Pituitary abscess is a rare disease with a high preoperative misdiagnosis rate. Magnetic resonance imaging is the primary method for confirming pituitary abscesses before surgery. We reported magnetic resonance imaging findings of four cases of pituitary abscess (three males and one female) aged from 33 to 72 years old. All four cases underwent transnasal sphenoid surgery and had a good prognosis. The findings on magnetic resonance imaging were analyzed. Three cases showed ring enhancement of the capsule wall. Four cases showed thickened pituitary stalk, with the diameter of the lower end larger than that of the upper end. The position of the pituitary stalk where it was inserted into the upper edge of the pituitary was located at the middle and posterior 1/3 junction of the upper edge of the pituitary two cases were with the enhancement of the dura mater at the bottom of the temporal lobe. Two cases were with the enhancement of the dura mater of the petroclival region, and one case was with the enhancement of the cavernous sinus. There are few articles concerning abnormal magnetic resonance imaging around the sellar region of pituitary abscess. The findings of the four cases in this article suggest that the pituitary abscess has characteristic abnormal magnetic resonance imaging of the sellar region and its surroundings.
Pituitary abscess is a clinically rare but life-threatening inflammatory disease of the pituitary gland [1]. More than 300 patients have been reported so far were misdiagnosed before surgery [2]. Before surgery, a preliminary diagnosis is essential for formulating surgical plans and perioperative management [3]. Imaging is the primary method to confirm the diagnosis of pituitary abscess before surgery. Magnetic resonance imaging (MRI) has a rich imaging series. It can provide imaging clues that computerized tomography (CT) and other examinations cannot provide. On enhanced pituitary MRI scan, the ring enhancement in the sellar region is a characteristic imaging manifestation of pituitary abscess [4]. However, some pituitary abscesses do not have a characteristic imaging manifestation of ring enhancement [5]. Therefore, more characteristic imaging manifestation should be explored by using MRI and other methods.
A 72-year-old female underwent transnasal resection of pituitary adenoma in
sellar in 2013. In July 2014, she was referred to our hospital due to a recurrence
of pituitary adenoma. After admission, MRI plain scan and an enhanced scan showed a
cystic lesion in the sellar region with the size of 1.2
 Fig. 1.
Fig. 1.Preoperative pituitary MRI and postoperative CT of Case 1. Preoperative coronal (A) and sagittal (B) MRI scans showed cystic lesions in the sellar. Both T1WI and T2WI were isointensity. There were sellar bone resorption and thickening of nasal septum mucosa. Preoperative coronal (C) and sagittal (D) enhanced MRI scan showed ring enhancement of the cyst wall, thickening and enhancement of the pituitary stalk, enhancement of the dura mater on both sides of the temporal lobe and petroclival region. Postoperative CT (E) showed no recurrence of the pituitary abscess.
