Academic Editor: Hongmin Wang
Background: Acute ischemic stroke (AIS) is the main cause of worldwide death and disability. Early neurological deterioration (END) can further increase the probability of death and disability in patients with ischemic stroke. Therefore, it is essential to find biomarkers to predict END early. Inflammatory response plays a crucial role in determining the course, outcome, and prognosis of END. Earlier studies focused on the relationship between routine hematological inflammatory markers and END, which limited the results. At present, relatively new and comprehensive markers of inflammatory response are relatively scarce. In this study, we investigate the predictive value of inflammatory markers in acute ischemic stroke cases for END which include systemic inflammatory response index (SIRI), platelet/lymphocyte ratio (PLR), lymphocyte/monocyte ratio (LMR), neutrophil/lymphocyte ratio (NLR), and then to establish a nomogram model. Methods: A total of 375 patients with AIS were analyzed who were admitted to the Second Affiliated Hospital of Harbin Medical University from September 2019 to June 2021. The associations between END and inflammatory markers were studied by employing the analysis of univariate. Following that, through regression models of the least absolute shrinkage and selection operator, the END risk model’s feature selection was optimized. The development of the model of prediction was carried out by applying the multivariable logistic regression analysis. The calibration, discrimination, and clinical efficacy of the prediction model were studied via calibration plot, C-index, and decision curve analysis (DCA). The bootstrapping validation method was used for the evaluation of internal validation. Results: We constructed a nomogram consisting of CRP, monocytes, NIHSS and SIRI. This model had desirable calibration and discrimination, with a C-index of 0.757 (95% confidence interval: 0.702–0.805). Interval validation could still achieve the higher C-index value of 0.747. When the risk threshold for END was greater than 13% but less than 84%, DCA proved to be clinically useful. Conclusions: Our research shows that SIRI can be used as a new predictor of END, as well as a monitor of treatment response. Compared with the traditional single inflammatory indicator, the integration of SIRI nomogram can predict the occurrence of END more objectively and reliably.
In general, one of the most key reasons for morbidity and mortality in the world is stroke [1], with an early neurological deterioration (END) rate of roughly 8.1–28.1% [2]. Studies have shown END may contribute to an increased probability of death and disability in acute ischemic stroke (AIS) patients [3, 4]. Increasing evidence shows that neuro-inflammatory response exerts critical roles in the occurrence and development of early neurological deterioration [5, 6]. In addition, the immune system is closely related to the pathogenesis and prognosis of stroke. Hypoperfusion and hypoxia may contribute to the release of M1 microglial subtype and pro-inflammatory cytokines, promoting the recruitment of surrounding immune cells and aggravating the damage of penumbra and blood-brain barrier [7]. Antibody production can also cause long-term damage to the central nervous system and affect patient outcomes. Routine blood tests used to assess inflammatory processes can often be used for early diagnosis of a variety of diseases. In particular, complete blood counts are easy to perform, inexpensive, and provide information on various cell types and morphologic parameters, such as white blood cell counts, lymphocytes, and neutrophils. At the same time, various new composite measures such as neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), systemic inflammatory response index (SIRI), can be used for various types of brain diseases, for instance, ischemic stroke [8], subarachnoid hemorrhage [9, 10], brain tumors [11]. SIRI is on the basis of peripheral blood counts of monocytes, lymphocytes, and neutrophils, which better reflect the balance between inflammatory response and immune state of the patient. SIRI’s predictive value in the occurrence of END in AIS patients is currently understudied. Furthermore, previous research has primarily focused on a single inflammatory marker, with few studies on hematological inflammatory markers.
The objective of this study is to investigate the association of inflammatory biomarkers with END in AIS patients, as well as assessment of predictive values of SIRI, PLR, NLR, and lymphocyte/monocyte ratio (LMR) for END. Additionally, a nomogram was developed in conjunction with SIRI to improve the discriminative capability of the inflammatory parameters for END.
