†These authors contributed equally.
Academic Editor: Giovanna Zamboni
Distraction and reinterpretation have been recognized as two different tactics of emotion regulation. As a tactic of attention deployment, distraction involves shifting attention to neutral information or performing a secondary task to distract attention from emotion stimuli of the primary task. Reinterpretation, a representative tactic of cognitive change, was defined as changing the meaning of a situation to enhance or reduce its emotional impact. Thus, there are significant differences between the two processes. We wondered if the neural mechanisms underlying distraction and reinterpretation are different. Even though their neural correlates have been widely studied with functional magnetic resonance imaging (fMRI), few studies were conducted to compare the two tactics directly. Here we conducted an activation likelihood estimation (ALE) meta-analyses to investigate the common or different neural bases of distraction and reinterpretation. Moreover, we also used the meta-analytic connectivity modeling (MACM) to identify the emotion regulation network of distraction and reinterpretation. Overall, we found that the left dorsal lateral prefrontal cortex (DLPFC) was consistently activated during distraction and reinterpretation, whereas the left amygdala and inferior frontal gyrus/ventrolateral prefrontal cortex (VLPFC) were specifically activated during reinterpretation alone. The results indicate that the neural basis of distraction and reinterpretation are similar but not identical. The MACM results showed that distraction and reinterpretation share a common emotion regulation network, including the bilateral DLPFC, the dorsal medial prefrontal cortex, the inferior parietal lobule, the insula, the left (pre) supplementary motor area, the left middle temporal gyrus, and the right superior temporal gyrus. However, that network may subserve different functions when adopting various emotion regulation strategies. In addition, we suggest that the emotion regulation network of the left VLPFC may be a specific regulatory network for reinterpretation.
Emotion regulation is critical for our mental health and daily social life [1, 2, 3, 4]. It has been defined as the ability to alter the way one experiences, influences, and expresses the emotions that we experience in our daily life [5]. Theoretically, the process of emotion generation can be divided into four stages over time, including situation; attention; appraisal; and response [6]. In line with the process of emotion generation, emotion-regulation strategies can be categorized as situation selection; situation modification; attentional deployment; cognitive change; and response modulation. Among these strategies, attentional deployment and cognitive change have been widely used and studied in clinical and scientific research.
Distraction is the most studied tactic of attentional deployment [7, 8]. It involves shifting attention to neutral information or performing a secondary task to distract attention from emotional stimuli of the primary task. Therefore, it is well known that distraction mainly involves selective attention. To date, most reports on the specific brain regions related to distraction are inconsistent and incomplete. Some neuroimaging studies have demonstrated that the anterior cingulate cortex, ventrolateral prefrontal cortex (VLPFC), and parietal cortex were associated with distraction [9, 10]. Others showed that the bilateral supplementary motor areas (SMA), dorsal lateral prefrontal cortex (DLPFC), right inferior parietal lobule (IPL), anterior insula, precuneus, and bilateral middle cingulate gyrus were activated during the distraction, whereas the bilateral amygdala and temporo-occipital cortex were deactivated [11]. Regarding the well-established role of the lateral prefrontal cortex (PFC) in selective attention, the neural mechanism of distraction was also explained as the interactions between the lateral orbital frontal cortex (OFC) and lateral PFC [12, 13, 14, 15, 16].
Compared to other regulatory strategies, cognitive change is considered as one of the most flexible and effective means of reducing the negative impact of an aversive event [17]. Cognitive change was defined as changing the meaning of a situation to enhance or reduce its emotional impact. It includes various tactics such as reinterpretation (changing the meaning of a stimulus), detachment (creating a sense of physical or psychological distance from the emotional event), and acceptance (invoking the justification that sometimes, bad things happen). Reinterpretation is the most representative tactic of cognitive change. In psychology, it mainly involves the process of cognitive control [18]. Several neuroimaging studies have shown that reinterpretation is associated with activation of a portion of the prefrontal cortex (left DLPFC, bilateral VLPFC, and dorsal medial prefrontal cortex (DMPFC)), the bilateral parietal lobule, and the left temporal gyrus [19, 20, 21].
