Relationships among language ability, arcuate fasciculus and lesion volume were
investigated by use of diffusion tensor tractography in patients with putaminal
hemorrhage. Thirty-three right-handed patients within six weeks of hemorrhage
onset were recruited. Correlation of the aphasia quotient with subset (fluency,
comprehension, repetition, naming) scores, diffusion tensor tractography
parameters and lesion volume of patients, aphasia quotient (r = 0.446)
with subset (naming: r = 0.489) score had moderate positive correlations
with fractional anisotropy of the left arcuate fasciculus. The aphasia quotient
subset (repetition) score had a strong positive correlation with fractional
anisotropy of the left arcuate fasciculus (r = 0.520), whereas, aphasia
quotient subset (fluency and comprehension) scores had no significant
correlations with fractional anisotropy of the left arcuate fasciculus after
Benjamini–Hochberg correction. Aphasia quotient (r = 0.668) with subset
(fluency: r = 0.736, comprehension: r = 0.739, repetition:
r = 0.649, naming: r = 0.766) scores had strong positive
correlations with the tract volume of the left arcuate fasciculus and strong
negative correlations with lesion volume (r = –0.521, fluency:
r = –0.520, comprehension: r = –0.513, repetition: r =
–0.518, naming: r = –0.562). Fractional anisotropy of the left arcuate
fasciculus had a moderate negative correlation with lesion volume (r =
–0.462), whereas the tract volume of the left arcuate fasciculus had a strong
negative correlation with lesion volume (r = –0.700). According to the
result of mediation analysis, tract volume of the left arcuate fasciculus fully
mediated the effect of lesion volume on the aphasia quotient. Regarding the
receiver operating characteristic curve, the lesion volume cut-off value was
29.17 cm
The arcuate fasciculus (AF), an important neural tract for language, connects
the posterior superior temporal cortex and posterior part of the inferior frontal
gyrus, often referred to as Wernicke’s and Broca’s areas [1]. Injury of the AF
such as discontinuation causes various language deficits, including conduction
aphasia [2, 3, 4, 5, 6]. Approximately 24–38% of acute stroke patients and 10–18% of
chronic stroke patients have been reported to exhibit aphasia [7, 8, 9, 10, 11, 12]. Therefore,
precise estimation of the AF state (preserved, discontinued, or
non-reconstructed) during the early stage of a stroke is clinically important as
it would help clinicians design rehabilitative strategies and predict aphasia
outcome. Additionally, such estimates provide useful data for determining the
necessity of surgical intervention during the acute stage of intracerebral
hemorrhage. Putaminal hemorrhage is the most common type of intracerebral
hemorrhage as the putamen is the site most involved with that type of hemorrhage
as a hematoma can expand to the internal capsule, corona radiata, centrum
semiovale, or temporal lobe [13]. Conservative treatment is considered when a
hematoma is smaller than 10 cm
The development of diffusion tensor imaging, which produces images based on measurements of the diffusion of water molecules in structures, has allowed investigation of the overall microstructural characteristics of brain white matter. In particular, diffusion tensor tractography in human brain tissue, derived from diffusion tensor imaging, enables three-dimensional reconstruction and visualization of structures, including the AF [18, 19]. Several diffusion tensor tractography based studies have demonstrated relationships between language ability and the severity of AF injury in the dominant hemisphere in stroke patients [20, 21, 22, 23, 24]. However, currently those relationships have not been fully elucidated. In particular, the relationships between LV, language ability and the state of the AF in the dominant hemisphere require characterization.
In this study, by using diffusion tensor tractography, the relationships among language ability, the AF state in the dominant hemisphere and LV were investigated in patients with putaminal hemorrhage.
