†These authors contributed equally.
Academic Editor: Simona Lattanzi
Objective: This study aimed to explore the diagnostic points and
treatment modes of the clinical characteristics of Japanese encephalitis (JE) in
the middle-aged and elderly population. Methods: Six patients aged
47–72 who were diagnosed with JE at the Beijing Chaoyang Hospital Affiliated
with the Capital Medical University between August 2018 and September 2019 were
enrolled in the study. Their clinical manifestations, biochemical indicators,
imaging data, diagnostic methods, and the evolution and outcomes of the
treatments they underwent were retrospectively analyzed. Results: (1)
All six patients had severe clinical symptoms and poor prognoses that were more
likely to be associated with other systemic diseases. (2) Lesions were most
commonly distributed in the thalamus, basal ganglia, and midbrain. The appearance
of hyperintensity in the corpus callosum, hippocampus, and subcortical white
matter was more specific. The hyperperfusion metabolism in the lesion area in
head computed tomography perfusion imaging indicated the state of inflammatory
activity in the lesion. In cranial magnetic resonance imaging (MRI), T2 and
fluid-attenuated inversion recovery (FLAIR) were more sensitive. (3) After a
patient has been systematically treated in the intensive care
unit (ICU), the patient gradually recovered and the level of consciousness
improved (p
Japanese encephalitis (JE) is an infectious disease of the central nervous
system caused by the JE virus (JEV). This acute infectious disease is spread
primarily by mosquito bites in the summer and autumn. The disease is endemic in
many countries in Asia, the Western Pacific, and Northern Australia. Most people
infected with the virus are asymptomatic, while
Symptomatic patients with JE are often clinically characterized by rapid onset, rapid progression, high fever, unconsciousness, and meningeal irritation. The diagnosis of JE in the acute phase therefore needs to be differentiated from viral encephalitis, meningitis, acute disseminated encephalomyelitis (ADEM). Viral encephalitis is more common in young and middle-aged people with a history of prodromal infection, epileptic seizures, abnormal behavior, and other manifestations, mostly involving the frontal and temporal lobes and limbic systems. Meningitis, a critical condition, is generally caused by meningococcus. It is more common in the winter and spring in children under the age of 14, and is characterized by a disturbance of consciousness, convulsions, and other manifestations. The cerebrospinal fluid (CSF) of patients with meningitis exhibits reduced sugar and chloride, and bacterial culture in the CSF can find meningococcus as the basis for diagnosis. ADEM usually begins acutely after infection or 1–2 weeks after vaccination, with symptoms of acute or subacute diffuse damage to the brain and spinal cord. Magnetic resonance imaging (MRI) shows scattered brain and spinal cord lesions for identification.
Previous literature has reported that the incidence of JE in children is high, but recent literature and clinical findings show that it is increasing every year in the middle-aged and elderly population. Some researchers have suggested that adults have more severe clinical manifestations and higher mortality rates, which may be due to a lack of JE vaccination in childhood or the higher mean age at onset [1]. In order to advance clinicians’ understanding of JE, this study reports the clinical data of six middle-aged and elderly patients with severe JE admitted to the Beijing Chaoyang Hospital Affiliated with the Capital Medical University between August 2018 and September 2019 and discusses their clinical features.
The cases of six patients aged 47–72 who were diagnosed with JE at the Beijing Chaoyang Hospital Affiliated with the Capital Medical University between August 2018 and September 2019 were collected. Samples of each patient’s serum and CSF were sent to the Chinese Center for Disease Control for immunoglobulin M (IgM) antibody detection for confirmation.
Enzyme-linked immunosorbent assay (ELISA) reagents and a detection kit (Beijing
Fangfang Biotechnology Co., Ltd., Beijing, China) were used to identify the JEV
IgM antibodies using the capture method. The test steps were used according to
the kit instructions. After color development, an enzyme-labeled colorimeter
(detection wavelength 450 nm) was used to zero-adjust with a blank well and read
the optical density (OD) value of each group. If the
sample/negative control OD value was
Inclusion criteria: All serum and CSF IgM antibodies were detected by enzyme-linked immunosorbent assay (ELISA). A positive serum or CSF test result was diagnosed as JE.
Exclusion criteria: In the case of multiple admissions, patients in the subacute or chronic phase were excluded from the analysis.
