As a gamma-aminobutyric acid type A receptor agonist sevoflurane is a common
general anesthetic used in anesthesia and affects the neural development in
offspring. We hypothesized that sevoflurane could regulate interneurons via the
neuregulin-1-epidermal growth factor receptor-4 (NRG1–ErbB4) pathway in the
entorhinal cortex (ECT) of the middle pregnancy. Six female rats in middle
pregnancy (14.5 days of pregnancy) were randomly and equally divided into
sevoflurane (SeV) and control groups. The rats in the SeV group were
exposed to 4% sevoflurane for 3 hours. The expression levels of NRG1 and ErbB4,
parvalbumin (PV) and glutamic acid decarboxylase (GAD67), and
N-methyl-D-aspartate receptor subunit 2A (NR2A) and subunit 2B (NR2B) in
offspring were examined through immunohistochemistry. The pyramidal neurons in
the ECT were examined via Golgi staining. The levels of NRG1 and ErbB4 were
significantly decreased (P
About 0.75–2% of pregnant women need non-obstetric surgery every year [1].
Middle pregnancy (namely, second trimester) is considered to be a safe period for
surgical anesthesia. With the development of fetal surgery and laparoscopic
technology, an increasing number of pregnant women undergo surgery under general
anesthesia during middle pregnancy [1]. However, recently, studies have shown
that middle pregnancy is a “busy period of neural development” in which neurons
proliferate, migrate, and differentiate [2]. Anesthetics commonly used in
clinical practice have been found to mainly affect two major neurotransmitter
receptors, N-methyl-D-aspartate (NMDA) receptors and gamma-aminobutyric acid
(GABA) receptors, during the development of the central nervous system (CNS).
Exposure to general anesthetics before and after birth could affect brain
development. A warning against prolonged and repeated exposure to general
anesthetics during pregnancy was proposed by the American Food and Drug
Administration. Sevoflurane, as a GABA type A (GABA
As an excitatory transmitter in the early stage of neural development, GABA is a
key neurotransmitter in the development of the brain that plays an important role
in the proliferation and migration of neurons [4, 5]. When neurons migrate to the
target cortex, the process of neuronal migration is terminated by contact of GABA
receptor. With the development of the brain, the GABA
The “vulnerability window” of neurotoxicity of general anesthetics is mainly at the peak of brain nerve cell proliferation, migration, or synaptic development (i.e., during the middle or late stage of pregnancy) [2]. In middle pregnancy, the fetus’ brain is extremely sensitive to changes in the environment. Middle pregnancy is a critical period for brain nerve development (neuron proliferation, migration, and the formation of neural connections), and most of these events occur in middle pregnancy [6]. In addition to unavoidable emergency operations in middle pregnancy, selective operations are also mostly carried out in this period. Prenatal sevoflurane exposure in middle pregnancy may affect fetal neural development and cause neural dysfunction in offspring.
Interneurons, located with GABA
Neuregulin-1 (NRG1), a member of the epidermal growth factor family, plays an
important role in promoting the phosphorylation of epidermal growth factor
receptor-4 (ErbB4) [12, 13]. ErbB4 receptors have a variety of isomers, including
ErbB (1–4). ErbB4 is highly expressed in the brain and has a strong affinity for
NRG1. Most ErbB4 receptors are expressed on parvalbumin (PV) interneurons, which
account for 40% of interneurons [12, 13, 14]. The NRG1–ErbB4 pathway can regulate
and interact with the secretion function of GABA
Herein, we hypothesize that sevoflurane could regulate interneurons via the NRG1–ErbB4 pathway in the ECT of offspring during the middle pregnancy. Gestational day 14.5 in pregnant rats is similar to middle pregnancy in humans [26]. In this study, pregnant mice were exposed to sevoflurane in middle pregnancy. The status of the NRG1–ErbB4 pathway, interneurons, NMDA receptors, and the dendrite morphology of pyramidal neurons were examined to test the hypothesis. Discovering the mechanism of sevoflurane-induced neurotoxicity is of great significance for guiding the standardized clinical use of general anesthetics and research into toxicity prevention and treatment.