As mentioned above, there are significant differences between distraction and reinterpretation in emotion regulation. These differences may suggest distinct neurobiological mechanisms underlying distraction and reinterpretation. However, studies directly comparing distraction and reinterpretation were limited and inconsistent. The differences in neural mechanism between distraction and reinterpretation, if any, are still elusive.
The present study aimed at further probing the two emotion regulation tactics to elucidate the following issues. (a) Previous research suggests that the activation evoked by distraction and reinterpretation are similar but not identical. Thus, the current study intends to make clear which brain regions are common to, or different in, reinterpretation and distraction. (b) Most of the existing meta-analyses of distraction and reinterpretation are indirect, and only include studies of a single tactic but do not compare the two [20, 22]. Here, we performed an activation likelihood estimation (ALE) meta-analysis, in which only studies comparing distraction and reinterpretation were included, to directly probe the common and different neural bases. (c) Meta-analytic connectivity modeling (MACM) was used to examine the functional connectivity of the specific brain regions of the two tactics. Moreover, we investigated the difference between the current direct and the previous indirect meta-analyses.
A comprehensive literature search was conducted using MEDLINE (PubMed), PsycARTICLES (EBSCOhost), EMBASE (Ovid), and Web of Science, searching for combinations of keywords: “emotion regulation”, “affective regulation”, “distraction”, “selective attention”, “reappraisal”, “reinterpretation”, “cognitive change”, “fMRI”, “neuroimaging”, “functional magnetic resonance imaging”, and “functional MRI”. The search was limited to (a) the English language, (b) fMRI studies on reinterpretation and distraction in (c) healthy human samples (d) published until March 2020. In addition, several studies were selected by manual searches from review article reference lists. If the same neuroimaging data were used in more than one study, only the first reported study was included. If the data we needed were incomplete or absent, we contacted the corresponding authors for the complete data. The literature search resulted in 1741 articles after removing duplicates (Fig. 1).
 Fig. 1.
Fig. 1.PRISMA flow diagram. PRISMA, Preferred reporting items for systematic reviews and meta-analyses (http://www.prismastatement.org/).
We included studies comparing blood oxygen level-dependent (BOLD) responses during reinterpretation and distraction tasks among healthy volunteers. Specifically, participants were presented with negative images from the International Affective Picture System (IAPS, University of Florida, Gainesville, FL, USA, http://www.csea.phhp.ufl.edu), and were instructed to down-regulate negative affect [23]. The task comprises three conditions: maintain, reinterpretation, and distraction. We included studies in which the comparison of reinterpretation and distraction was conducted. Studies were considered only when the whole-brain analyses and standard anatomical reference space (Talairach/Tournoux; Montreal Neurological Institute (MNI)) were used.
Studies that conducted either functional-connectivity analyses or region-of-interest analyses were excluded. We also excluded studies in which whole-brain statistical results were not reported, or in which peak coordinates or statistical parametric maps (SPMs) could not be obtained. In addition, to ensure the reliability of our result, the studies were excluded if statistical thresholds varied in different conditions.
Four studies were included in our research (Table 1, Ref. [8, 11, 24, 25]). The number of subjects and peak coordinates were extracted from the original articles or supplementary material. The literature search, and decisions on inclusion and data extraction, were all conducted independently by two authors (Yuluo Liu & Xiangyu Wang).
