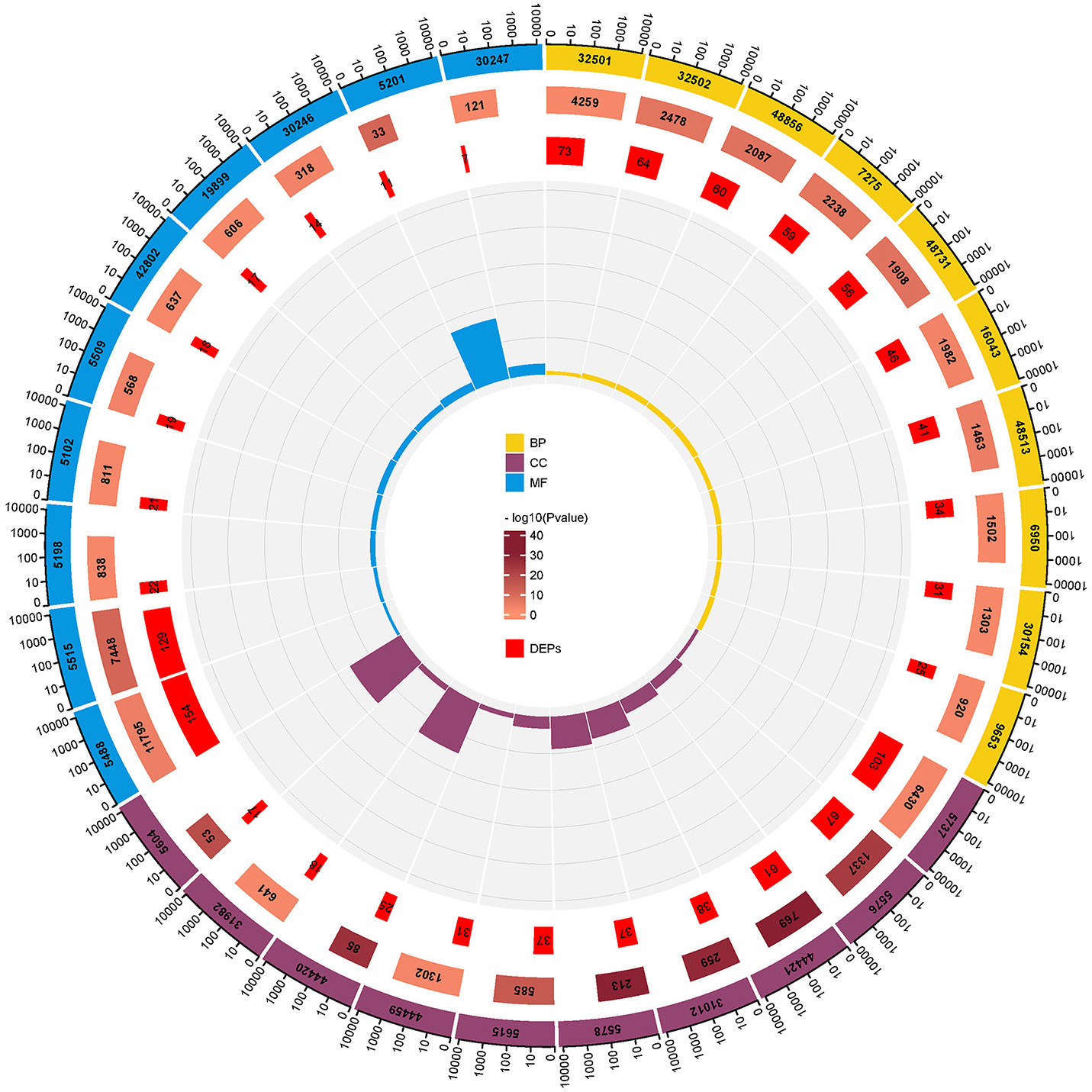 Fig. 3.
Fig. 3.GO functional annotation circle map of 250 DEPs. From outside to inside: GO classification annotation (yellow, purple and blue parts represent BP, CC and MF of 250 DEPs), the total number of proteins involved in this term in the database, the TMT quantitative proteome in this study The ratio of the number of DEPs screened out and involved in the term, the ratio of the number of DEPs involved in the term in the DEPs screened in this study to the total number of proteins involved in the term in the database.
