1. Introduction
Sleep exists in all organisms despite evolutionary pressure, from C.
elegans, to Drosophila, to human, they all need to sleep or in a sleep-like
state, and poor sleep can have deleterious effects on development cognitive
abilities and life span [1]. Sleep is a resting behavioral state characterized by
reduced response to weak stimuli and rapid reversal of strong stimuli [2].
General anesthesia is a state of unresponsiveness which is like sleep to a
certain extent, although sleep and anesthesia have similar behavioral
characteristics, there are obvious behavioral and physiological differences
between the two states. For example, unlike the natural occurrence of sleep,
general anesthesia is induced by anesthetics and does not response to external
stimuli. After considering the similarities and differences between anesthesia
and sleep, a hypothesis was proposed that anesthesia and sleep might share a part
of neuronal network [3].
Sleep deprivation, as a manipulation for studying the function of sleep, has
been widely used in various sleep-related experiments. It has been shown that
sleep deprivation reduced the time to loss of righting reflex for propofol and
isoflurane and prolonged the time to recovery [3]. Nevertheless, these related
studies did not evaluate the 50% effective dose for loss of righting reflex
(LORR ED). In addition, the effect of duration of sleep deprivation on
anesthetic potency is also unknown. LORR ED was often used to explore the
potency of anesthetics in inducing unconsciousness in mice, since loss of
righting reflex resembled sleep compared with the minimal alveolar concentration
(MAC).
The purpose of this study was to determine the effects of sleep deprivation on
the potency of sevoflurane in inducing unconsciousness in mice. We hypothesized
that sleep deprivation can increase the potency of sevoflurane and the
enhancement was positively correlated with the duration of sleep deprivation.
2. Methods
In this study, we first determined the basal sevoflurane LORR ED in mice
as control, followed by three behavioral interventions in the order of 24-h,
48-h, and 72-h sleep deprivation in the same mice. Sevoflurane LORR ED was
tested immediately after each behavioral intervention and the mice rested for
three days to recover (Fig. 1). The sleep deprivation model was established by
using modified multiple platform method. The selection of three days for recovery
was based on previous research with slight modification [3], we also tested LORR
ED in another group of mice after 3-day recovery from sleep deprivation.
 Fig. 1.
Fig. 1.
Flowchart showing the study design and the temporal order (from
top to bottom) of behavior interventions.
2.1 Animals
C57BL/6 male mice (7 weeks; weight range, 22–25 g) were ordered from Beijing
Vital River Laboratory Animal Technology Co., Ltd., Beijing, China. Mice were
bred in a temperature- and humidity-controlled room with a 12-h light/dark cycle.
All mice had free access to standard mouse chow and tap water before the
experiments. The animals used for the behavioral experiments were housed in the
room for a week.
2.2 Sleep Deprivation
Sleep deprivation in mice was achieved by modified multiple platform method [4].
The mice were placed on 9 circular and 3.5 cm-diameter platforms in a ventilated
transparent cage (50 30 17 cm, available food and water, 4
mice/cage) filled by water up to 1 cm of the platforms’ surface enabling the mice
to jump between the platforms. Whenever the mice reached the rapid eye movement
(REM) sleep, they fall into the water because of muscle atonia and then wake up
and tried to climb back to the platforms to avoid being drowned. The water in the
cage was refreshed every day due to the excreta of mice. The sleep deprivation
periods were timed to begin and end at 8:00 AM. The non-sleep deprived mice were
maintained under normal feeding conditions.
2.3 LORR ED Determination
The LORR ED of sevoflurane in mice was determined according to our
previously described method [5] with slight modifications. Briefly, all the
behavioral interventions LORR ED of each mouse was determined, and all the
values were compared. For either the control group (ad libitum activity) or the
sleep deprivation group LORR ED determination, mice were individually
placed in independent plastic grid V-shaped trough fixed in a transparent plastic
chamber (205 134 69 mm) with an electrical fan to mix
gases. One side of the chamber was connected to a sevoflurane vaporizer (Aika,
Ichikawa Shiseido, Tokyo, Japan). The other side was connected to an infrared gas
monitor (BeneView T5, Mindray Bio-Medical Electronics, Shenzhen, China) to
measure the sevoflurane, oxygen, and carbon dioxide concentrations in real time.
The monitor can monitor the sevoflurane concentration with a precision of 0.01%.