Six adult female Sprague-Dawley (SD) rats, weighing 180–220 g, were raised with free diet and water intake in polypropylene cages for 7 days. Then the female SD rats were mated with male SD rats with sexual experience at 7:00 PM after adaptive feeding. Vaginal smears were performed the next morning and pregnancy day 0, G0, was defined by sperm detection. The pregnant rats were randomly divided into two groups: a control group (control, n = 3) and a sevoflurane group (SeV, n = 3). The six female SD rats were raised to G14.5 (middle pregnancy).
On pregnancy day 14.5, the rats allocated to sevoflurane exposure were put
inside a 30 cm
Three offspring were randomly selected from each group with one offspring/dam. The ex vivo brain samples of the offspring rats were harvested at the day 30 of postpartum (P30) for histology, immunostaining and Golgi staining. Rats in both the control and SeV groups were executed and perfused through the left ventricle with precooling saline followed by 4% paraformaldehyde in 0.01 M phosphate buffered saline (PBS) pH 7.35. The brain tissue of the rats was taken and post-fixed for 24 hours for paraffin and frozen sections.
To analyze the status of the NRG1–ErbB4 pathway in the interneurons, the expression levels of NRG1 and ErbB4 in LII and III of the ECT were examined via immunohistochemistry. The NRG1–ErbB4 pathway plays an important role in the genesis, migration, differentiation, maturation, and neurotransmitter synthesis of GABAergic interneurons. We assumed that the number of GABAergic interneurons the LII/LIII ECT of offspring in could be affected by alterations of the NRG1–ErbB4 pathway. Therefore, we label GABAergic interneurons with PV to represent PV interneurons. We also used glutamic acid decarboxylase 67 (GAD67) to label GABAergic interneuron (the key enzyme of GABA neurotransmitter synthesis) positive cells to represent the total GABAergic interneurons in the LII/LIII ECT. The expression levels of PV and GAD67 in LII and LIII of the ECT were examined via immunohistochemistry. That is, Interneurons were identified by immunoreactivity to PV and GAD67. To investigate whether NRG1–ErbB4 pathway changes in offspring after prenatal sevoflurane exposure affect the formation of subunits during maturation, we detected NMDA receptor subunit 2A (NR2A) and NMDA receptor subunit 2B (NR2B) by immunofluorescence. The expression levels of NR2A and NR2B in LII and LIII of the ECT were examined via immunohistochemistry to analyze the status of NMDA receptors in the ECT. There is a fixed pattern of neurite growth in the developing brain. We assumed that prenatal sevoflurane exposure could affect the inherent growth pattern of dendrites and dendritic spines in pyramidal neurons through NRG1–ErbB4 alterations. Therefore, we used Golgi silver staining to investigate the length of dendrites and the number of branches and dendritic spines. Golgi staining was performed to analyze the total dendrite length, number of dendritic branches, spatial distribution of dendrites, and density of dendritic spines in the pyramidal neurons in the ECT.
The coronal sections of the brain were deparaffinized, rehydrated, and immersed
in 3% H
150
All the data was expressed as mean
The results showed that the expression of NRG1 in the SeV group was
significantly lower than that in the control group (control group: 9.94
 Fig. 1.
Fig. 1.Exposure of sevoflurane to maternal rats impaired the
NRG1–ErbB4 signaling pathway in the LII/LIII entorhinal cortex of offspring.