Detailed disease-related information was collected from the medical records. The
demographics, vaccination history, past medical history, exact
date of diagnosis, and any incidences of high fever (
The neurologically accepted Glasgow Coma Scale (GCS) was used to assess the
level of each patient’s consciousness. Categorical variables were expressed as
percentages (%). Analysis was performed using SPSS version 19.0 (SPSS Inc.,
Chicago, IL, USA). Differences between groups of categorical variables were
compared using a P-test, and p
All six patients exhibited acute onset, with clinical manifestations of headache, high fever, confusion, and neck stiffness; five of these patients also had respiratory failure. Routine blood leukocyte counts, neutrophil percentages, CSF leukocyte counts, and CSF pressure were elevated in all six patients. The comorbidities included three patients with abnormal liver function, one with cardiac insufficiency, one with myocardial infarction, one with renal insufficiency, and three with epilepsy. Five patients were not vaccinated against JE, and one had an unknown vaccination history. The pupillary light reflex was dull or absent in five patients. Six patients tested positive for anti-JEV IgM antibodies in their blood and/or CSF. Four patients underwent MRI head scans, which showed abnormal signals in the thalamus, brainstem, and corpus callosum. The CT scans of two patients showed hypodensity in the thalamus and basal ganglia. In one patient, perfect head CT perfusion imaging indicated that the metabolism of the hippocampus was increased (Table 1).
| Item | Characteristic factor | Middle-aged and elderly population | |
| (N = 11) | |||
| Demographics | Age | 60.83 (47,72) | |
| Gender (female/male) | 5/1 | ||
| Nationality | Han | ||
| Common diseases | 1.83 | ||
| Routine blood examination | leukocyte counts | 5 | |
| neutrophil percentages | 5 | ||
| Biochemical examination | abnormal liver function | 3 | |
| renal insufficiency | 1 | ||
| cardiac insufficiency | 1 | ||
| myocardial infarction | 1 | ||
| Lumbar puncture results | CSF pressure elevated | 6 | |
| trace total protein elevated | 5 | ||
| Polycythemia | Leukocytosis | 6 | |
| monocyte |
6 | ||
| CSF appearance | Clear | 5 | |
| muddy | 1 | ||
| Imaging examination | thalamus | 6 | |
| basal ganglia | 4 | ||
| midbrain | 2 | ||
| cortex | |||
| WMH | 1 | ||
| corpus callosum | 1 | ||
| hippocampus | 1 | ||
| Secondary complication | UGB | 1 | |
| DVT | 2 | ||
| Treated with tracheal intubation/tracheostomy assisted ventilation | 5 | ||
| Hypoproteinemia | 2 | ||
| epileptic seizures | 3 | ||
| Note: WMH, white matter hyperintensity; DVT, deep venous thrombosis; UGB, upper gastrointestinal bleeding; CSF, cerebrospinal fluid. | |||
In terms of treatment, all six patients were treated in the intensive care unit (ICU), and all were treated with anti-infection, anti-virus, and mannitol treatments to lower intracranial pressure. Five patients also underwent tracheal intubation/tracheostomy-assisted ventilation, and five were treated with mild hypothermia for brain protection. Two patients were treated with hormone replacement therapy (Table 2). Five patients improved and were discharged from the hospital, while the family of one patient chose to cease treatment; this patient was transferred to a different clinical center, and their prognosis is unknown.
| Case number | Treated in the ICU | Regular treatment | Hormone replacement therapy | Mild hypothermic cerebral protection therapies |
| 1 | Yes | anti-infection, anti-virus, and mannitol treatments | Sou-Medrol | No |
| 2 | Yes | anti-infection, anti-virus, and mannitol treatments | No | Yes |
| 3 | Yes | anti-infection, anti-virus, and mannitol treatments | No | Yes |
| 4 | Yes | anti-infection, anti-virus, and mannitol treatments | No | Yes |
| 5 | Yes | anti-infection, anti-virus, and mannitol treatments | No | Yes |
| 6 | Yes | anti-infection, anti-virus, and mannitol treatments | Sou-Medrol | Yes |
After the patients were treated in the ICU, hormones combined with
anti-inflammatory, antiviral, and mild hypothermia cerebral protection therapies
were administered to actively promote recovery. The patients’
GCS scores suggested that the implementation of this treatment plan improved
their level of consciousness (p
| Case number | Pupillary light reflex | Mosquito bite history | History of JE vaccination | GCS score at admission※ | The exact date of diagnosis | Length of stay | Days requiring mechanical ventilation | GCS score at discharge※ | mRS score at discharge |
| 1 | sensitive | Yes | unknown | 10 | 14 | 21 | 0 | 15 | 4 |
| 2 | disappeared | No | No | 7 | 7 | 4 | 4 | 3 | 5 |
| 3 | dull | No | No | 7 | 10 | 23 | 18 | 13 | 4 |
| 4 | dull | No | No | 5 | 12 | 38 | 36 | 14 | 4 |
| 5 | dull | No | No | 6 | 10 | 18 | 19 | 13 | 4 |
| 6 | dull | Yes | No | 5 | 11 | 19 | 22 | 13 | 4 |
| p = 0.0434 | 10.67 |
20.50 |
16.50 |
4.17 | |||||
| Note: Comparison of GCS score between admission and discharge, | |||||||||
Based on the imaging data of the six patients, it was found that lesions were most commonly distributed in the thalamus, basal ganglia, and midbrain lesions. The appearance of hyperintensity in the corpus callosum, hippocampus, and subcortical white matter was more specific. The hyperperfusion metabolism in the lesion area in head CT perfusion imaging indicated the state of inflammatory activity in the lesion (Fig. 1). In the acute phase of JE, CT indicated brain tissue swelling, unclear gray and white matter boundaries, and multiple low-density foci in the bilateral basal ganglia (Fig. 2).