Thirty-three right-handed consecutive subjects with putaminal hemorrhage (23
men, 10 women; mean age 50.49
| Subjects | |
| Age (years) | 50.49 |
| Number (n) | 33 |
| Male:Female | 23:10 |
| Mean duration to AQ (days) | 18.36 |
| Mean duration to DTI (days) | 18.36 |
| AQ value | 47.38 |
| FA | 0.42 |
| TV | 832.12 |
| LV (cm |
29.1 |
| AQ, aphasia quotient; DTI, diffusion tensor imaging; FA, fractional anisotropy;
TV, tract volume; LV, lesion volume; Values presented are mean | |
The aphasia quotient (AQ) of the Western Aphasia Battery was used to assess the
language ability of subjects (range, 0–100 percentiles). The AQ value comprises
four subset scores (fluency, comprehension, repetition and naming) and higher
scores indicate better function [27]. The reliability and validity of the Western
Aphasia Battery has been well established [27, 28]. Subject AQ scores were
obtained at an average of 18.4
Brain LV was determined by examination of a T2-weighted turbo single echo (TSE)
sequence in brain magnetic resonance images (18.4
 Fig. 1.
Fig. 1.Result of lesion volume measurement by applying the following
formula: [A (cm)
Diffusion tensor imaging data were acquired on the same day as the language (AQ)
evaluation (18.4
Statistical analysis was performed using SPSS 21.0 for Windows (SPSS, Chicago,
IL, USA). Multiple comparisons were corrected by the Benjamini–Hochberg (BH)
procedure to control the false discovery rate [33]. Pearson correlation analysis
was used to estimate the significance of the correlations among AQ values with
subset (fluency, comprehension, repetition, and naming) scores, diffusion tensor
tractography parameters (FA: fractional anisotropy; TV: tract volume) and LV;
p
In diffusion tensor tractography findings, 13 subjects showed a discontinuation
of the left AF, while 20 subjects exhibited an intact left AF. Correlations among
the AQ values with subset scores, diffusion tensor tractography parameters and
the LV of subjects are summarized in Table 2. Moderate positive correlations were
detected between the AQ value (r = 0.446) with subset (naming:
r = 0.489) score and the FA value of the left AF (p
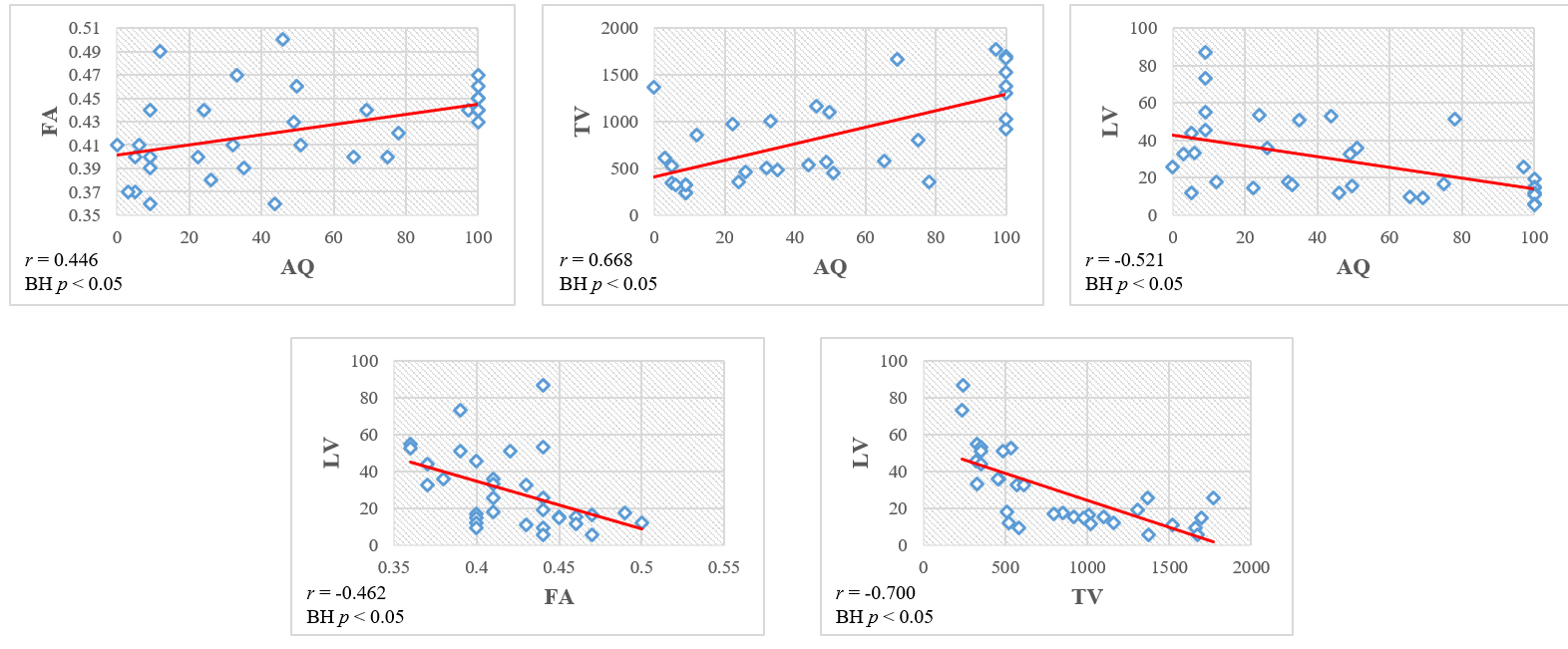 Fig. 2.