KOBAS software was used for enrichment analysis of the KEGG pathways of the 250
identified DEPs, and a total of 15 KEGG pathways were selected with p
 Fig. 4.
Fig. 4.Statistical bubble diagram of the Kyoto Encyclopedia of Genes and Genomes enrichment pathway. Enrichment is the ratio of the number of genes in the pathway to the total number of genes; the color and size of the dots represent the p value and the number of differentially expressed proteins in the pathway.
| Pathway | Input number | –log10 (Corrected p-value) | Input proteins |
| rno01100:Metabolic pathways | 25 | 3.84 | Gpi, H2afx, Oxct1, Cox7c, Glb1, Gpx3, Dpyd, Sms, Elovl1, Gstm4, Gclc, Pla2g2a, Ldha, Upp1, Spr, Nt5e, Akr1a1, Rpe, Abat, Naga, Glo1, Cox7a2, Pgk1, Hibch, Pafah1b2 |
| rno04512:ECM-receptor interaction | 21 | 23.40 | Lama2, Col6a2, Lama4, Lama5, Fn1, Col6a1, Hspg2, Agrn, Lamb1, Col4a5, Lamc1, Col1a2, Col4a2, Col4a1, Col1a1, Sv2a, Vwf, Lamb2, Col6a5, Sv2c, Col6a6 |
| rno04510:Focal adhesion | 19 | 14.48 | Lama2, Lamb2, Lama4, Lama5, Fn1, Col6a1, Col6a2, Lamb1, Col4a5, Lamc1, Col1a2, Col4a2, Col4a1, Col1a1, Myl12b, Vwf, Myl9, Col6a5, Col6a6 |
| rno05165:Human papillomavirus infection | 19 | 10.34 | Lama2, Col6a2, Lama4, Lama5, Fn1, Stat2, Col6a1, Col4a5, Lamc1, Col1a2, Col4a2, Col4a1, Col1a1, Lamb1, Prkaca, Vwf, Lamb2, Col6a5, Col6a6 |
| rno04151:PI3K-Akt signaling pathway | 17 | 8.80 | Lama2, Col6a2, Lama4, Lama5, Fn1, Col6a1, Col4a5, Lamc1, Col1a2, Col4a2, Col4a1, Col1a1, Lamb1, Vwf, Lamb2, Col6a5, Col6a6 |
| rno05200:Pathways in cancer | 15 | 4.89 | Lama2, Lamc1, Lama4, Stat2, Fn1, Gstm4, Slc2a1, Col4a5, Prkaca, Col4a2, Col4a1, Lamb1, Calm2, Lamb2, Lama5 |
| rno05146:Amoebiasis | 14 | 13.03 | Lama2, Lamc1, Lama4, Lama5, Fn1, Prkaca, Col4a5, Col1a2, Col4a2, Col4a1, Col1a1, Lamb1, Col3a1, Lamb2 |
| rno04974:Protein digestion and absorption | 13 | 12.02 | Col15a1, Col7a1, Col14a1, Col4a5, Col1a2, Col4a2, Col4a1, Col1a1, Col6a1, Col3a1, Col6a2, Col6a5, Col6a6 |
| rno05222:Small cell lung cancer | 10 | 8.33 | Lama2, Lamc1, Lama4, Lama5, Fn1, Col4a5, Col4a2, Col4a1, Lamb1, Lamb2 |
| rno04611:Platelet activation | 9 | 6.11 | Myl12b, Col1a2, Col1a1, Prkaca, Col3a1, Vwf, Fgg, Fga, Fgb |
| rno04926:Relaxin signaling pathway | 8 | 5.09 | Arrb2, Col4a5, Col1a2, Col4a2, Col4a1, Col1a1, Prkaca, Col3a1 |
| rno04933:AGE-RAGE signaling pathway in diabetic complications | 7 | 4.82 | Fn, Col4a5, Col1a2, Col4a2, Col4a1, Col1a1, Col3a1 |
| rno05145:Toxoplasmosis | 6 | 3.55 | Lama2, Lamc1, Lama4, Lama5, Lamb1, Lamb2 |
| rno04142:Lysosome | 6 | 3.35 | Lgmn, Ctsb, Glb1, Lamp1, Naga, Ap1g2 |
| rno04270:Vascular smooth muscle contraction | 6 | 3.25 | Myl6, Calm2, Pla2g2a, Prkaca, Cald1, Myl9 |
To study the interaction between the 250 identified DEPs, the STRING online database and Cytoscape software were used to construct the PPI of the DEPs, with nodes representing the DEPs and lines representing interactions between proteins. Removing the DEPs without interactions, there were 229 nodes and 5374 edges in the PPI graph (Fig. 5). we screened out 7 clusters by using MCODE and performed KEGG pathway enrichment analysis on the 7 clusters using cluego. Cluster 1 was found to be mainly involved in signaling pathways such as ECM-receptor interaction, Focal adhesion, PI3K-Akt signaling pathway and Relaxin signaling pathway, while cluster 2 was involved in Amyotrophic lateral sclerosis (ALS) and Phototransduction pathway, cluster 3 is involved in Vascular smooth muscle contraction pathway, cluster 4 is mainly involved in Synthesis and degradation of ketone bodies and PPAR Cluster 4 is mainly involved in signaling pathways such as Synthesis and degradation of ketone bodies and PPAR signaling pathway, cluster 5 is mainly involved in signaling pathways such as Drug metabolism, cluster 6 is involved in signaling pathways such as Axon guidance, and cluster 7 is involved in Huntington disease pathway (Fig. 6).