| Author | Participants | Cognitive reappraisal tactics used | Attention deployment tactics used | Stimulus material | Coordinate space | |
| Number (female) | Age, y, mean (SD) | |||||
| McRae et al. [24] | 18 (18) | 24.4 (3.5) | Reinterpretation | Distraction by six-letter string | Negative images (IAPS) | MNI |
| Kanske et al. [8] | 30 (17) | 21.8 (2.1) | Reinterpretation | Distraction by arithmetic problems | Negative images (IAPS) | MNI |
| Dörfel et al. [11] | 35 (35) | 23.15 (N) | Reinterpretation | Distraction by arithmetic problems | Negative images (IAPS) | MNI |
| Hermann et al. [25] | 27 (27) | 21.59 (2.58) | Reinterpretation | Distraction by thinking neutrual situation | Negative images (IAPS) | MNI |
| Notes: SD, Standard deviation; IAPS, International Affective Picture System (https://csea.phhp.ufl.edu/Media.html). | ||||||
All meta-analyses were performed using the revised ALE
algorithm for coordinate-based analyses as implemented in Ginger ALE software
(BrainMap, San Antonio, TX, USA, version 3.0.2,
http://www.brainmap.org/ale/) [26, 27]. The ALE meta-analysis algorithm has four
main steps: ALE scores; null distribution; thresholding; and cluster statics.
Before performing meta-analysis, we classified and summarized the coordinate data
of each experiment into four datasets: co-activation; co-deactivation;
reinterpretation versus distraction; and distraction versus reinterpretation.
Within each dataset, there were different foci groups according to different
experiments. To describe the convergence of the assessed experiments for each
dataset, a whole-brain modeled activation map (MA-map) was obtained by estimating
the activation probabilities of each voxel in the brain [28].
The ALE image was a union of all of the
MA-maps. Then, the MA-maps of each experiment were calculated to determine a
voxel-wise ALE score. In the steps of null distribution, the
p-values table of ALE scores was obtained by
comparison to an empirical null distribution. The ALE image and the
p-values table were used to create a 3D p-value image. In the
steps of thresholding, a permutation test was adopted to identify the true
convergence of foci across different experiments from random spatial association
by comparing the ALE scores to an empirical null distribution [29, 30].
Histograms of the ALE scores obtained under the permutation distribution were
then employed to assign p-values to compute the ALE map threshold [29].
The resulting ALE maps were determined at a cluster-level Family Wise Error (FWE)
rate corrected threshold of p
To investigate the neural network of distraction and reinterpretation, we selected the activation areas of meta-analysis as volumes of interest (VOIs) for further analysis by using the meta-analytic connectivity modeling (MACM). Therefore, the left middle frontal gyrus/DLPFC, which was activated during both distraction and reinterpretation, was employed to uncover the common emotion regulation network. The left amygdala and the left inferior frontal gyrus/VLPFC, which were activated during the reinterpretation in the absence of distraction, were used to identify the neural network of reinterpretation. All VOIs were imported into the BrainMap database to search for the studies that reported activation within each VOI boundary [31]. The search criteria in the current study included: “context: normal mapping”; “behavioral domain matches: cognition attention or emotion”; “imaging modality: fMRI”; and “activations: activation only”.
We then downloaded whole-brain coordinates of activations from identified contrasts: left middle frontal gyrus/DLPFC = 502 subjects, 30 experiments, 494 foci; left amygdala = 764 subjects, 47 experiments, 821 foci; and left inferior frontal gyrus = 494 subjects, 29 experiments, 456 foci. These foci were then used to determine co-activated brain areas by ALE meta-analyses.
The pooled functional meta-analyses included 4 comparative studies with 110 subjects and 102 brain foci (Table 1). Brain clusters of the pooled analysis include the frontal gyrus, inferior parietal lobe, precuneus, insula, amygdala, and superior and middle temporal gyrus. Areas demonstrating common and different activation between reinterpretation and distraction are presented in Table 2 and illustrated in Fig. 2.