Fig. 2.Scatter plots of the correlation among the aphasia quotient (AQ) values, diffusion tensor tractography parameters (FA: fractional anisotropy; TV: tract volume) and lesion volume (LV) of subjects.
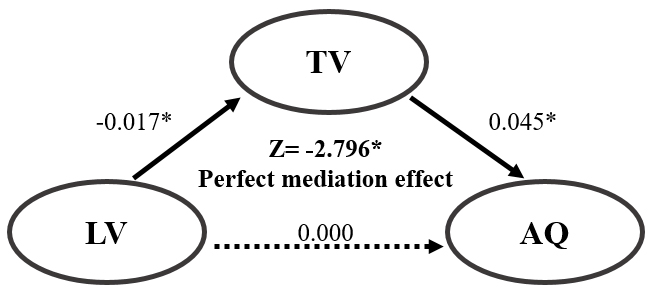 Fig. 3.
Fig. 3.Mediation analysis of the role of the tract volume (TV)
as a mediator of the relationship between the aphasia quotient (AQ) and lesion
volume (LV). * p
 Fig. 4.
Fig. 4.Regions of interest and diffusion tensor tractography for the
arcuate fasciculus (AF). (A) The seed and target regions of interest are applied
to the posterior parietal area of the superior longitudinal fascicle where the
longitudinal aspect of the AF and the posterior temporal lobe are expected. (B)
(1) T2-weighted brain magnetic resonance images at the time of diffusion tensor
imaging scanning in representative patients with a lesion volume (LV) larger than
29.17 cm
| AQ (total) | FA | TV | LV | ||
| AQ (total) | r-value | - | 0.446 | 0.668 | –0.521 |
| p-value | 0.009* | 0.000* | 0.002* | ||
| BH p-value | 0.017** | 0.003** | 0.008** | ||
| AQ (fluency) | r-value | - | 0.388 | 0.736 | –0.520 |
| p-value | 0.026* | 0.000* | 0.002* | ||
| BH p-value | 0.019 | 0.003** | 0.008** | ||
| AQ (comprehension) | r-value | - | 0.370 | 0.739 | –0.513 |
| p-value | 0.034* | 0.000* | 0.002* | ||
| BH p-value | 0.022 | 0.003** | 0.008** | ||
| AQ (repetition) | r-value | - | 0.520 | 0.649 | –0.518 |
| p-value | 0.002* | 0.000* | 0.002* | ||
| BH p-value | 0.008** | 0.003** | 0.008** | ||
| AQ (naming) | r-value | - | 0.489 | 0.766 | –0.562 |
| p-value | 0.004* | 0.000* | 0.001* | ||
| BH p-value | 0.011** | 0.003** | 0.006** | ||
| LV | r-value | –0.521 | –0.462 | –0.700 | - |
| p-value | 0.002* | 0.007* | 0.000* | ||
| BH p-value | 0.008** | 0.014** | 0.003** | ||
| AQ, aphasia quotient; FA, fractional anisotropy; TV, tract volume; LV, lesion
volume; *: correlation is significant at p | |||||
| Step | Dependent variable | Independent variable | B | SE | t | p | R | |
| 1 | TV | LV | –0.017 | 0.003 | –0.700 | –5.455 | 0.000 |
0.490 |
| 2 | AQ | LV | –0.001 | 0 | –0.521 | –3.401 | 0.002 |
0.272 |
| 3 | AQ | LV | 0.000 | 0.000 | –0.106 | –0.559 | 0.580 | 0.451 |
| TV | 0.045 | 0.014 | 0.593 | 3.135 | 0.004 | |||
| Sobel test | –2.796 | |||||||
| TV, tract volume; LV, lesion volume; AQ, aphasia quotient, *: p | ||||||||
| Cut-off value | AUC | Sensitivity | Specificity | |
| AQ (total) | 34.05 | 0.14 | 0.23 | 0.25 |
| AQ (fluency) | 36.00 | 0.09 | 0.23 | 0.25 |
| AQ (comprehension) | 41.75 | 0.13 | 0.15 | 0.20 |
| AQ (repetition) | 50.15 | 0.05 | 0.15 | 0.20 |
| AQ (naming) | 29.30 | 0.06 | 0.23 | 0.25 |
| FA | 0.42 | 0.10 | 0.15 | 0.25 |
| TV | 575.50 | 0.14 | 0.23 | 0.25 |
| LV | 29.17 | 0.74 | 0.77 | 0.80 |
| AUC, area under the curve; AQ, aphasia quotient; FA, fractional anisotropy; TV, tract volume; LV, lesion volume. | ||||
This study investigated the relationships among language ability, the AF state
in the dominant hemisphere, and LV in patients with putaminal hemorrhage. Results
can be summarized as follows. First, the AQ value with subset (naming) score had
moderate positive correlations with the FA value of the left AF. The AQ subset
(repetition) score had a strong positive correlation with the FA value of the