When a mouse was placed in the chamber, pure oxygen was immediately supplied at
a rate of 600 mL min. When the chamber’s oxygen concentration increased
to 99%, sevoflurane gas mixed in pure oxygen was provided by the vaporizer. The
initial sevoflurane concentration in the chamber was 1.00%, which was maintained
for 15 minutes to equilibrate the mouse with sevoflurane gas. Then, the chamber
was rotated 180 to place the mouse on its back in the V-shaped trough,
and its righting reflex was observed. LORR was defined as the supine mouse unable
to turn itself onto all 4 paws three times within 1 min. According to the mouse’s
righting reflex, a stepwise increase or decrease of 0.10% sevoflurane in the
chamber was applied. Specifically, if the mouse’s righting reflex disappeared,
the sevoflurane concentration was decreased 0.10%; otherwise, it was increased
0.10%. After 15 minutes of equilibration at each sevoflurane concentration, the
mouse’s righting reflex was observed again. The LORR ED was the average of
the two critical sevoflurane concentrations at which the mouse either lost or
regained its righting reflex. All determinations were made between 8:00 and
18:00. For the sleep deprivation group, LORR ED was determined immediately
after sleep deprivation.
2.4 Statistical Analysis
Sample sizes were predetermined according to our previous study [5]. GraphPad
Prism software (version 8.0.2 for Windows, GraphPad Software
Inc., San Diego, CA, USA) was used for statistical analyses. The acquired values
of LORR ED were expressed as mean SD,
repeated-measures analysis of variance (RMANOVA) was used to
determine the significance of our behavioral interventions. Post hoc multiple
comparisons were made using the Bonferroni test. A p value less than
0.05 (two-tailed) was considered to be statistically significant.
3. Results
3.1 Effects of Sleep Deprivation of Sevoflurane LORR ED
The LORR ED of sevoflurane was 1.08 0.11% (95%
CI, 1.01%–1.16%), 0.88 0.12% (95% CI, 0.80%–0.96%), 0.89