| Case | 1 | 2 | 3 | 4 | |
| T1 signal | Isointense | Low | Slightly high | Low | |
| T2 signal | Isointense | Slightly high | Slightly high | High | |
| Ring enhancement | Thickened wall, uneven | Thickened wall, uneven | Thickened wall, uneven | N/A | |
| Pituitary stalk | The position of pituitary stem inserted into the upper edge of the pituitary gland | The middle and posterior 1/3 junction of the upper edge of the pituitary gland | The middle and posterior 1/3 junction of the upper edge of the pituitary gland | The middle and posterior 1/3 junction of the upper edge of the pituitary gland | The middle and posterior 1/3 junction of the upper edge of the pituitary gland |
| Diameter of upper end (mm) | 1.6 | 5.1 | 3.1 | 2.1 | |
| Diameter oflower end (mm) | 4.4 | 6.2 | 3.7 | 3.6 | |
| Length (mm) | 9.0 | 8.6 | 7.3 | 6.7 | |
| Shape | Right deviation, uniform thickening | Right deviation, uniform thickening | Right deviation, uniform thickening | Bend and thickened evenly | |
| Meningeal enhancement | Clivus, bilateral temporal lobe dura enhancement | Clivus, dura enhancement | Bilateral temporal lobe dura enhancement | N/A | |
| Cavernous sinus involvement | N/A | Bilateral cavernous sinus enhancement | N/A | N/A | |
| Peritumoral enhancement | N/A | N/A | N/A | N/A | |
| Paranasal sinus inflammation | N/A | N/A | Thicking of sphenoid sinus mucosa | Thickening of sphenoid sinus mucosa, submucosal empyema | |
| Others | Thinning of the sellar bone and thickening of the mucosa of the nasal septum | N/A | Intrasaccular fluid level | N/A | |
A 45-year-old male was admitted to a local clinic in February 2009 due to
headache, fever and weakness of the limbs. After admission, he had a body
temperature as high as 39.5
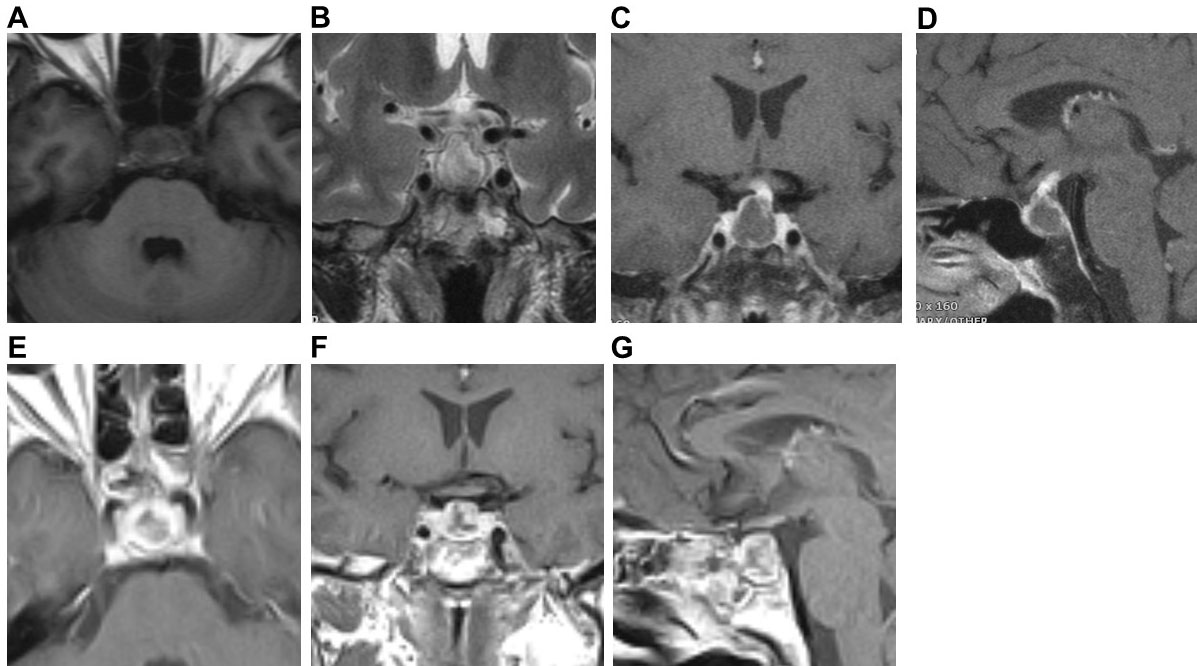 Fig. 2.
Fig. 2.Preoperative pituitary MRI and postoperative MRI of Case 2. Preoperative axial (A) and coronal (B) MRI scans showed cystic lesions in the sellar, T1WI low signal, and T2WI slightly high signal. Preoperative coronal (C) and sagittal (D) MRI enhanced scan showed ring enhancement of the cyst wall, thickening and enhancement of the pituitary stalk, and bilateral cavernous sinus enhancement. Postoperative axial (E), coronal (F), and sagittal (G) MRI showed no recurrence of the pituitary abscess.