This is a single-center retrospective cohort research.
From September 2019 to June 2021, consecutive AIS patients admitted to the
Neurology department, Harbin Medical University’s Second Affiliated Hospital,
were retrospectively analyzed. Following was the inclusion criteria: (1) age
Following data was collected: patients’ age, sex, previous history (drinking,
smoking, hypertension, coronary heart disease, diabetes mellitus, acute
myocardial infarction, cerebral infarction) and NIHSS scores at admission.
Furthermore, laboratory parameters such as triglyceride (TG), total cholesterol
(TC), high-density lipoprotein cholesterol (HDL), low-density lipoprotein
cholesterol (LDL), high sensitivity C-reactive protein (CRP), baseline blood
glucose (G), homocysteine (HCY), and blood routine (white blood cell, lymphocyte,
neutrophil, and monocyte counts) were obtained upon admission [12]. Calculations
of NLR, SIRI, LMR, and PLR, were performed as (NLR = neutrophil count/lymphocyte
count; SIRI = neutrophil count
The NIHSS was employed for assessing the stroke severity on the day of admission
and every day for 7 days after admission. The NIHSS consists of 15 items with a
total score of 42, the greater score associated with the more severe the stroke
[13]. All neurologists received unified training in NIHSS scoring evaluation.
When the overall score of NIHSS is enhanced by
Median (quartile) or mean
Using the method of least absolute shrinkage and selection operator (LASSO)
[17], the variables p
We collected 375 consecutive cases suffering from AIS processed at our hospital
from September 2019 to June 2021. The cohort included 164 patients with early
deterioration in the neurological system and 211 cases with non-early
deterioration in the neurological system. There were 128 (34.1%) female and 247
(65.9%) male patients. The average age of the cases was 61.9
| parameter | END group (n = 164) | Non-END group (n = 211) | ALL (n = 375) | p value |
| Age (years) | 63 (56–71) | 63 (53–68) | 63 (55–69) | 0.02 |
| Male (%) | 111 (67.7%) | 136 (64.5%) | 249 (64.5%) | 0.51 |
| Smoking (%) | 73 (44.5%) | 85 (40.3%) | 158 (42.1%) | 0.49 |
| Drinking (%) | 41 (25.0%) | 57 (27.0%) | 98 (26.1%) | 0.84 |
| Hypertension (%) | 109 (66.5%) | 154 (73.0%) | 263 (70.1%) | 0.05 |
| Diabetes mellitus (%) | 51 (31.1%) | 58 (27.5%) | 109 (29.1%) | 0.52 |
| CHD (%) | 17 (10.4%) | 24 (11.4%) | 41 (10.9%) | 0.76 |
| CI (%) | 46 (28.0%) | 55 (26.1%) | 101 (26.9%) | 0.67 |
| AMI (%) | 5 (3.0%) | 3 (1.4%) | 8 (2.1%) | 0.31 |
| NIHSS (score) | 4 (3–7) | 3 (1–6) | 4 (2–6) | |
| TG (mmol) | 1.6 (1.1–2.3) | 1.4 (1.1–2.1) | 1.5 (1.1–2.2) | 0.24 |
| TC (mmol) | 4.5 (3.9–5.3) | 4.5 (3.8–5.2) | 4.5 (3.9–5.2) | 0.89 |
| HDL (mmol) | 1.0 (0.9–1.1) | 1.1 (0.9–1.2) | 1.0 (0.9–1.2) | 0.17 |
| LDL (mmol) | 2.94 |
2.78 |
2.8 (2.2–3.4) | 0.73 |
| CRP (mg/L) | 3.1 (1.3–7.3) | 1.8 (0.8–4.5) | 2.4 (0.9–5.7) | |
| G (mmol) | 6.0 (5.2–8.2) | 5.8 (5.1–7.2) | 5.9 (5.1–7.8) | 0.16 |