0.08% (95% CI, 0.83%–0.95%) and 0.88 0.12% (95% CI,
0.80%–0.96%) for the control, sleep deprivation (24 h), sleep deprivation (48
h) and sleep deprivation (72 h) group, respectively. By comparing the LORR
ED of different groups using RMANOVA, we found a statistically significant
effect of different durations of sleep deprivation on LORR ED (F = 10.6,
p = 0.0003).
The results of Bonferroni multiple comparisons test showed a decrease in LORR
ED after 24-h (p = 0.0174), 48-h (p = 0.0043) and 72-h
(p = 0.0023) sleep deprivation compared with the control group.
Nevertheless, there are no significant statistical differences in LORR ED between sleep deprivation groups of different durations (p 0.9999)
(Fig. 2). In other words, the sleep deprivation duration did not affect the LORR
ED. Because a decreased ED means increased potency of anesthetic,
these findings indicated that sleep deprivation can increase anesthetic potency.
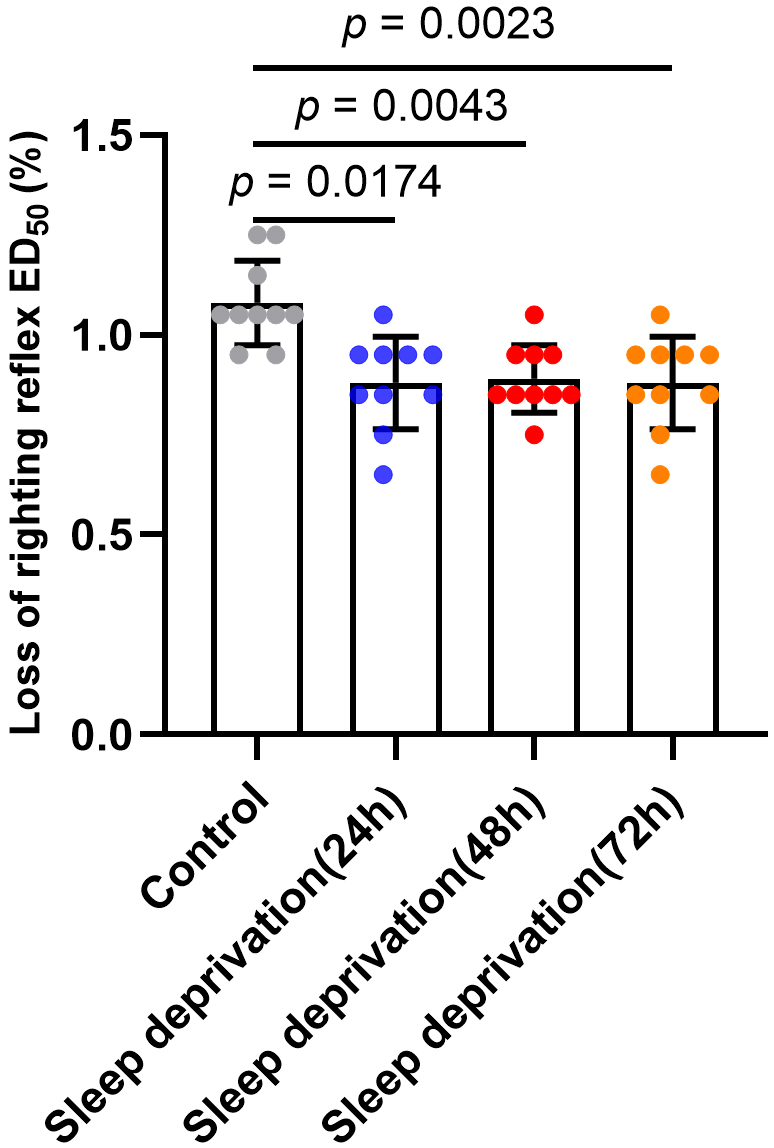 Fig. 2.
Fig. 2.
Effects of sleep deprivation on the LORR ED of sevoflurane in
mice. The LORR ED of sevoflurane was 1.08% (95% CI, 1.01%–1.16%),
0.88% (95% CI, 0.80%–0.96%), 0.89% (95% CI, 0.83%–0.95%) and 0.88%
(95% CI, 0.80%–0.96%) for the control, sleep deprivation (24 h), sleep
deprivation (48 h) and sleep deprivation (72 h) group, respectively. Data are
shown as mean SD (n = 10/group).
3.2 LORR ED Determination after 3-Day Recovery from Sleep
Deprivation
The LORR ED of sevoflurane was 1.12 0.08% (95% CI,
1.03%–1.20%), 1.12 0.08% (95% CI, 1.03%–1.20%),
1.15 0.09% (95% CI, 1.06%–1.24%) for the control, 3-day
recovery from sleep deprivation (24 h) and 3-day recovery from
sleep deprivation (48 h) group, respectively (Fig. 3). By comparing the LORR
ED of different groups using RMANOVA, we found there was no statistically
significant difference in LORR ED after 3-day recovery from sleep
deprivation (F = 0.6250, p = 0.5549).
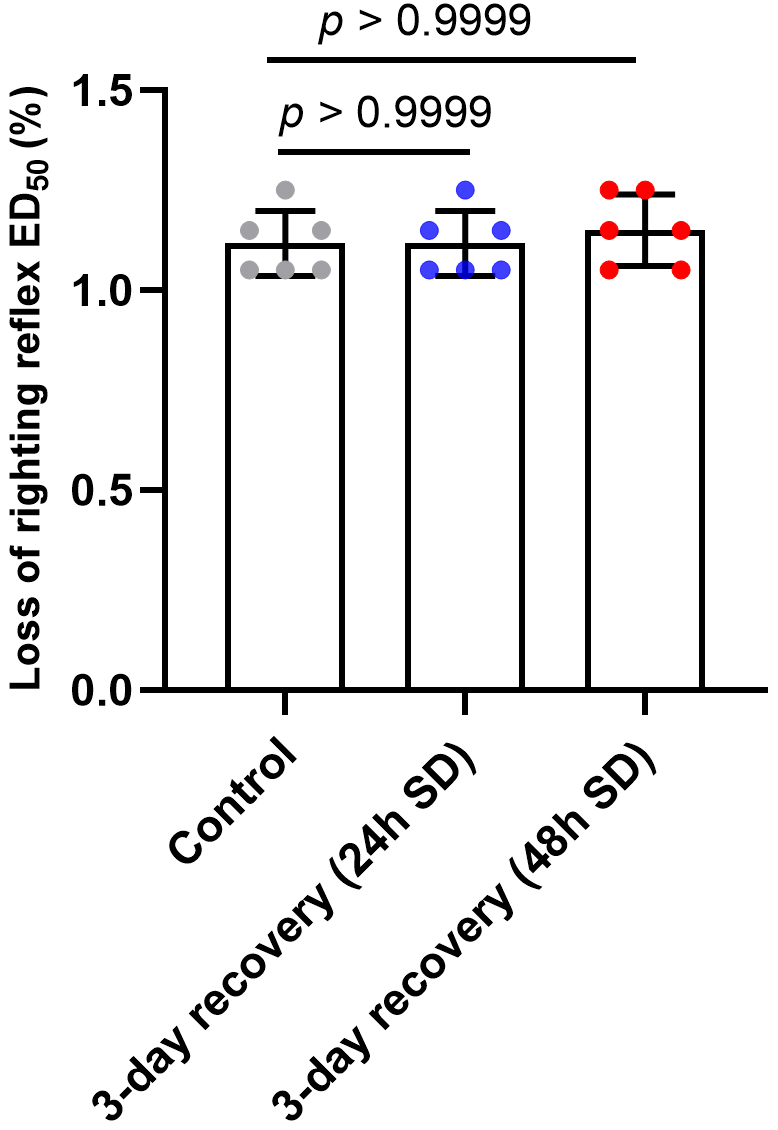 Fig. 3.