Inverted fluorescence microscopy showed that the NRG1 and ErbB4 positive cells
were stained with green fluorescence. The top picture shows the field of vision
under the 100
The results showed that the number of PV positive cells in the SeV group was
significantly lower than that in control group (control group: 23.41
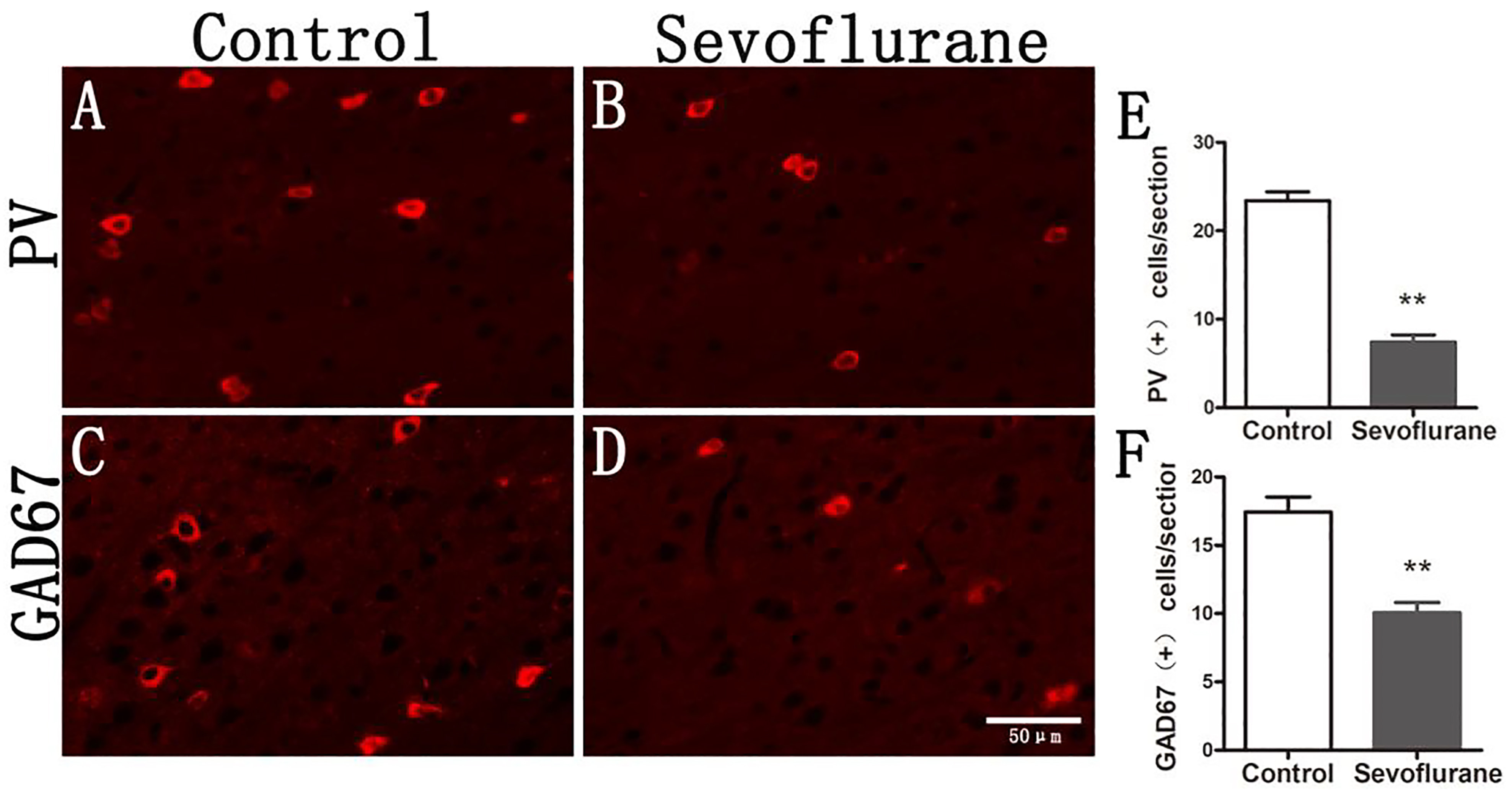 Fig. 2.
Fig. 2.The GABAergic interneurons of offspring in the LII/LIII entorhinal cortex were significantly reduced. PV and GAD67 positive cells were stained with red fluorescence, so in the inverted fluorescence microscope at 20X magnification, these cells are indicated by red fluorescence. (A) The PV positive cells in the LII/LIII entorhinal cortex of the P30 offspring in the control group. (B) PV immunofluorescence (red) on a P30 offspring rat in the sevoflurane group. (C) GAD67 immunofluorescence (red) on a P30 offspring rat in the control group. (D) GAD67 immunofluorescence (red) on a P30 offspring rat in the sevoflurane group. (E) The numbers of PV cells were significantly decreased in the sevoflurane compared to the control group. (F) The numbers of GAD67 cells were significantly decreased in the sevoflurane group compared to the control group. Note: The symbol ‘**’ represented there was significant difference between groups under the significant level of 0.01.