left AF, the AQ subset (fluency and comprehension) score had no significant
correlations with the FA value of the left AF. By contrast, the AQ value with
subset (fluency, comprehension, repetition, and naming) scores had a strong
positive correlation with the TV value of the left AF, and a strong negative
correlation with the LV. The FA value of the left AF had a moderate negative
correlation with the LV, whereas the TV value of the left AF had a strong
negative correlation with the LV. Second, the TV value of the left AF fully
mediated the effect of the LV on the AQ value. Third, the LV cut-off value was
29.17 cm
Regarding the assessed diffusion tensor tractography parameters, the FA value is indicative of the integrity of white matter microstructures, such as axons, myelin and microtubules and represents the degree of directionality of water diffusion [18]. Therefore, the FA value reflects the fiber density, axonal diameter and white matter myelination [40]. The TV value indicates the average volume of a neural tract including the total number and thickness of neural fiber and white matter microstructures such as myelin, oligodendrocytes and dendrites obtained by probabilistic tractography segmentation [40, 41]. Therefore, low FA and TV values for a neural structure represent low structure density, diameter, axonal myelination and low fiber numbers and thickness of the neural tract, respectively [18, 40]. Consequently, the result showing a moderate positive correlation between the AQ value with subset (naming) score and the FA value of the left AF indicates that language ability (especially, the naming ability) is closely related to the fiber density, axonal diameter and myelination of the left AF. By contrast, the strong positive correlations between the AQ subset (repetition) score and the FA value of the left AF and between the AQ value with subset (fluency, comprehension, repetition, and naming) scores and the TV value of the left AF suggest that repetition ability is more closely associated with fiber density, axonal diameter and myelination of the left AF than the other language (fluency, comprehension, and naming) abilities, and comprehensive language ability is more closely associated with the remaining number and thickness of neural fibers within the left AF than with the fiber density, axonal diameter and myelination of the left AF. Consequently, this suggests that the level of language disability is closely related to injury severity of the left AF. On the other hand, the correlations of the LV with the AQ value with subset (fluency, comprehension, repetition, and naming) scores (strong negative), FA value (moderate negative) of the left AF and TV value of the left AF (strong negative) suggest that the size of the lesion negatively affects comprehensive language ability and the density, diameter, axonal myelination, number and thickness of the remaining neural fibers of the left AF. Considering the significance of these correlations, language ability and the remaining neural fibers and thickness of the left AF are more affected by LV than by AF density, diameter and myelination. Thus, LV is closely related to language disability and severity of AF injury.