A 49-year-old male was admitted in 2013 due to headache and sexual dysfunction
lasting half a year and worsening one week. Pituitary MRI imaging (plain and
enhanced scan) (Fig. 3A–D) showed cystic lesions in the sellar, about 2.0
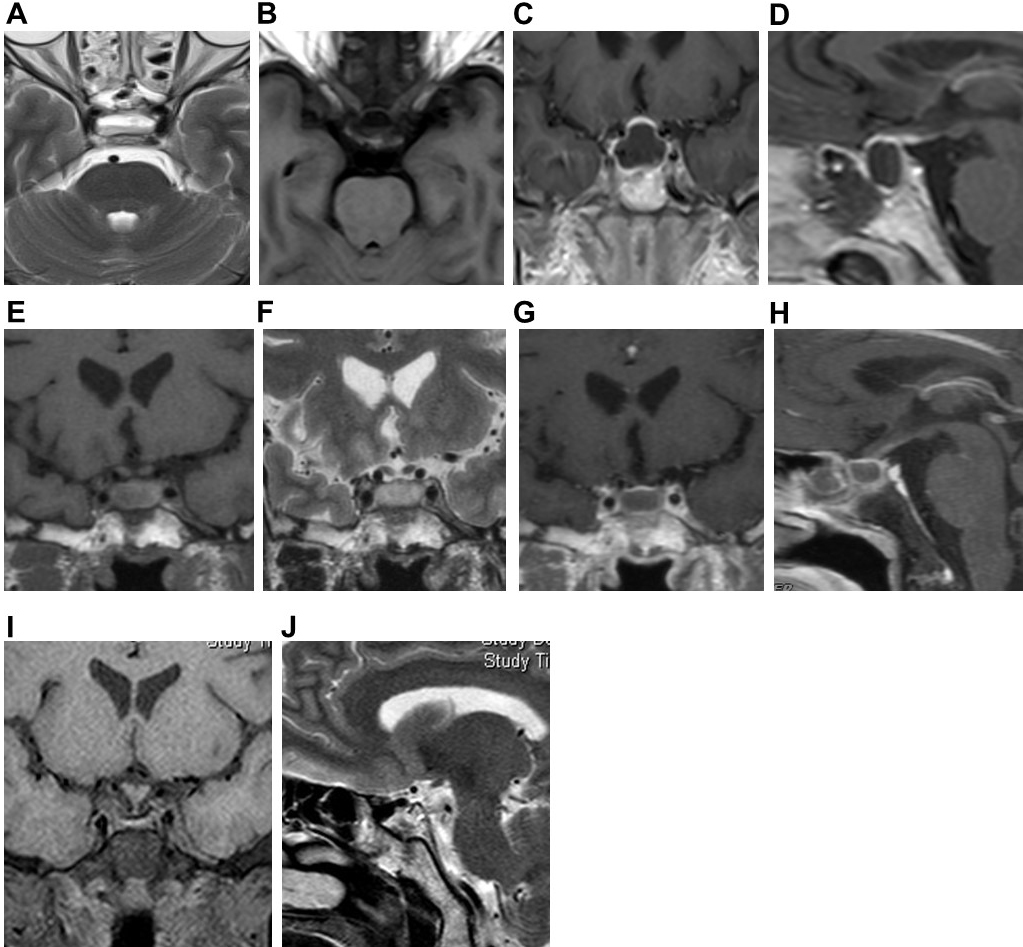 Fig. 3.