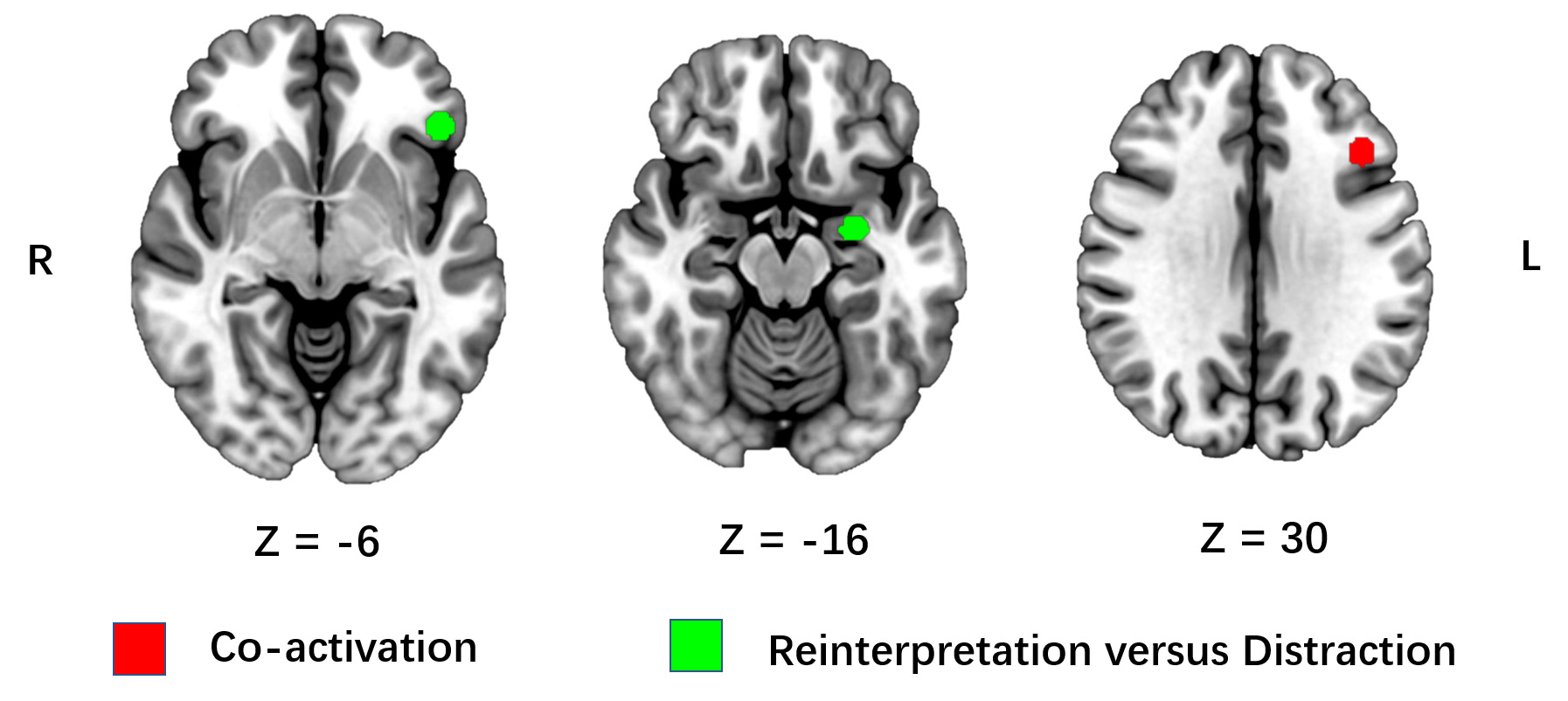 Fig. 2.