| HCY (µmol/L) | 13.0 (10.1–17.8) | 14.4 (12.0–20.5) | 13.9 (10.7–18.9) | 0.01 |
| WBC (10 |
7.5 (6.4–8.8) | 7.6 (6.1–7.6) | 7.6 (6.3–8.8) | 0.72 |
| Neutrophils (10 |
5.1 (4.2–6.5) | 5.0 (4.1–6.2) | 5.1 (4.1–6.4) | 0.23 |
| Lymphocytes (10 |
1.8 (1.4–2.2) | 2.1 (1.6–2.4) | 1.9 (1.5–2.3) | 0.001 |
| Monocytes (10 |
0.3 (0.3–0.4) | 0.3 (0.2–0.3) | 0.3 (0.2–0.4) | |
| SIRI | 1.0 (0.6–1.5) | 0.6 (0.4–1.0) | 0.7 (0.5–1.2) | |
| PLR | 121.8 (90.2–156.8) | 117.7 (89.7–159.2) | 119.1 (90.1–157.5) | 0.96 |
| NLR | 3.0 (2.2–4.0) | 2.5 (2.0–3.2) | 2.7 (2.0–3.5) | |
| LMR | 5.4 (3.9–7.1) | 9.0 (5.8–11.5) | 6.9 (4.6–10.0) | |
| Note: CHD, coronary heart disease; NLR, neutrophil-lymphocyte ratio; CI, cerebral infarction; AMI, acute myocardial infarction; HDL, high-density lipoprotein cholesterol; TG, triglyceride; LDL, low-density lipoprotein cholesterol; NIHSS, National Institute of Health Stroke Scale; TC, total cholesterol; G, baseline blood glucose; CRP, high sensitivity C-reactive protein; WBC, white blood cell; HCY, homocysteine; PLR, platelet lymphocyte ratio; SIRI, systemic inflammation response index; LMR, lymphocyte/monocyte ratio. | ||||
Analysis for all the variables was performed via the univariate binary logistic
regression. In order to prevent omissions, we set the p-value as 0.1 as
the cut-off value, and determined 7 variables related to END for further
analysis, as shown in Table 2. By performing the LASSO analysis,
a 4 variable analysis was constructed according to the optimum
| Variable | OR (95% CI) | p value |
| Age (years) | 1.03 (1.01–1.05) | |
| Male (%) | 0.87 (0.56–1.33) | 0.51 |
| Smoking (%) | 1.11 (0.75–1.63) | 0.61 |
| Drinking (%) | 0.86 (0.56–1.32) | 0.49 |
| Hypertension (%) | 1.02 (0.86–1.22) | 0.79 |
| Diabetes mellitus (%) | 1.13 (0.73–1.73) | 0.59 |
| CHD (%) | 0.90 (0.47–1.74) | 0.76 |
| AMI (%) | 1.11 (0.47–1.74) | 0.67 |
| CI (%) | 2.18 (0.51–9.26) | 0.29 |
| NIHSS (score) | 1.15 (1.07–1.23) | |
| TG (mmol) | 1.04 (0.9–1.21) | 0.56 |
| TC (mmol) | 0.99 (0.83–1.17) | 0.88 |
| HDL (mmol) | 0.56 (0.27–1.18) | 0.13 |
| LDL (mmol) | 1.22 (0.97–1.53) | 0.10 |
| CRP (mg/L) | 1.11 (1.05–1.16) | |
| G (mmol) | 1.06 (0.99–1.13) | 0.08 |
| HCY (µmol/L) | 0.97 (0.95–1.02) | 0.46 |
| WBC (10 |
1.02 (0.91–1.16) | 0.7 |
| Neutrophils (10 |
1.11 (0.97–1.27) | 0.12 |
| Lymphocytes (10 |
1.02 (0.95–1.08) | 0.62 |
| Monocytes (10 |
33.3 (7.82–141.8) | |
| SIRI | 3.14 (2.09–4.43) | |
| PLR | 1 (1–1) | 0.47 |
| NLR | 1.15 (1.03–1.27) | 0.01* |
| LMR | 0.92 (0.88–0.97) | |
| Note: *Variables with p | ||
| Intercept and variable | OR (95% CI) | p value |
| Intercept | 0.08 (0.04–0.16) | |
| CRP | 1.06 (1.01–1.120 | 0.02 |
| Monocytes | 8.30 (1.81–40.30) | |
| NIHSS | 1.12 (1.04–1.21) | |
| SIRI | 2.52 (1.68–3.95) | |
| Note: CRP, high sensitivity C-reactive protein; NIHSS, National Institute of Health Stroke Scale; SIRI, systemic inflammation response index. | ||
 Fig. 1.