Fig. 3.
LORR ED determination after 3-day recovery from sleep
deprivation. SD means sleep deprivation. There was no statistically significant
difference in LORR ED after 3-day recovery from sleep deprivation
(p 0.9999). Data are shown as mean SD (n = 6/group).
4. Discussion
In this paper, we present two main findings. First, sleep deprivation can
increase the anesthetic potency of sevoflurane. Second, the duration of sleep
deprivation did not affect the anesthetic potency of sevoflurane. These findings
showed that sleep homeostasis affects the potency of anesthetics, suggesting that
general anesthesia, sleep may share a common mechanism. So, our future work is to
explore what this common mechanism is and how it works.
4.1 Sleep Homeostasis and Circadian Clock
Previous study had shown that circadian clock genes can affect sleep
homeostasis [6] and there are circadian differences in the
minimal alveolar concentration (MAC) for recovery of righting reflex
(MAC) and the time to recovery of righting reflex (Time) [7],
REM sleep deprivation can induce circadian clock gene abnormalities of rats’
hippocampus after sevoflurane inhalation [8]. So, there may be a shared component
between sleep, anesthesia and circadian rhythms. We designed the above experiment
to explore the relationship between sleep and anesthesia, in this animal
experiment, we found an approximate 20% reduction in the LORR ED after a
behavioral intervention of sleep deprivation, however the reduction did not
change with the duration of sleep deprivation.
4.2 Sleep Deprivation and Neurotransmitters
Our findings indicated that sleep deprivation can increase the anesthetic
potency of sevoflurane. Previous research has been done to evaluate the time to
loss of righting reflex and recovery by propofol and isoflurane after sleep
deprivation, the results also suggested that sleep deprivation can potentiate the
effect of anesthetics. Then how might sleep deprivation affect the anesthetic
potency? Although the underlying mechanism of sleep deprivation is still unclear,
there have been some discoveries about the role of neurotransmitters. For
example, the concentration of adenosine in the basal forebrain of cats increased
after 6 hours of sleep deprivation and decreased after recovery [9]. However,
administration of adenosine antagonist cannot completely reverse the effects of
sleep deprivation on righting reflex, suggesting that the effects of sleep
deprivation may not be mediated by adenosine [10]. In addition, another widely
concerned neuropeptides produced by the posterior hypothalamic region, which
plays a key role in the maintenance of sleep and arousal, include orexin
and melanin concentrating hormone (MCH). Orexin is a
neuropeptide family secreted by hypothalamus that promotes appetite and regulates
sleep and wakefulness, it consists of two peptides orexin-A and orexin-B. Dong
et al. [11] found that intrabasalis microinjection of orexin-A can
shorten the emergence time to sevoflurane anesthesia and the
orexin receptor antagonist (SB-334867A) prolonged the emergence time to
sevoflurane anesthesia. The decrease of orexin-A is also the reason for the
delayed recovery of sleep deprived rats under isoflurane anesthesia [12]. Whereas
MCH perform opposite roles to orexin in sleep and wakefulness [13]. In the
rebound phase of REM sleep after 72 hours of REM sleep deprivation, MCH
neurons were strongly active, and rapid eye movement sleep was prolonged after
lateral ventricular injection of MCH [14]. Systemic application of MCHR-1
antagonist resulted in a dose-dependent reduction of slow wave (SW) sleep and REM
sleep, and a corresponding increase in wakefulness [15]. Orexin and MCH were also
found to be related with the effects of ketamine and propofol on sleep structure
in the period of postanethesia [16]. Other neurotransmitters include glutamate
(Glu), and -amino butyric acid (GABA) also play an important role in
the wake/sleep cycle, Xie et al. [17] found that 24 h
sleep deprivation significantly increased the concentration of
Glu and GABA in the rat’s hippocampus and propofol anesthesia can normalized the
upregulated GABA and Glu levels like natural sleep.