Fig. 2.The common and different activation for reinterpretation and
distraction. ALE meta-analysis of comparative neuroimage studies on
reinterpretation and distraction. The co-activation of distraction and
reinterpretation is represented by red clusters, whereas specific activation of
reinterpretation is represented by green clusters. Results are displayed at a
cluster-level Family Wise Error (FWE) rate corrected threshold of p
| Contrasts | Cluster number | Cluster size (mm |
Region | BA | Side | Maximum ALE score | p | Z-score | MNI coordinates | ||
| X | Y | Z | |||||||||
| Co-activation of distraction and reinterpretation | |||||||||||
| 1 | 592 | Middle Frontal Gyrus/DLPFC | 9 | L | 0.016 | 4.17 |
5.36 | –42 | 24 | 30 | |
| Reinterpretation versus distraction | |||||||||||
| 1 | 864 | Amygdala | L | 0.022 | 1.25 |
5.96 | –28 | –6 | –16 | ||
| 2 | 552 | Inferior Frontal Gyrus/VLPFC | 47 | L | 0.017 | 1.51 |
5.12 | –46 | 30 | –6 | |
| Notes: BA, Brodmann’s area; MNI, Montreal Neurological Institute; p, p-value; DLPFC, Dorsolateral prefrontal cortex; VLPFC, Ventrolateral prefrontal cortex. | |||||||||||
The ALE analyses revealed activation of the left middle frontal gyrus/DLPFC during both reinterpretation and distraction (Table 2). The results also showed that no brain regions were co-deactivated during both reinterpretation and distraction. When compared to distraction, reinterpretation specifically recruited the left amygdala and the left inferior frontal gyrus (Table 2). In contrast, there were no specific brain structures activated in the condition of distraction when compared to reinterpretation.
MACM analysis for the left DLPFC revealed convergent co-activation in bilateral DLPFC, insula, IPL, DMPFC, left (pre)SMA, left middle temporal gyrus, and right superior temporal gyrus (see Table 3 and Fig. 3).
| Cluster number | Cluster size (mm |
Region | BA | Side | Maximum ALE score | p | Z-score | MNI coordinates | ||
| X | Y | Z | ||||||||
| 1 | 11320 | Middle Frontal Gyrus/DLPFC | 9 | L | 0.128 | 0 | 15.62 | –42 | 24 | 30 |
| Insula | 13 | L | 0.033 | 6.45 |
6.07 | –32 | 22 | 2 | ||
| Precentral Gyrus | 6 | L | 0.020 | 1.03 |
4.26 | –42 | 6 | 36 | ||
| 2 | 4920 | Middle Frontal Gyrus/DLPFC | 9 | R | 0.026 | 1.68 |
5.10 | 48 | 20 | 30 |
| Middle Frontal Gyrus/DLPFC | 8 | R | 0.023 | 1.51 |
4.67 | 46 | 32 | 36 | ||
| 3 | 4464 | Inferior Parietal Lobule | 40 | L | 0.030 | 1.29 |
5.57 | –36 | –52 | 42 |
| Middle Temporal Gyrus | 39 | L | 0.017 | 1.18 |
3.68 | –42 | –60 | 36 | ||
| 4 | 2632 | Inferior Parietal Lobule | 40 | R | 0.032 | 2.42 |
5.85 | 38 | –52 | 46 |
| Precuneus | 7 | R | 0.015 | 2.60 |
3.47 | 32 | –64 | 42 | ||
| 5 | 2352 | Superior Frontal Gyrus/DMPFC | 6/88/32 | L/R | 0.038 | 1.94 |
6.61 | –2 | 20 | 50 |
| 6 | 1672 | Middle Frontal Gyrus/pre(SMA) | 6 | L | 0.029 | 1.64 |
5.53 | –26 | 2 | 54 |
| 7 | 1560 | Insula | 13 | R | 0.034 | 5.63 |
6.09 | 32 | 24 | 4 |
| 8 | 1064 | Medial Frontal Gyrus/DMPFC | 8 | R | 0.021 | 8.71 |
4.30 | 4 | 36 | 38 |
| 9 | 992 | Superior Temporal Gyrus | 22 | R | 0.026 | 2.44 |