Fig. 3.Pituitary gland MRI of Case 3. Preoperative axial (A,B) MRI plain scans showed cystic lesions in the sellar region. T1WI showed uneven and slightly high signal, and T2WI showed a slightly high signal, with a fluid plane inside. Preoperative coronal (C) and sagittal (D) MRI enhanced scan showed ring enhancement of the cyst wall, thickened and enhanced pituitary stalk. Postoperative coronal (E,F) MRI plain scan showed cystic lesions in the sellar. T1WI showed a high signal, T2WI showed a slightly high signal. There was sphenoid Sinus mucosal thickening with effusion. Postoperative coronal (C) and sagittal (D) enhanced MRI scans showed ring enhancement of the capsule wall. Coronal (I) and sagittal (J) MRI plain scans after the second operation showed no recurrence of the pituitary abscess.
A 33-year-old male was admitted in February 2006 due to decreased libido for
several years and headache for two months. Pituitary MRI imaging (plain and
enhanced scan) (Fig. 4A–D) showed cystic lesions in the sellar, about three
 Fig. 4.
Fig. 4.Preoperative pituitary MRI, intraoperative image and postoperative CT of Case 4. Preoperative coronal (A), sagittal (B), and axial (C) MRI plain scans showed cystic lesions in the sellar region, low signal on T1WI, high signal on T2WI. The mucosa of the sphenoid sinus at the sellar floor was significantly thickened. Submucosal effusion was observed. Preoperative coronal (D) enhanced MRI scan showed partial enhancement of the cyst wall. During operation (E), the pus was yellow and viscous. Postoperative head CT (F) showed no recurrence of the pituitary abscess.
The incidence of pituitary abscess is extremely low, which accounted for no more than 1% of pituitary diseases [6, 7]. Moreover, pituitary abscess is slightly more common in females. Most of the published literature focusing on pituitary abscess were case reports [1, 3], with the most significant number of cases (66 cases) in [8]. It has been shown that preoperative diagnosis of a pituitary abscess is challenging [9, 10].
The characteristic clinical manifestations of a pituitary abscess include pituitary hypofunction (the adrenal axis, thyroid axis, and gonadal axis are simultaneously affected) and symptoms of the neurohypophysis and hypothalamus damage (such as diabetes insipidus, lethargy, abnormal behavior, etc.) [2, 11]. Among four patients, only one case had total hypopituitarism. No patient had symptoms of neurohypophysis or hypothalamus damage. However, three had a headache, one had a fever, and three had partial hypopituitary. Therefore, while paying attention to the clinical manifestations of hypopituitarism, neurohypophysis and hypothalamus damage, a comprehensive analysis should be carried out in conjunction with the symptoms of headache, fever, and invasion of surrounding tissues caused by inflammation.
Recently, with the increase in the number of reported cases of pituitary abscess and the development of MRI technology, some characteristic MRI manifestations of pituitary abscess have been observed [12, 13, 14]. The pituitary abscess may show ring enhancement without internal enhancement during an enhanced scan. The abscess cavity may show a high signal in diffusion-weighted imaging, with a decreased apparent diffusion coefficient, and the abscess wall may show low signal. However, not all pituitary abscesses have characteristic imaging manifestations such as ring enhancement [15]. Among the four patients, one case did not have ring enhancement. In addition, some pituitary abscesses have atypical ring enhancement or only have small cysts [5, 16], which are not easily distinguished from cystic pituitary adenomas and Rathke cysts in the sellar. This highlights the importance of exploring other imaging features of the pituitary abscess.
Thickening of the pituitary stalk is also considered an essential imaging manifestation of a pituitary abscess. Still, no detailed characteristics of thickening of the pituitary stalk caused by pituitary abscess have been reported. We observed the shape of the pituitary stalk, the position of the pituitary stalk where it was inserted into the upper edge of the pituitary and measured the length, upper and lower diameter of the pituitary stalk. We found that the length of the pituitary stalk of these four patients ranged from 6.7 mm to 9.0 mm. Within the range of normal pituitary stalk length, 3 cases of the pituitary stalk were rightward, one case was bent, three cases were tapered and thickened, and 1 case was cylindrical. In all four cases, the diameter of the lower end of the pituitary stalk was more significant than that of the upper end, which was different from the standard pituitary stalk and the thickened pituitary stalk related to the posterior pituitary ectopic, lymphoma, solid cancer metastasis and other diseases [17].