Fig. 1.Lasso regression analysis based on Selection of predictors. (a) LASSO regression of the 7 variables. (b) Cross-validation for tuning the selection of parameters in the regression of LASSO.
Table 3 summarizes the findings of the analysis of logistic regression amongst CRP, Monocytes, NIHSS, and SIRI. A model was developed by incorporating these independent predictors and presented in the form of a nomogram (Fig. 2).
 Fig. 2.
Fig. 2.Advancement of a model of risk prediction for END. CRP, high sensitivity C-reactive protein; NIHSS, National Institute of Health Stroke Scale; SIRI, systemic inflammation response index.
Apparent performances of the risk nomogram for early deterioration in the neurological system within the cohort.
The assessment of the ROC curve was applied to ascertain SIRI’s predictive capability for END. The SIRI optimal cut-off value on admission was 0.767 with 0.712 the area under the curve (AUC), the specificity and the sensitivity were 65.2%, and 67.3%, respectively, 50.3% negative predictive value, and 77.5% positive predictive value. Furthermore, SIRI was better to predict END risk than other inflammatory markers (Fig. 3).
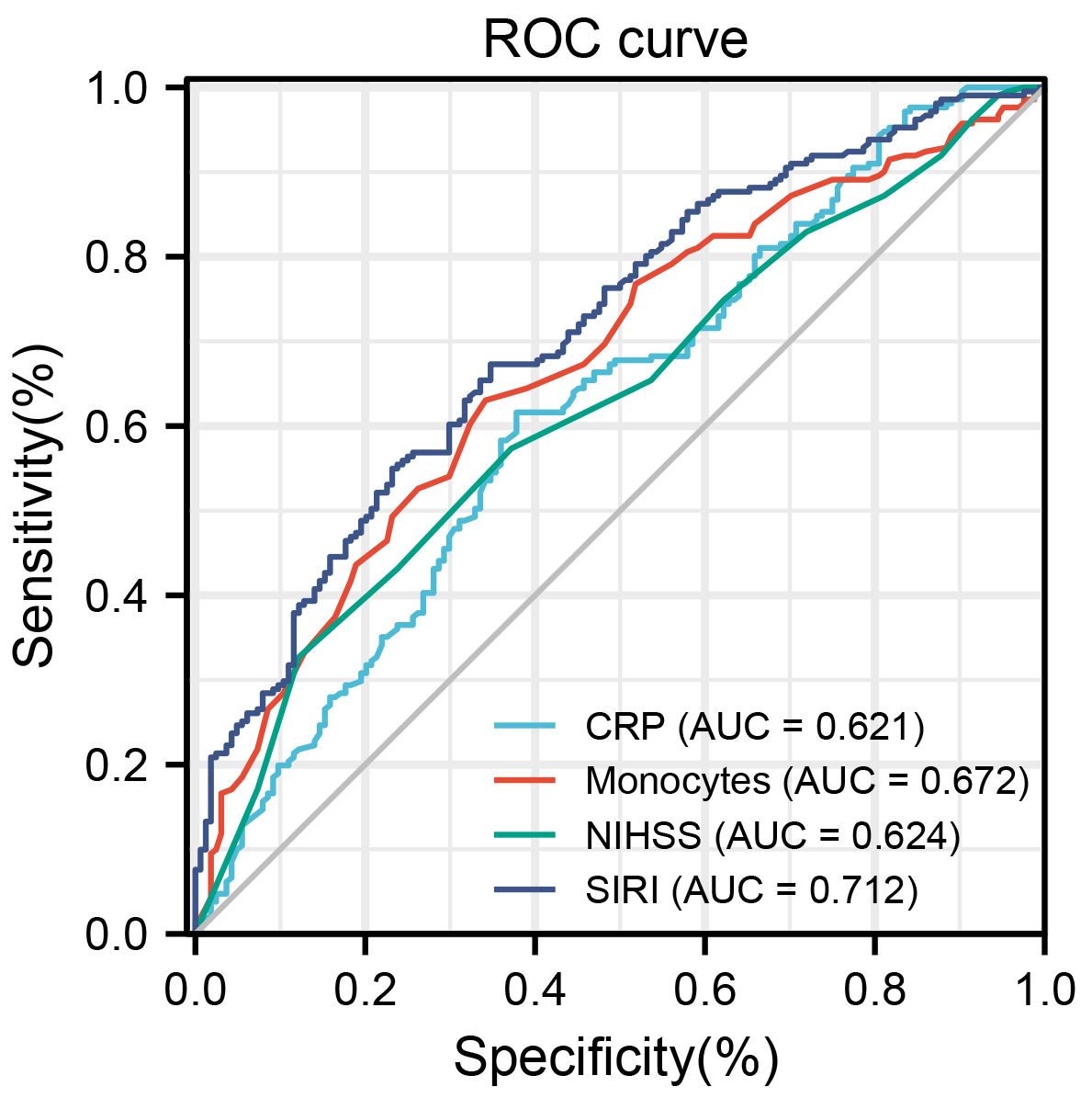 Fig. 3.
Fig. 3.Analysis of ROC curve for the predictive values of SIRI and other markers (CRP, Monocytes, NIHSS) for END. ROC, receiver operating characteristic; END, early neurological deterioration; CRP, high sensitivity C-reactive protein; NIHSS, National Institute of Health Stroke Scale; SIRI, systemic inflammation response index.