5.03 | 54 | –26 | 0 |
| Notes: BA, Brodmann’s area; MNI, Montreal Neurological Institute; p, p-value; DLPFC, Dorsolateral prefrontal cortex; DMPFC, Dorsomedial prefrontal cortex; SMA, Supplementary motor areas. | ||||||||||
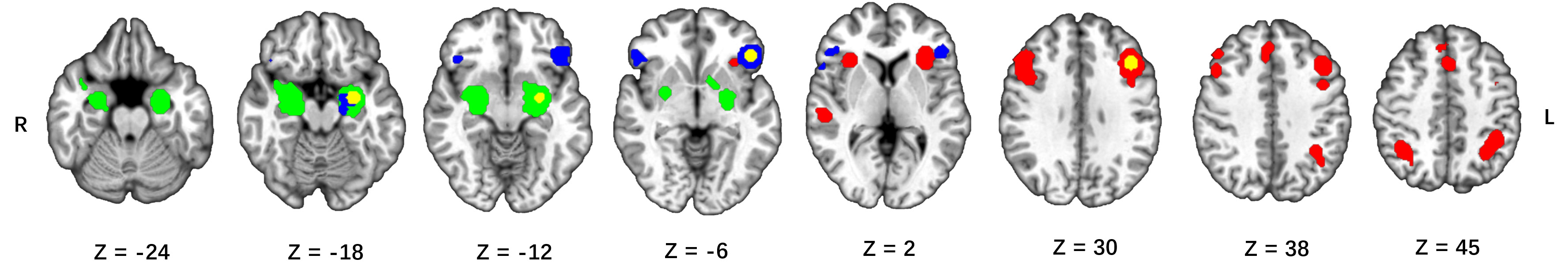 Fig. 3.
Fig. 3.Connectivity maps corrected for multiple comparisons (FWE
Co-activation maps for the left amygdala included the bilateral amygdala, left lentiform nucleus, right superior temporal gyrus (see Table 4 and Fig. 3).
| Cluster number | Cluster size (mm |
Region | BA | Side | Maximum ALE score | p | Z-score | MNI coordinates | ||
| X | Y | Z | ||||||||
| 1 | 9192 | Amygdala | L | 0.185 | 0 | 18.68 | –24 | –6 | –16 | |
| Lentiform Nucleus | L | 0.024 | 1.54 |
4.17 | –14 | 6 | –8 | |||
| 2 | 7464 | Amygdala | R | 0. 077 | 4.72 |
9.82 | 26 | –4 | –16 | |
| Superior Temporal Gyrus | 38 | R | 0.022 | 5.89 |
3.85 | 38 | 4 | –18 | ||
| Notes: BA, Brodmann’s area; MNI, Montreal Neurological Institute; p, p-value. | ||||||||||
For the left inferior frontal gyrus, significant co-activation was observed in the bilateral VLPFC, the left amygdala and the left parahippocampal gyrus (see Table 5 and Fig. 3).
| Cluster number | Cluster size (mm |
Region | BA | Side | Maximum ALE score | p | Z-score | MNI coordinates | ||
| X | Y | Z | ||||||||
| 1 | 4680 | Inferior Frontal Gyrus/VLPFC | 47 | L | 0.121 | 0 | 14.87 | –46 | 30 | –6 |
| 2 | 1936 | Inferior Frontal Gyrus/VLPFC | 45 | R | 0.031 | 6.41 |
5.69 | 54 | 30 | –4 |
| Inferior Frontal Gyrus/VLPFC | 47 | R | 0.019 | 3.45 |
3.98 | 44 | 26 | –14 | ||
| 3 | 1384 | Amygdala | L | 0.025 | 4.14 |
4.93 | –22 | –8 | –18 | |
| Parahippocampal Gyrus | 28 | L | 0.021 | 1.09 |
4.25 | –18 | –16 | –20 | ||
| Notes: BA, Brodmann’s area; MNI, Montreal Neurological Institute; p, p-value. | ||||||||||
Humans employ multiple strategies to down-regulate negative emotion that include attention deployment (e.g., distraction), cognitive reappraisal (e.g., reinterpretation), and response modulation. Here, we compared the neural bases of distraction and reinterpretation using meta-analysis and data from four comparative fMRI studies of a total of 110 participants. Our study showed that there were significant commonalities in the neural correlates of the two tactics as well as differences. We found that the left middle frontal gyrus/DLPFC was the only brain area involved in both distraction and reinterpretation to rise above the statistical threshold. The activation of DLPFC during both distraction and reinterpretation was consistent with previous literature [32, 33, 34]. The DLPFC was hypothesized to play a critical role in the cognitive control process of emotion regulation [19, 35]. But the DLPFC may subserve different functions when adopting various emotion regulation strategies. In the process of reinterpretation, the DLPFC was reported to be associated with emotional information reprocessing and guiding the goals of regulation [16, 36, 37]. Although when combined with the OFC, the DLPFC is involved in selective attention during distraction [12, 14].