We observed for the first time that the diameter of the lower end of the pituitary stalk was larger than that of the upper end in patients with pituitary abscess regardless of the shape of the thickened pituitary stalk. This may be because the inflammatory damage of the pituitary stalk gradually starts from the lower end of the pituitary stalk. In normal anatomy, the position of the pituitary stalk where it is inserted into the upper edge of the pituitary is mainly at the junction of the middle and posterior 1/3 of the upper edge of the pituitary. There is no report on the influence of the pituitary abscess on the position of the pituitary stalk where it is inserted into the upper edge of the pituitary. In all four cases of this study, the position of the pituitary stalk where it is inserted into the upper edge of the pituitary was all at the middle and posterior 1/3 junction of the upper edge of the pituitary, which may suggest that the abscess has extensive damage to the pituitary tissue.
The enhancement of surrounding tissues of the sellar, including that of the dura mater at the base of the temporal lobe, the petroclival region, the cavernous sinus, the optic chiasm, etc., is caused by inflammation of the pituitary glands [15]. Two cases had temporal lobe base dura mater enhancement, two cases had petroclival region dura mater enhancement, and one case had cavernous sinus involvement and enhancement.
In this article, MRI images of one case showed severe inflammation in the mucosa of the sphenoid sinus at the bottom of the sellar. It has been reported that inflammation of the structures around the pituitary, such as sphenoid sinusitis, meningitis, and cavernous sinus thrombophlebitis, is one of the causes of pituitary abscess [18, 19, 20, 21]. This type of abscess is called a primary pituitary abscess. Cases three and four had sphenoid sinusitis, which may be a causative factor for their pituitary abscess. The inflammatory manifestations of the sphenoid sinus and other parts have particular significance for diagnosing pituitary abscesses before surgery. In addition, pituitary adenomas, craniopharyngiomas, and Rathke’s cysts secondary to sellar lesions after surgery are another major cause of the pituitary abscess. Abscesses that occur in the pituitary gland with lesions are called secondary pituitary abscesses. Here, case 1 had a history of pituitary adenoma surgery, which may cause her secondary pituitary abscess. However, the cause of secondary pituitary abscess in case 2 was unclear.
Although it has been reported that some patients have been cured by drug treatment [22], incision and drainage of the pituitary abscess through the sphenoid sinus approach combined with postoperative anti-inflammatory therapy is still the first choice. The four patients in this study were treated with incision and drainage of the pituitary abscess through the nasal sphenoid approach. Three cases did not have a recurrence, and one case relapsed one year after surgery and recovered. Recurrence is considered to be related to persistently low hormone levels and insufficient drainage [23].
In conclusion, there were 3 cases with ring enhancement of the capsule wall, 4 cases with a thickened pituitary stalk (with the diameter of the lower end larger than that of the upper end), two cases with enhancement of dura mater at the bottom of the temporal lobe, two cases with enhancement of dura mater of the petroclival region, and one case with enhancement of cavernous sinus. These abnormal imaging findings around the sellar region are reliable presentations for diagnosing a of pituitary abscess, often unavailable in other sellar lesions. Combined with the ring enhancement of the lesion, the medical history, and clinical manifestations, most cases of pituitary abscess can be confirmed before surgery.
FSH, follicle-stimulating hormone; FT3, free triiodothyronine; LH, luteinizing hormone; TSH, thyroid stimulating hormone.
ZW and SW designed the research study. ZW and YQ performed the research. ZW and HL analyzed the data. ZW wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The Ethics Committee approved this study of Fujian Armed Police Corps Hospital. Written informed consent was obtained from each patient.
Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