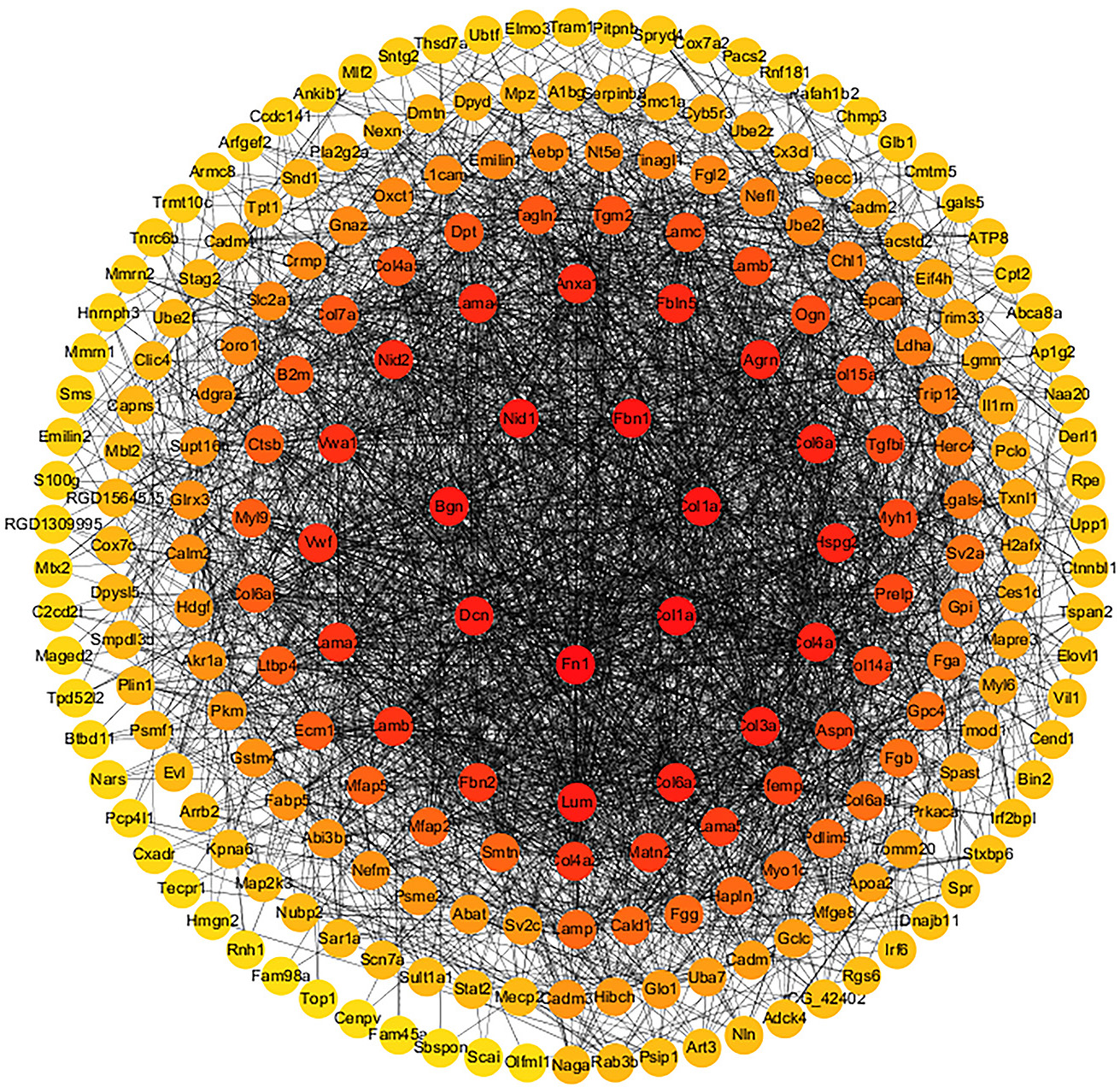 Fig. 5.