Regarding the differential activation of distraction and reinterpretation, we observed that the left amygdala and inferior frontal gyrus/VLPFC were specifically activated during reinterpretation. The well-established role of the amygdala is that it receives processed information from the prefrontal and parietal cortex and regulates emotion responses during the last stage of emotion regulation [38, 39]. Deactivation of the amygdala has been recognized as a feature of successful emotion regulation [40, 41]. However, our results showed that the left amygdala was activated during reinterpretation in the absence of distraction. By careful review of the included studies, we found that the bilateral amygdala was deactivation during both reinterpretation and distraction in three of four studies [8, 11, 24], although the bilateral amygdala was activated during reinterpretation in the absence of distraction in two of the three studies [8, 24]. Therefore, we suggest that this may result in the greater deactivation of the left amygdala during distraction. In other words, our results suggest that distraction is more effective in regulating negative emotion than reinterpretation. This can be confirmed by the self-reported negative affect in the included studies [24]. However, given the small sample size of our study, much more research is needed to confirm whether distraction is more effective than reinterpretation.
The activation of the left inferior frontal gyrus/VLPFC during reinterpretation was consistent with previous findings [19, 21]. A wealth of studies suggests that the left VLPFC is involved in various cognitive functions such as selection and retrieval processes, semantic and phonological processing, inner speech generation, and semantic generation [42, 43, 44, 45]. The VLPFC has been reported also to be involved in the selection process of appropriate reappraisals and in language processes [21, 46]. In view of the different psychological processes of reinterpretation and distraction, we prefer to connect the specific activation of the left VLPFC with the semantic processing of reinterpretation.
One of the striking results was that no brain region was involved in distraction to a degree that rose above the statistical threshold (compare to reinterpretation). Nevertheless, a previous meta-analysis showed a similar result [20]. Thus, the results indicate that distraction shares a common core with reinterpretation during emotion regulation, which includes the left DLPFC. In addition, reinterpretation also recruits activation of the left VLPFC during emotion regulation.
As the MACM analyses result showed, significant co-activation of the left DLPFC was mainly observed in bilateral DLPFC; DMPFC; inferior parietal lobule; insula; left (pre)SMA; left middle temporal gyrus; and right superior temporal gyrus. This indicates that distraction and reinterpretation share a common emotion regulation network. Moreover, our findings are similar to the results of previous meta-analyses on emotion regulation strategy [19, 20]. As outlined before, we suggest that the prefrontal cortex (DLPFC and DMPFC) may play a role in information reprocessing and selective attention during emotion regulation [36, 37]. The (pre)SMA is involved in the process of executive control [47]. Specifically, it plays a role in inhibiting emotion responses and switching tasks during emotion regulation. The anterior insula has been associated with representing emotional state and self-reported arousal [48, 49, 50]. The insula is also considered as a pivotal region for the detection of emotional stimulus during the emotion process [51]. Besides, it was reported play a role of the supervisory attentional control during the distraction [52, 53]. The functions of these brain regions play a fundamental role in the process of emotional regulation. We suggest that this emotion regulation network is the core network of reinterpretation and distraction. In addition, the MACM analysis results also indicate an emotion regulation network of the left VLPFC, encompassing the bilateral VLPFC, left amygdala, and parahippocampal gyrus. The VLPFC is mainly involved in the selection of appropriate reappraisals and language processes during reinterpretation [21, 46], and the activity between amygdala and parahippocampal gyrus increases during facial information processing [54]. Semantic processing and facial information processing play an important role in reinterpretation. We propose that the emotion regulation network of the left VLPFC may be a specific regulatory network for reinterpretation.