The results were as follows (Fig. 3): compared to the control group, the number
of NR2A positive cells in the SeV group was decreased (control group: 24.00
 Fig. 3.
Fig. 3.Maternal exposure of sevoflurane leads to impaired maturation of NMDA receptors in offspring. NR2A and NR2B positive cells are indicated by green fluorescence. (A) The NR2A positive cells in the LII/LIII entorhinal cortex of the P30 offspring in the control group. (B) NR2A immunofluorescence (green) on a P30 offspring rat in the sevoflurane group. (C) NR2B immunofluorescence (green) on a P30 offspring rat in the control group. (D) NR2B immunofluorescence (green) on a P30 offspring rat in the sevoflurane group. (E) The numbers of NR2A cells were significantly decreased in the sevoflurane compared to the control group. (F) The numbers of NR2B cells were significantly increased in the sevoflurane compared to the control group. Note: The symbol ‘**’ represented there was significant difference between groups under the significant level of 0.01.
The results showed that compared to that in the control group, the number of
dendritic spines of the pyramidal neurons in LII/LIII of the ECT in the SeV group
was significantly increased (control group: 8.88
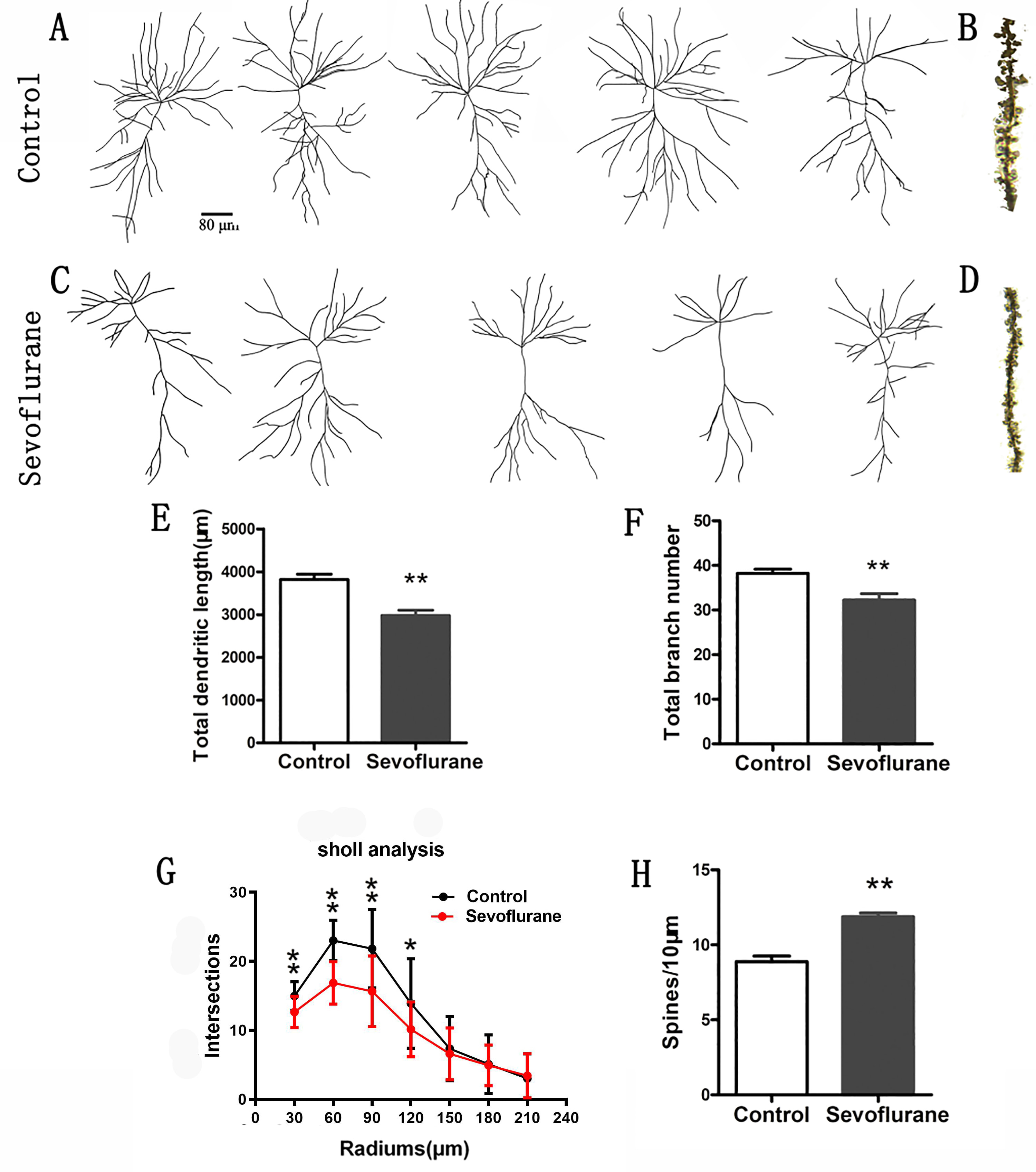 Fig. 4.