Fig. 5.The protein–protein interaction network of 244 differentially expressed proteins (DEPs); the red nodes indicate DEPs participating in Kyoto Encyclopedia of Genes and Genomes signaling pathways.
 Fig. 6.
Fig. 6.ECM-receptor interaction, focal adhesion, phosphoinositide 3-kinase/protein kinase B signaling pathway, vascular smooth-muscle contraction and relaxin signaling pathway.
After spinal cord injury above T10, the bladder sphincter, which is innervated by the T10-L2 sympathetic nerves, loses the control from the higher nerve center and has non-inhibitory contraction, resulting in bladder urination dysfunction [16]. The greatest clinical risk is the sudden increase in bladder pressure and large residual urine volume during voiding due to the inability of the bladder sphincter to relax at the same time as the contraction of the detrusor muscle, which can lead to recurrent urinary retention, urinary reflux, hydronephrosis and urinary tract infection, and even to renal failure. Because of the important role of the bladder sphincter in the voiding process, it is clinically useful to target it as a target site in the treatment of the detrusor-sphincter synergistic bladder disorder.
Therefore, we prepared a rat model of detrusor-sphincter synergistic dysfunctional bladder after transection injury of the T10 spinal cord segment, and the bladder sphincter (bladder neck) was selected as the object of observation in this study. The histomorphological results showed that, compared with the blank group, the mucosal epithelial layer of the bladder sphincter was thicker in the model group, with mucosal epithelial detachment in some areas; the lamina propria was heavily infiltrated with inflammatory cells and the elastic fibers were reduced; the smooth muscle cell nuclei in the muscle layer were enlarged, the muscle fibers were hypertrophied, and the muscle fibers were disorganized. In addition, urodynamics showed that both LPP and MCC were significantly higher in the bladder of the model group compared with the blank group, which suggested that the bladder sphincter and/or external urethral sphincter were excessively contracted after modeling, resulting in obstruction of urinary drainage, and thus LPP and MCC were significantly increased. Thus, a rat NB model of sphincter overactivity can be successfully caused by transection of the T10 spinal cord segment. We selected bladder sphincter (bladder neck) tissue and performed proteomic identification using TMT quantitative labeling technology to screen for differentially expressed proteins in bladder sphincter tissue, and the results showed that 250 DEPs were identified in the model/blank group of bladder sphincter. To explore potential clinical intervention targets for the disease, bioinformatic analysis was next performed on the differentially expressed proteins, and the results showed that these 250 DEPs were significantly enriched in 15 KEGG pathways. We focused on pathways closely related to the regulation of smooth muscle contraction, and the relevant DEPs were significantly enriched in extracellular matrix (ECM)-receptor interactions, adherent plaques, PI3K-Akt signaling pathway, cytosolic relaxin signaling pathway, and vascular smooth muscle contraction signaling pathway.
The three signaling pathways, extracellular matrix (ECM) receptor interactions, PI3K-Akt signaling pathway and adherent plaques, are very closely linked (Fig. 7A). Extracellular matrix (ECM) is a macromolecular material that is synthesized and secreted into the cell membrane or interstitial matrix, including collagen fibers, non-collagen fibers, elastic fibers, proteoglycans and aminoglycans. The complex network of ECMs not only has physical roles such as support, protection, and connectivity, but also exerts a full range of biological effects on cellular activities through signal transduction pathways in vivo. It has been shown that in bladder smooth muscle, alterations in collagen fibers, elastic fibers, and adhesion proteins in the ECM seriously affect the function of bladder smooth muscle cells. We found that in cluster 1 Lama2, Lama4, Fn1, Fbn1, Lamb1, and Col3a1 are all ECM components (Fig. 6), and by analyzing the KEGG pathway of DEPs in cluster 1, we found that these DEPs mainly focus on ECM-receptor interaction, Focal adhesion, PI3K-Akt signaling pathway, relaxin signaling pathway and etc (Fig. 6). Previous studies have also found significant alterations in ECM components in bladder smooth muscle bundles after spinal cord injury, which remains consistent with our study. In normal bladder tissue, the contractility of smooth muscle cells is closely related to ECM, which may be related to the fact that ECM connects to the smooth muscle cell intracellular actin filaments through the corresponding receptors on the smooth muscle cell membrane, converting extracellular mechanical signals into chemical signals that ultimately affect the contractile properties of smooth muscle cells.