The previous meta-analyses study of emotion regulation strategies showed that the bilateral anterior insula, the left (pre)SMA, and the VLPFC were activated during both distraction and reinterpretation. The contrast analysis did not reveal a significant difference between distraction and reinterpretation [20]. In contrast to the previous study, we found that the left middle frontal gyrus/DLPFC was activated during both distraction and reinterpretation, whereas the left amygdala and inferior frontal gyrus/VLPFC were activated in the condition of reinterpretation in the absence of distraction. The difference may be due to two potential reasons. On the one hand, it may due to the fact that the current meta-analyses study was more direct, because only the comparative studies of distraction and reinterpretation are included. The previous meta-analyses were based on comparing the ALE results of the individual meta-analyses with each other. On the other hand, the sample of experiments in the current study was so small that makes it hard to avoid biased results. The current study may not have been comprehensive enough to investigate the common and different neural bases of distraction and reinterpretation. Further research is needed to investigate the difference between direct and indirect meta-analyses.
Although the present study maintains consistency in the emotion regulation goals, strategy, and stimulus material, it is not without limitations. First, to investigate the common and different neural bases of distraction and reinterpretation, our meta-analyses relied solely on the contrast of reinterpretation and distraction in healthy volunteers. Therefore, only four experiments meeting the inclusion criterion were incorporated into the current study. The results were not comprehensive enough to cover all of the common and different brain regions of distraction and reinterpretation. Second, another potential limitation stems from the gender imbalance of the participants. In the current study, 97 of 110 participants were female. It is generally assumed that women are more emotionally responsive to negative stimuli than are men [55, 56], and women are more likely to employ a frontal top-down network to down-regulate negative emotion, whereas men may redirect attention away from stimulus by using posterior regions of the ventral attention network [57, 58]. Therefore, the large number of female participants in current study may be one of the reasons for activation of the DLPFC. Third, the ALE analysis approach assigns weight to each experiment according to the number of participants, so the larger the sample, the stronger the weight in the meta-analyses. The results will deviate when there is a significant difference in the number of participants among the included experiments. In the current study, the n of Dörfel’s et al. [11] study is nearly twice as large as that of McRae’s et al. [24] study. We suggest this imbalance may lead to a bias in the results.
Our results indicate that the neural basis of distraction and reinterpretation are similar but not identical. Although they share a common core, encompassing the left DLPFC, they also involve distinct regions, including the left amygdala, and inferior frontal gyrus/VLPFC. The left DLPFC seems to play a critical role of cognitive control in the process of emotion regulation, whereas the left VLPFC seems mainly involved in the semantic processing of reinterpretation. In addition, the activation of the left amygdala may suggest that distraction is more effective in regulating negative emotion than in reinterpretation. From the perspective of emotion regulation networks, we suggest that distraction and reinterpretation share a common emotion regulation network. However, the network may subserve different functions when adopting various emotion regulation strategies. The results also indicate that the emotion regulation network of the left VLPFC may be specific for reinterpretation. Our results point to the common and different neural bases of distraction and reinterpretation, which should provide a direction for further exploration of the neural mechanisms of the two tactics. Future work should pay more attention to the contrast of the two tactics in patients with emotion regulation disorders.
Author contributions included conception and study design—YL, XW, data collection or acquisition—YL, XW, SG, statistical analysis—YL, FT, interpretation of results—YL, ZL, LY, drafting the manuscript work or revising it critically for important intellectual content—YL, LY, YZ and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work—All authors.
Not applicable.
Not applicable.
Funding for this study was provided by National Key Research and Development Program of China (2018YFC0807203).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