Calibration curves used for estimating the early neurological deterioration risk in AIS patients showed good agreement across the cohort (Fig. 4). For the nomogram prediction, the C-index was 0.757 (95% CI: 0.702–0.805) for the cohort, and further validated through bootstrapping validation as 0.747, indicating the model had good discriminant ability.
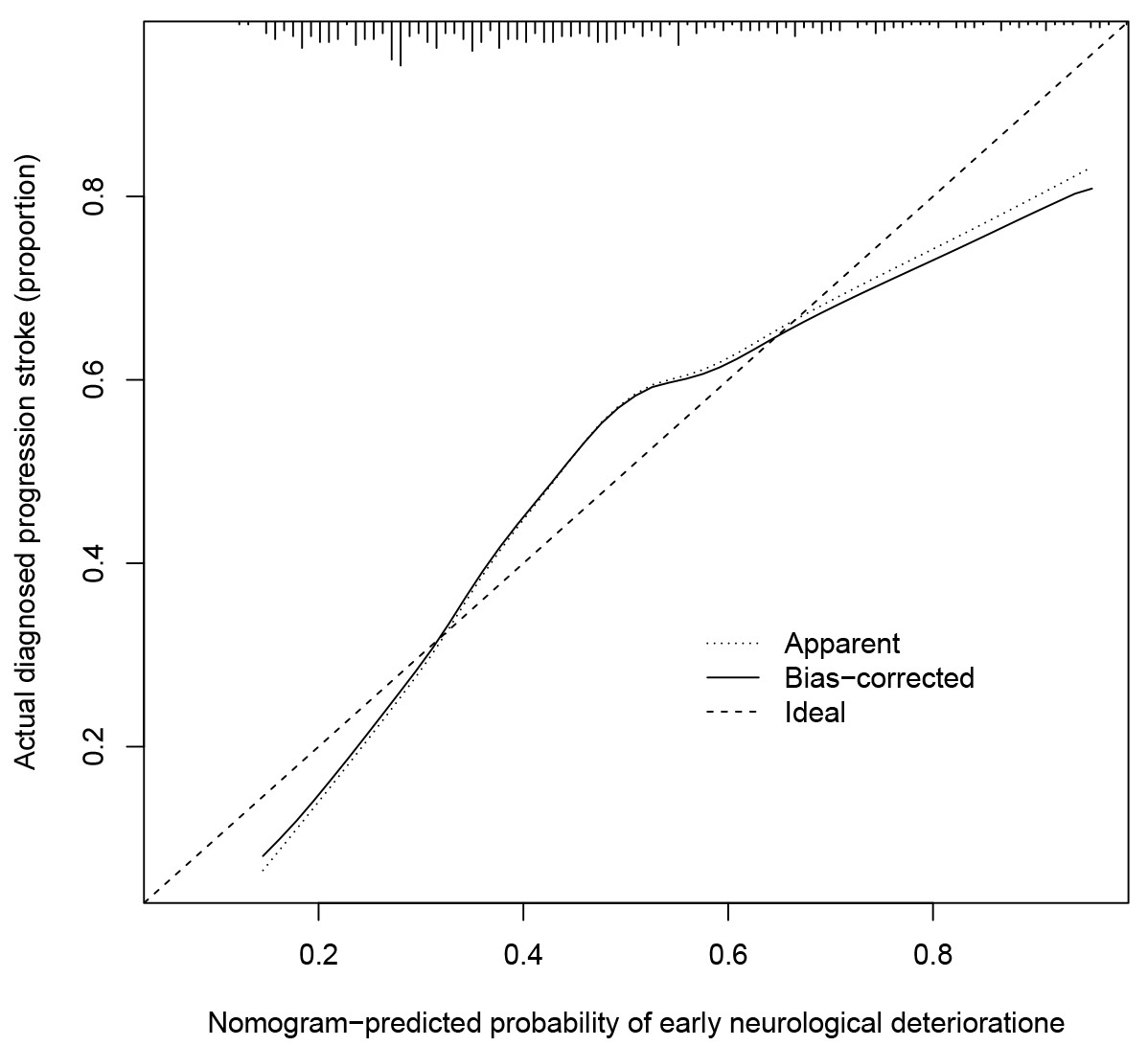 Fig. 4.
Fig. 4.Calibration curves of the END risk nomogram. The x-axis shows the estimated early neurological deterioration risk while the y-axis signifies the actually diagnosed early neurological deterioration. Dashed diagonal lines demonstrate the excellent estimations of the ideal model. The nomogram performance represents through the solid line, and the closer the solid line is to the diagonal dotted line results in more desirable estimation.
The clinical application value of DCA in assessing the risk of END nomogram.