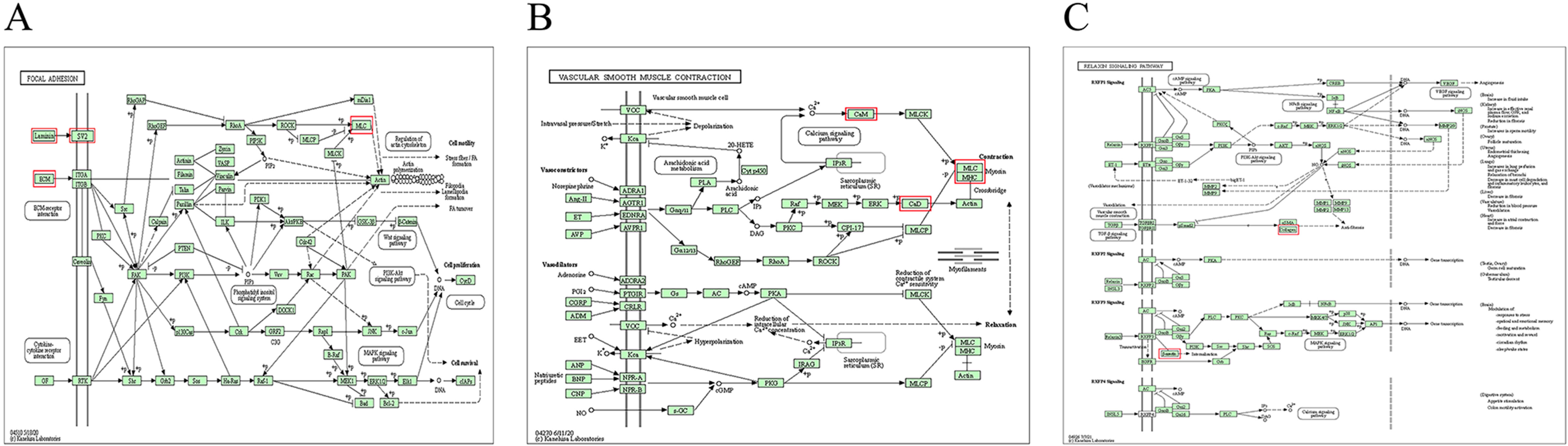 Fig. 7.
Fig. 7.KEGG signaling pathway diagram. The red boxes marked are the DEPs screened in this study. (A) is the relationship between ECM-receptor interaction, Focal adhesion and PI3K-Akt signaling pathway, (B) is the Vascular smooth muscle contraction pathway in which DEPs are involved, (C) is the relaxin signaling pathway in which DEPs are involved. Relaxin signaling pathway.
ECM has been found to activate focal adhesion kinase (FAK) in situ
after binding to the integrin receptor on the cell membrane; FAK further binds
and activates many signal proteins to regulate physiological activities, such as
actin polymerization and cell migration, proliferation, and apoptosis [17].
Previous studies have revealed that, in the gastric fundus smooth muscle, the
phosphorylation of FAK can activate the calcium-sensitive signaling pathway and
promote the contraction of the gastric fundus smooth muscle cells [18];
furthermore, in physiologically stretched smooth muscle in the bladder, the
activation of FAK can enhance the contraction and proliferation of the smooth
muscle cells [19]. Phosphorylated FAK can further activate the PI3K/Akt signaling
pathway, which is related to biological processes such as cell proliferation,
differentiation, protein synthesis, and actin polymerization [20]. Activated PI3K
generates a second messenger, phosphatidylinositol-3,4,5-triphosphate (PIP3), on
the cell membrane, which further activates p21-activated protein kinases (PAKs).