 Fig. 1.
Fig. 1.Head magnetic resonance imaging. (A) Asymmetrical long abnormal T2 signal from the bilateral thalamus. (B) Abnormal T2 signal from the bilateral hippocampus and midbrain. (C) Abnormal diffusion-weighted imaging high signal from bilateral thalamus. (D) Abnormal fluid-attenuated inversion recovery high signal from the bilateral thalamus, midbrain, and hippocampus. Brain functional magnetic resonance (ASL). (E,F) bilateral thalamus and left hippocampus hyperperfusion.
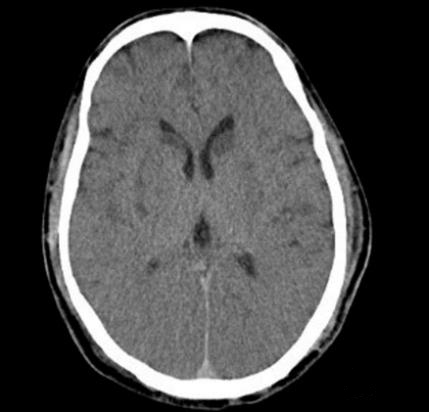 Fig. 2.
Fig. 2.Computed tomography. Brain tissue swelling, unclear gray and white matter boundaries, multiple low-density foci in the bilateral basal ganglia.
Cranial MRI has a stronger sensitivity than head CT in detecting brain tissue damaged by the JEV, especially in the early hours after onset. T2 and fluid-attenuated inversion-recovery (FLAIR) are more sensitive, while diffusion-weighted imaging (DWI) is relatively less sensitive. After treatment improves, the scope of inflammatory lesions will shrink or even disappear (Fig. 3; Table 3).
 Fig. 3.
Fig. 3.Before cranial magnetic resonance imaging treatment. (A) Bilateral thalamus long T2 signal. (B) Abnormal bilateral thalamus and midbrain fluid-attenuated inversion recovery high signal. (C) Abnormal corpus callosum pressure DWI high signal. After 23 days of treatment (D–F), abnormal signals of the bilateral thalamus, midbrain, and corpus callosum disappeared.
The window of the JEV antibody detection by IgM captured by ELISA is 3 days 2 two weeks, and multiple findings can improve the positive detection rate.
JE is a mosquito-borne infectious disease, and its pathogen, JEV, was first
isolated by Japanese scholars in 1935 [2]. In China, the primary vector of JE is
Culex tritaeniorhynchus. The disease has strict seasonality and is more
common in the summer and autumn. A previous study showed that the estimated
overall incidence of JE in 25 endemic countries (including China and Taiwan) was
2.2 cases per 100,000 people each year, with half of the cases occurring in China
[3]. In China, the early vaccination stage of JE began in the 1960s, but the
vaccination rate was not high (
Between 2011 and 2016, the incidence of JE per 100,000 people was
The six cases reported in this study were all from the same area, and the month
of onset was between August and September. All were adult onset, with an average
age of 59.80
The clinical manifestations of JE are characterized by high fever, headache, vomiting, disturbance of consciousness, and cognitive impairment. JE can be divided into three stages [8]: (1) the prodromal stage, manifesting as fever, headache, nausea, and vomiting; (2) the JE stage, occurring more than 3–5 days after the prodromal period, with confusion, meningeal irritation, muscle stiffness, and movement disorders; (3) the recovery period (sequelae period). Severe cases may be associated with pulmonary edema and respiratory failure [9]. Some patients have other symptoms after being infected with JEV, such as acute myelitis caused by transverse spinal cord injury [10] and Guillain–Barré syndrome caused by nerve root damage [11]. The patients in the present study all had acute onset, with fever and headache as the first symptoms; the disease then progressed rapidly, with high fever and unconsciousness, including five cases with respiratory failure.