Fig. 4.Sevoflurane exposure disturbs the maturation of pyramidal neurons in the LII/LIII entorhinal cortex of offspring. Six P30 brains in each group were processed for Golgi–Cox impregnation, and pyramidal neurons in the LII/LIII entorhinal cortex were studied in the control (examples in A) and sevoflurane (examples in C) groups. The left panels (A and C) show two examples of Golgi-impregnated neurons. The total branch number and dendritic length were significantly decreased in the sevoflurane group compared to the control group (E and F) (n = 24 neurons in each group). In addition, higher spine density was observed in the sevoflurane group had than the control group (see B, D, and H) (n = 24 dendrites in each group). Sholl analysis showed that the complexity of dendritic trees was lower in the sevoflurane group than the control group (G). Note: The symbol ‘*’ represented there was significant difference between groups under the significant level of 0.05. The symbol ‘**’ represented there was significant difference between groups under the significant level of 0.01.
We previously speculated that prenatal sevoflurane, as an exogenous GABA
With the development of surgical technology and fetal surgery, an increasing
number of pregnant women need to be exposed to general anesthetics. Sevoflurane
mainly acts as GABA
In this study, we simulated clinical practice. The pregnant rats were
anesthetized with 4% sevoflurane for 3 hours at 14.5 days of gestation to reach
a MAC of 1.8 (lower than normal rats, considering that pregnant rats are more
sensitive to sevoflurane), which was equivalent to that of most surgical
operations for pregnant females. We found that sevoflurane, as an exogenous
GABA
The NMDA receptor (NMDAR) is mainly composed of two NR1 and two NR2 subunits. The distribution of NMDAR subunits (NR1, NR2, NR3) also changes with the process of neurodevelopment. NR1 begins to increase after birth until puberty and reaches a peak level in the third week after birth. NR2B and NR2D are the main subunits of NR2 in the embryonic stage. The expression of NR2 changes significantly in the two weeks after birth. NR2A begins to partially replace NR2B until the brain matures. The development of NMDAR subunits marks the transition from immature to mature neurons and is closely related to the development of learning and memory ability. In our experiment, exposure to sevoflurane was found to decrease the expression of NR2A and increase the expression of NR2B in offspring, which is consistent with the findings of previous studies [31, 32]. NRG1–ErbB4 changes could increase the phosphorylation of NR2B by Fyn and, thus, reduce the internalization of NR2B and delay the transition from NR2B to NR2A. NRG1–ErbB4 can regulate not only the release and activity of GABAergic neurotransmitters but also the excitatory synapses on inhibitory neurons (interneurons). The intracellular segment of the ErbB4 protein contains post-synaptic postsynaptic dens 95 (PSD-95)/DiscsLarge/zonula occludens protein-1 (ZO1) (PDZ) domains, which are anchored to the postsynaptic membrane by interacting with other proteins (such as PSD-95) that also contain PDZ [41, 42, 43]. Thus, ErbB4 regulates the function of the NMDAR by the connection of PDZ [41, 42, 43]. The NRG1–ErbB4 pathway phosphorylates the phosphorylation site of NR2B through a member of the Src family of Fyn (SRC/Fyn), which blocks the internalization of NR2B by protein AP-2 [44, 45]. In this way, the NRG1–ErbB4 pathway increases the expression of NR2B on the postsynaptic membrane [44, 45]. Thus, the decreased NRG1–ErbB4 level results in increased NR2B and decreased NR2A levels (Fig. 3). This alteration may lead to the abnormal development of learning and memory ability.