PAK can inhibit the phosphorylation of myosin light chain kinase (MLCK)
stimulated by acetylcholine to inhibit the contraction of smooth muscle cells
[21]. Yiming Wang et al. [22] found that PAK regulates contraction
mediated by
Synaptic vesicle glycoprotein 2A (Sv2A) in DEPs is involved in the signaling pathway of ECM-receptor interaction (Fig. 7A). Sv2A is a glycoprotein, specifically expressed in synaptic vesicles, that can regulate the release of neurotransmitters dependent on action potential. Recently, a study has reported that Sv2A has many potential functions, including vesicle transport, stabilizing the vesicle load of neurotransmitters, and regulating calcium sensitivity [24]. A previous study found that Sv2A-deficient mice had persistent seizures; levetiracetam, an anti-epileptic drug that targets Sv2A, has been widely used in clinical practice [25]. Another previous study identified that Sv2A overexpression can reduce the release of neurotransmitters [26]. This indicates that the abnormal increase or decrease of Sv2A expression may affect the release of neurotransmitters in synapses. Sv2A is not only expressed in the central nervous system but also controls the release of neurotransmitters by regulating the size of the easy-release pool in peripheral sympathetic synapses [27]. However, the bladder sphincter is mainly innervated by sympathetic nerves. In the present study, the expression of Sv2A in the bladder sphincters in the model group was significantly lower than in the blank group, which may be one of the reasons for the involuntary contraction of the bladder sphincter after T10 spinal cord injury.
The results of the present study found that the vascular smooth muscle
contraction signaling pathway was significantly enriched, and we identified
Calm2, Cald1 as the main DEPs involved in this signaling pathway (Fig. 6). In the
study results, Calm2, a gene encoding calmodulin (CaM), and Cald1, a gene
encoding calmodulin binding protein (CaD), were significantly elevated in the
bladder sphincter of the model group. The change in Ca
The abnormal expression of the CALM2 gene leads to the abnormal opening of the
voltage-gated Ca
In the relaxin signaling pathway, G-protein coupled receptor (GPCR) and relaxin
family peptide receptor 1 (RXFP1) play an important role in promoting
vasodilation and antifibrosis, activating nitrogenous nerve fibers and protecting
the heart [35, 36]. Edward et al. [37] found that RXFP1 was expressed in
the smooth muscle cell layer of the human bladder triangle (where the bladder
sphincter is located) and fornix, and expression of transforming growth factor
Arrestin
It is of great significance to explore the pathological mechanism of
non-inhibitory contraction of the bladder sphincter caused by spinal cord injury
above the T10 segment from the perspective of
ECM-receptor interaction, the focal
adhesion-activated PI3K-Akt signaling pathway, the smooth muscle contraction
signaling pathway, and the cell-relaxation signaling pathway. Sv2A, which is
involved in the release of neurotransmitters from synaptic vesicles, arrestin
It has been found that in patients with incomplete suprasacral spinal cord injuries (such as ASIA B, C spinal cord injuries) there is still a dysfunction of the detrusor-vesical sphincter synergy, and overactivity of the bladder sphincter may occur. For these conditions, clinical management can include inhibition of excessive bladder sphincter contraction as an important therapeutic idea. Therefore, incomplete spinal cord injury with overactive bladder sphincter can also consider the above-mentioned targets for intervention.
In this study, we screened for DEPs in the bladder sphincter after T10 spinal segment injury by TMT quantitative proteomics, however, we have not yet validated those DEPs that potentially modulate bladder sphincter contraction. We will continue to validate the screened DEPs to further explore these potential therapeutic targets and pathways for bladder sphincter overactivity.
The data have been uploaded to ProteomeXchange with identifier PXD034595 (http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD034595).
Study design—KA, LZ. Project administration—KA, MX. Experiment implementation—QRQ, QL, XW. Data analysis—LYT, YYL, FQ. Paper writing—QRQ, LYT, KA. Paper review & editing—HZ. Experimental support—KA, LZ. All authors approved the final version of the manuscript.
Experimental animal ethics committee of Hunan University of Chinese Medcine. Ethical approval number: LL2019092303.
Not applicable.
General program of National Natural Science Foundation of China (81874510); University Student Innovation and Entrepreneurship Training Program Project of Hunan Province (S202110541019).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