Mediation analysis showed that the TV value of the left AF fully mediated the relationship between the AQ value and LV. This indicates that the effect of the LV on the language ability operates through the TV value of the left AF. It suggests that surgical intervention to remove a relevant hematoma considering the TV value of the left AF is necessary to maximize the recovery of language ability in the early stage of putaminal hemorrhage.
The receiver operator analysis suggest that an LV cut-off value of 29.17
cm
Previous studies using brain mapping techniques, have reported that a patient’s language ability, such as speech rate, informativeness, efficiency, fluency and naming ability, could be predicted at the chronic stage by the lesion load (a variable combining LV and lesion site) of the AF in the dominant hemisphere [42, 43, 44, 45]. Moreover, several diffusion tensor tractography-based studies have demonstrated that language ability is related to the injury severity of the AF in the dominant hemisphere in stroke patients [20, 21, 22, 23, 24]. Breier et al. [20] have reported that speech-repetition ability was associated with the FA value of the AF in the dominant hemisphere in stroke (hemorrhage and ischemia) patients at the chronic stage. Kim et al. [21] observed that AQ values were higher in stroke (hemorrhage and ischemia) patients with preserved integrity of the AF in the dominant hemisphere than in those patients with a non-reconstructed or disrupted AF at the early stage after onset. Tak et al. [22] demonstrated that the AQ value was positively correlated with the TV value of the AF in the dominant hemisphere in chronic stroke patients and was higher if the integrity of the AF in the dominant hemisphere was preserved. Wang et al. [23] reported that speech-repetition disability within six months of onset was related to a low FA value for the AF in the dominant hemisphere in stroke (hemorrhage and ischemia) patients. Subsequently, Noh et al. [2021] reported that the AQ value and an AQ subset score (naming) were positively correlated with the FA value of the AF in the dominant hemisphere and the AQ subset scores for fluency, repetition and naming were negatively correlated with LV in stroke (hemorrhage and ischemia) patients at the early stage (within two months of onset) [24]. However, to the best of the author’s knowledge, the present study is the first to demonstrate a relationship between language ability and LV and report a quantitative LV value helpful in discriminating discontinuation of the left AF. A relationship is also described between language ability and diffusion tensor tractography parameters of the AF in the dominant hemisphere in the early stage of putaminal hemorrhage.
Some limitations of this study should be considered. First, diffusion tensor tractography analysis is operator-dependent and thus can elicit false-positive and false-negative results, mainly due to areas of crossing fibers or the partial volume effect [46]. Second, although the language dominant hemisphere is assumed to be the left hemisphere, the functional localization of individual language dominance was not confirmed. Third, only a small number of subjects were enrolled in this study. Fourth, diffusion tensor imaging scanning was performed during the early stage of putaminal hemorrhage. If diffusion tensor imaging scanning was performed during the acute or subacute stages, those results might be useful when deciding on the necessity of surgical intervention to preserve the AF in the dominant hemisphere. Fifth, because the AF might consist of anterior, posterior and long segments, the location of regions of interest of the AF may be controversial [47]. Sixth, although the AF is related to language ability, there are other neural tracts such as the superior longitudinal fasciculus, uncinate fasciculus and extreme capsule related to language function that are affected by putaminal hemorrhage. Hence, further prospective studies that include assessment of the functional localization of the language dominance, large numbers of subjects, acute or subacute stage diffusion tensor imaging scanning, precise placement of region of interests of the AF, and additional neural tracts related to language ability are encouraged.
The use of diffusion tensor tractography allowed the conclusion that language
disability was closely related to LV and to the severity of the AF injury in the
dominant hemisphere in subjects with putaminal hemorrhage. In particular, the TV
value of the AF in the dominant hemisphere fully mediated the effect of the LV on
language ability. Additionally, following putaminal hemorrhage, an LV of
approximately 30 cm
AF, arcuate fasciculus; LV, lesion volume; AQ, aphasia quotient; FA, fractional anisotropy; TV, tract volume; BH, Benjamini–Hochberg.
MJC and SHJ designed the research study. MJC performed the research. SHJ provided help and advice on the study. MJC analyzed the data. MJC and SHJ wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All participants signed a written informed consent beforehand, which abided by the Helsinki Declaration.
Not applicable.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (No. 2021R1A2B5B01001386).
The authors declare no conflict of interest.