Pyramidal neurons are responsible for information transmission, and their function is very important. Therefore, it has always been a research hotspot in the topic of the neurotoxicity of general anesthetics during development. Interneurons play an important role in regulating pyramidal neurons. We found that prenatal sevoflurane exposure in middle pregnancy resulted in a significant increase in the number of dendritic spines, a significant decrease in the total length of dendrites, and an abnormal spatial distribution of dendrites in offspring (Fig. 4). There could be three possible mechanism behind the increased number of dendritic spines. One explanation is that the decrease in the number of interneurons leads to the weakening of their regulation of pyramidal neurons, resulting in abnormal numbers of dendritic spines. The second explanation is that the change of the NRG1–ErbB4 pathway could regulate the dendritic spines of pyramidal neurons. Barros et al. [35] found that the number of pyramidal dendritic spines decreased after ErbB4 and ErbB2 knockout in the nervous system. However, the number of pyramidal dendritic spines did not decrease in the experiment of ErbB4 knockout by pyramidal cells [44, 46]. It is suggested that ErbB2 may play a role of functional compensation, and there is an over-compensation in the number of pyramidal dendritic spines [37, 47]. The third explanation is that the NR2B subunit of NMDAR combines with PSD-95 and calmodulin-dependent kinase II (CaMKII), which activates a series of downstream signaling pathways and leads to an increase in the number of dendritic spines [48]. The NR2B/PSD95/kalirin-7 pathway is very important in the development of neuronal dendritic spines. Recent studies have shown that PSD-95, kalirin-7, and NR2B form a complex with a postsynaptic membrane through the PDZ domain. NR2B can activate kalirin-7, and then activate Rac1, a downstream RhoGTPase family member [49]. In this way, NR2B could dynamically regulate actin cytoskeleton rearrangement, promote the growth of dendritic spines, induce the formation of spinous structures in neuronal bodies, and cause the excessive formation of dendritic spines in pyramidal neurons and interneurons [49]. The decrease of total length and branches of dendrites in offspring may be explained by the change of the NRG1–ErbB4 signaling pathway and NR2B subunit, which mainly involve the growth and pruning of dendrites [36, 37]. Further, the plasticity of dendritic spines affects learning and memory function, especially the formation of long-term memory [50, 51].
The initial experimental hypothesis has been verified in this study. However, to test the hypothesis, this study focused on the study of the ECT with a concentration on the memory transfer station. Thus, this study did not involve an investigation of the dentate gyrus area of the hippocampus, which was directly projected by pyramidal neurons in the ECT. The time (3 h) for sevoflurane exposure may be too long in actual clinical situation. However, despite the hard exploratory work we’ve done, there is a few results we assumed except for that sevoflurane exposed to fetal brain for at least 3 hours or neonatal brains for more than 6 hours were neurotoxic. These results suggested that the safe concentration and exposure time in most clinical practice is safe.
Prenatal sevoflurane exposure in middle pregnancy could lead to the disorder of
the interneurons by activating the GABA
NMDA, N-methyl-D-aspartate; GABA, gamma-aminobutyric acid; CNS, central nervous
system; GABA
XS, YG and TZ designed research; YG, TZ, YC, ZS, ZS and JL performed experiments; YC analyzed data; YG wrote the paper; TZ and XS critically revised the paper.
This experiment was approved by the Animal Ethics Committee of Guangzhou Medical University, and the researchers strictly followed the relevant provisions of the “Guidelines for the Care and Use of Laboratory Animals” issued by the National Institutes of Health in 1996 (ethic code: 2016-029).
We thank Xiaolong Zeng for assistance in manuscript revision preparation.
This work was granted by the National Natural Science Foundation of China (Granted no. 81870823).
The authors declare no conflict of interest.