Fig. 5 exhibits the DCA of the END risk nomogram. According to the decision
curve, the threshold probability
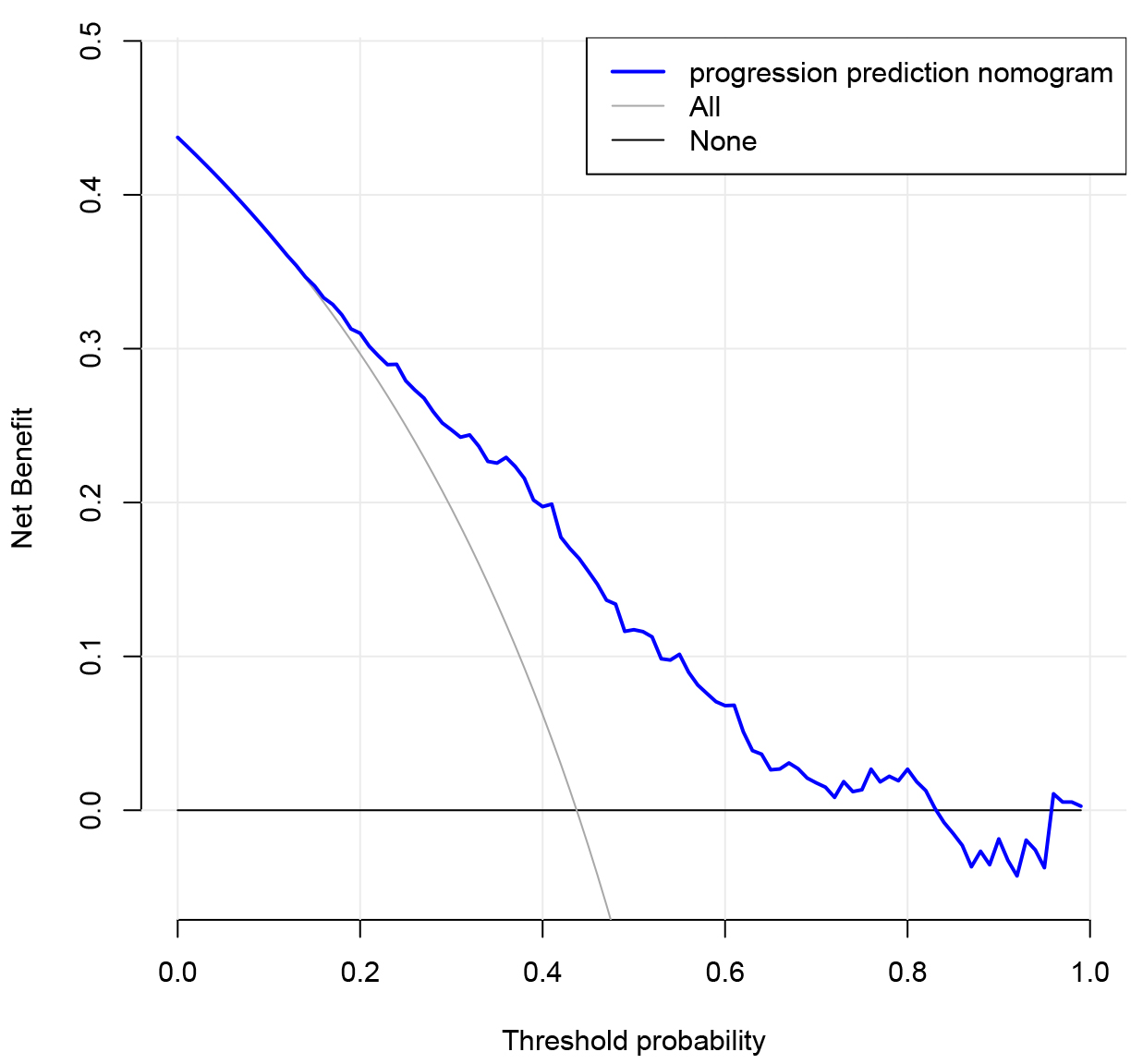 Fig. 5.
Fig. 5.Decision curve assessment for the END prediction model. The benefit is represented by the y-axis. The thin line indicates the assumption that all cases possessed early deterioration in the neurological system; the bold line shows that no patient experienced early deterioration in the neurological system while the blue line denotes the risk nomogram of early deterioration in the neurological system.
END is one of the leading causes of a patient’s poor prognosis suffering from AIS [25]. Recently, new inflammatory markers such as PLR, NLR, and SIRI have attracted the attention of clinicians in assessing the disease severity and prognosis of AIS patients. PLR and NLR were both independent predictors of 3-month functional outcomes of AIS [26]. Sharma confirmed that PLR was positively linearly correlated with NIHSS scores in patients with AIS, which could help predict disease severity and prognosis in terms of functional outcome [8]. Jin found that the increased SIRI was related to increased stroke risk [27]. Lattanzi found that higher SIRI at admission is associated with an increased risk of poor functional outcome at 3 months in ischemic stroke patients treated with EVT and successfully recanalized [28]. However, previous studies mainly focused on a single inflammatory marker. Therefore, we further developed the inflammatory factors-based nomogram to estimate the END incidence in AIS cases, so as to identify the END risk factors earlier and promote the communication between patients and clinicians as well as patient management.