4.3 Selection of Methods
We chose LORR ED as an observed index because this index was more stable
compared with the time to loss of righting reflex or the emergence time from
anesthesia, and it only takes 0.5–1 h to complete a LORR ED test, which
is not too long. Induction time is not an index to evaluate the potency of
inhaled anesthetics, for example, sevoflurane induces anesthesia faster than
isoflurane, but sevoflurane is less potent than isoflurane [18]. LORR ED has been commonly used to evaluate the potency of inhaled anesthetics in
inducing unconsciousness in mice [5, 19]. Furthermore, although studies
demonstrated that prolonged sedation with propofol can discharge the sleep debt
[20, 21], when exposed to the inhaled anesthetics such as sevoflurane, isoflurane
and halothane, rapid eye movement sleep debt accrues in mice [22, 23]. Pal
et al. [24] also found that sevoflurane induction time was shortened
after sleep deprivation and REM sleep could not be restored during sevoflurane
anesthesia. Therefore, we did not need to consider the recovery of REM sleep that
may be caused by the duration of LORR ED test. We did not use the
minimal alveolar concentration (MAC) as a measure to evaluate
the anesthetic potency for following reasons. At first, MAC involves the painful
stimulation during tail-clamp, while sleep loss would increase the pain
sensitivity in mice [25, 26, 27]. In addition, MAC was used to evaluate the
anesthetic potency of inducing immobility, and the value was not altered
after spinal cord transection in rats which means that MAC
associated body movements may be in the spinal cord [28], nevertheless, the
potency of anesthetics to induce hypnosis is measured by loss of righting reflex
and hypothalamic nuclei and cerebral cortex may be involved in this process [29].
There are many ways to model sleep deprivation, in this study we chose the
modified multiple platform method for several reasons. Above all, we want to
explore the effects of REM sleep deprivation on anesthetic potency as the
determination of LORR ED required a period of 0.5–1 h though it is not
too long, we do not know that if the sleep debt can be discharged during
anesthesia, whereas as mentioned above, inhaled anesthetics cannot satisfy the
homeostatic need for REM sleep, so we can exclude the effect of anesthetic time
on our results. Also, modified multiple platform method was
widely used for REM sleep deprivation [4, 30, 31] and it is convenient to model.
4.4 Clinical Significance
Our results demonstrated that preoperative sleep disorders may affect
perioperative anesthetic managements. Nowadays, sleep disorders are regarded as
an independent risk factor of postoperative cognitive dysfunction (POCD) which is
a severe postoperative neurological sequela. So, for the anesthesia of such
patients, we can consider reducing the dose of anesthetics during operation, this
may help to reduce the incidence of POCD, however, this hypothesis needs to be
further verified.
4.5 Limitations and Future Directions
This study only presents a behavioral finding and does not further investigate
the mechanism. Therefore, the next step is to explore the basic mechanism of
sleep deprivation leading to the increased anesthetic potency
and try to uncover the relationship between sleep and anesthesia.
In addition, it can also be clinically explored whether reducing the dosage of
anesthetics in patients with sleep disorders can reduce the incidence of POCD.
5. Conclusions
In summary, we report that sleep deprivation can increase the anesthetic potency
of sevoflurane regardless of duration of sleep deprivation, suggested that
general anesthesia may share a common mechanism with sleep. More researches are
needed to explore the mechanisms that how do sleep and anesthesia interact with
each other.
Author Contributions
SZ designed this study. HQ, QZ and NC performed the experiments. HQ and QZ wrote
the manuscript. All authors have read and approved the final manuscript.
Ethics Approval and Consent to Participate
All animal operations and experimental protocols conformed to the US National
Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH
Publications No. 8023, revised 1978) and were approved by the Institutional
Animal Care and Use Committee (approval No: S164) at Tongji Medical College,
Huazhong University of Science and Technology.
Acknowledgment
Not applicable.
Funding
This work was supported by a National Natural Science Foundation of China
(81670068 to SZ).
Conflict of Interest
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3.