The JEV can induce an inflammatory response as various immune cells accumulate
in the peripheral spleen, lymph nodes, and blood. Leukocytes, mainly neutrophils,
increase in the blood (Table 4). This response differs from that of other forms of viral
encephalitis [12]. The present study found that CSF pressure increased in
patients with JE. The CSF white blood cell count also increased, mostly in
(10–100)
| Case number | Gender | Age | Clinical manifestation | Routine blood | CSF | Antibody to JE | MRI or CT | ||
| Leukocyte counts (10 |
neutrophil percentages (%) | leukocyte counts (µL) | Trace total protein (mg/dL) | ||||||
| 1 | Female | 47 | high fever, headache, consciousness disorder | 11.42 | 89.2 | 35 | 68 | IgM (+) | MRI: Abnormal signals of thalamus, hippocampus and midbrain |
| 2 | Male | 57 | high fever, headache, consciousness disorder, respiratory failure | 14.21 | 86.4 | 100 | 66 | IgM (+) | CT: brain tissue swelling, multiple low-density lesions in bilateral basal ganglia |
| 3 | Male | 58 | high fever, headache, consciousness disorder, respiratory failure | 9.77 | 85.3 | 41 | 37 | IgM (+) | MRI: Abnormal signals of bilateral thalamus, midbrain and corpus callosum |
| 4 | Male | 65 | high fever, consciousness disorder, respiratory failure | 14.24 | 93.8 | 99 | 96 | IgM (+) | MRI: abnormal signal of right thalamus |
| 5 | Male | 72 | high fever, consciousness disorder, respiratory failure | 14.7 | 94.4 | 82 | 71 | IgM (+) | CT: right thalamus, right basal ganglia punctate low-density focus |
| 6 | Male | 66 | high fever, consciousness disorder, respiratory failure | 15.3 | 92.4 | 78 | 83 | IgM (+) | MRI:abnormal signals in the right thalamus, bilateral subcortical white matter and basal ganglia |
The brain MRI and CT scans of patients with JE show characteristic changes. The JEV enters the central nervous system through the blood–brain barrier and can extensively affect the brain and spinal cord, with thalamic and midbrain lesions being the most serious [14]. Low-density foci, such as unilateral or bilateral thalamus, basal ganglia, and brainstem, can be seen on cranial CT scans, and in some patients, obvious cerebral edema can be seen. The brain MRI is more sensitive, usually manifesting as high T2 weighted-imaging (T2WI) and FLAIR signals and T1WI and other signals at the affected site; DWI is mainly an equal or high signal in the acute phase [15, 16, 17] (Table 4). After comprehensive and symptomatic treatments, the inflammatory lesions shrink or even disappear. All the patients in the present study were diagnosed with JE, and there were no abnormal imaging findings in the thymus of any of the patients. Four of them underwent cranial CT examination, with two showing low-density foci in the thalamus and basal ganglia. Four patients underwent MRI examination of the brain, which showed an abnormally high signal of T2WI and FLAIR in the thalamus and/or midbrain. The MRI scan of the one of the patients showed cytotoxic edema of the corpus callosum pressure, and the DWI signal was high. Man et al. [18] reported the first reversible corpus callosum pressure lesion caused by JEV infection. The lesion was a cytotoxic edema with reversible recovery. The thalamus, especially with bilateral thalamus involvement, is a typical imaging manifestation of JE, with the midbrain, basal ganglia, and hippocampus also being common damage sites. However, when JE is strongly suspected in patients with bilateral thalamus and midbrain involvement, other diseases, such as Wernicke encephalopathy, Wilson’s disease, hypoxic encephalopathy, and thalamus infarction, should also be excluded.