A major advantage of this study is the combination of SIRI, PLR, NLR and other novel inflammatory markers that can reflect immune-inflammation of AIS. Another advantage is the integration of many inflammatory indicators such as C-reactive protein and homocysteine, which is more comprehensive than other single indicators. In addition, based on the above indicators, a novel nomogram was developed, which could accurately predict the END risk in AIS patients through the score of nomogram, DCA and C index, etc.
Our prospective single-center study showed that higher age, NIHSS, CRP, monocytes, SIRI, NLR, and LMR on admission were associated with END risk in AIS patients. After multivariable logistic regression analysis, we developed a novel nomogram, including CRP, monocytes, NIHSS and SIRI. The cohort’s internal validation revealed good discrimination and correction ability. The obtained high C-index in interval validation, in particular, demonstrated that the nomogram could be frequently and precisely used [29].
Previous studies showed that CRP, monocytes and initial NIHSS were related to the occurrence of END in AIS patients [30, 31, 32, 33]. In accordance with previous research, these variables were also included in our nomogram as predictors of END. Moreover, the study confirmed that SIRI may be a risk factor for END. SIRI, the only comprehensive marker of inflammation on the nomogram, was also a significant predictor of END. Yi and colleagues discovered that a lesser SIRI was directly correlated with more desirable clinical outcomes after mechanical thrombectomy (MT) [34]. Yun also demonstrated that elevated SIRI could be independent estimating factors for an undesirable prognosis following subarachnoid hemorrhage [10]. These studies indirectly support our findings, and larger studies will be needed in the future to further verify. Therefore, to appraise the risk of END in AIS cases, these predictors should be integrated into the risk calculator in the construction of the risk prediction model.
Our construction of the END risk nomogram can be used as an intuitive scoring
system. For example, an AIS patient had a NIHSS of 7, a CRP of 10.08 and a
monocyte count of 0.29
Despite the promising results, the following limitations should be noted in this study. As a retrospective study, this study is subject to selection bias. Besides, temporal variations in these biomarkers could also be a significant factor that may mediate their prognostic value. We are currently prospectively collecting and amplifying predictors to verify the predictive power of the model in great samples and approve the related clinical value.
SIRI is a novel predictor of END and serves as a potential marker for monitoring responses on treatment in AIS cases. Compared with a single indicator of inflammation, the integration of SIRI nomogram can predict the END risk in AIS patients more accurately and reliably, and make a reasonable individualized treatment regimen. The findings of this study should be interpreted in the context of the study design and study population. Further studies are required to validate the findings of this study.
AIS, acute ischemic stroke; END, early neurological deterioration; SIRI, systemic inflammatory response index; PLR, platelet/lymphocyte ratio; LMR, lymphocyte/monocyte ratio; NLR, neutrophil/lymphocyte ratio; DCA, decision curve analysis; LASSO, least absolute shrinkage and selection operator; ROC, receiver operating characteristic; AUC, area under the curve. MT, mechanical thrombectomy.
JW wrote the main manuscript text. XZ is also involved in subject design and statistical analysis. JT, HL and HT studied the design and key revisions to the manuscript. CY was involved in key revisions of the manuscript.
This research was reviewed and confirmed by the Ethics Committee of Harbin Medical University (KY2021-238), and exempted from informed consent.
We would like to thank the researchers and study participants for their contributions.
The project was funded by the Postgraduate Research & Practice Innovation Program of Harbin Medical University (Grant No. YJSCX2020-99HYD).
The authors declare no conflict of interest.
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