In terms of the current methodology for detecting JEV antigens and antibodies, the virus mainly exists in the brain tissue. It can be isolated from the brain tissue of those who die in the early stage of the disease, however, so it is not suitable for the diagnosis of JE in the acute stage. In addition, the reverse indirect hemagglutination method can be used to measure antigens in the early CSF to determine the JEV antigen. Although this method is sensitive, simple, and fast, the positive rate is only 66.7%. The JE-specific IgM antibody has a high positivity rate, up to 97%, and has the characteristic of rapid sensitivity. Immunofluorescence is used to detect the antibodies in cells or tissues, which is more suitable for qualitative detection, while ELISA is used to detect serum and tissue homogenates. The detection of antigen or antibody levels in bodily fluids, such as cell culture fluid and urine, can obtain specific values and achieve the purpose of quantitative detection. An ELISA can realize the quantitative analysis of multiple samples and has higher sensitivity. Although the plaque-neutralizing antibody method is relatively quantitative, it requires cultured cells. The cycle takes about 28 days and is very long, so this method should only be considered for patients in the convalescent period.
In the clinical diagnosis of acute JE, the capture method ELISA is used because the specific IgM and IgG for some antigens in the serum often coexist, and the latter interferes with the determination of IgM antibodies. First, all serum IgM (including heterogeneous and non-specific IgM) are immobilized in the solid phase, then the specific IgM is determined after removing the IgG. In this way, the detection of JE-specific IgM has a strong effect. The sensitivity and specificity positivity rate of this method is 74.4%, of which 93% of cases are positive on the fourth day of the disease and can be used for early diagnosis. This method can also distinguish other flavivirus-specific antibodies (forest encephalitis and dengue viruses). The Center for Disease Control uses this method to routinely detect JE IgM antibodies.
The JEV anti-IgM antibodies are an important basis for diagnosing JE. These antibodies in the blood generally appear 3–4 days after disease onset. Clinically, for patients with a high suspicion of JE, both serum and CSF JEV IgM antibodies should be tested at the same time, as the antibodies can be detected in the serum of patients with asymptomatic infection and vaccination, but they cannot be detected in the CSF [15]. Due to their poor condition and lack of coordination, some patients do not have CSF IgM antibodies at the same time, so only serum IgM antibodies are tested. Although the diagnostic value of serum IgM is lower than that of CSF, when combined with clinical and imaging performance, a diagnosis can still be made [19]. The serum and CSF JEV antibodies of the first patient in this study were negative on the second day of onset, which may be related to the shorter detection time from the onset of the disease. On the fourteenth day following onset, the serum was sent to the Beijing Disease Control Center for testing, and the result returned positive for serum JE IgM antibodies. This shows that clinicians must remain vigilant for patients with high clinical suspicion of JE but with negative serum and CSF antibody tests. The possible reasons for this must also be considered, and the patient should be tested again to confirm the diagnosis.
There is no specific antiviral treatment for JE. Controlling high fever, cerebral edema, and respiratory failure and preventing and treating complications are the keys to current treatment. Previous research has found that the fatality rate of inpatients with JE is 20–30%, and approximately 33–50% of survivors exhibit long-term sequelae [20]. The patients in the present study were treated with dehydration (to reduce intracranial pressure), infection control, and symptomatic support. Four of the patients with high fever and respiratory failure were also treated with mild hypothermia, brain protection, and mechanical ventilation. One patient ceased their treatment and was transferred to a local hospital (their prognosis is unknown). The remaining five patients achieved better treatment results and were discharged.
In conclusion, JE may be considered in patients presenting with symptoms of central nervous system infection if the following criteria are met: (1) the onset is prevalent in July–September, with a history of mosquito bites; (2) the symptoms are acute onset, high fever, headache, vomiting, consciousness disorder, convulsions, status epilepticus, and slow or absent bilateral pupillary light reflex; (3) on MRI, lesions mostly involve the cerebral cortex, midbrain, basal ganglia, and thalamus. In the hippocampus, corpus callosum, and brainstem, especially when abnormal signals appear on T2 and FLAIR images, the possibility of JE should be considered. It also needs to be differentiated from viral encephalitis, meningitis, ADEM, and encephalopathy caused by autoimmune brain and systemic diseases. It is necessary to improve the determination of serum-specific antibodies and JEV antigens as soon as possible. Patients who test negative for JE need to be retested 3–5 days later to confirm the positivity rate of their tests.
HG and LS conceived the idea and conceptualised the study. HG and XS collected the data. LS and WH analysed the data. HG and WH drafted the manuscript, then LS and XS reviewed the manuscript. All authors read and approved the final draft.
This study was retrospective and did not require ethical approval. Informed consent from the patient is not required.
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Beijing Natural Fund General Project, 7152058, IL-17 in the pathogenesis of optic neuromyelitis animal model Research on the role of control.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
