Twenty-seven healthy subjects were randomly assigned to 1 of 2 equal groups
: (1) experimental group (active stimulation) and (2) control group (sham stimulation). A
total of 10 Hz repetitive transcranial magnetic stimulation was delivered to the
left dorsolateral prefrontal cortex at 80% of the resting motor threshold. The
reaction time of the correct response, omission error, and commission error of
the auditory and visual continuous performance test scores were measured. The
motor evoked potentials, resting motor threshold, short-interval intracortical
inhibition, and intracortical facilitation was recorded in the right first dorsal
interosseous muscle to determine motor cortex excitability. The reaction time and
commission error of the auditory continuous performance test were reduced
significantly after 10 Hz repetitive transcranial magnetic stimulation (P
Attention is an essential function in our daily lives that is closely related to learning, perception, memory, and executive function. The essential function of attention is the selection of a particular subset of the available stimuli and the simultaneous suppression of currently irrelevant information. There are many types of attention. Focused attention is the ability to respond discretely to specific visual, auditory, or tactile stimuli. Sustained attention is the ability to maintain a consistent behavioral response during continuous and repetitive activity. Selective attention is the ability to maintain a behavioral or cognitive set in the face of distracting or competing stimuli. Alternating attention is mental flexibility that allows individuals to shift their focus of attention and move between tasks having different cognitive requirements. Lastly, divided attention refers to respond simultaneously to multiple tasks or multiple task demands (Sohlberg and Mateer, 1990).
Attention often deteriorates in various neuropsychiatric disorders. It is considered an important target of treatment (Evers et al., 2001). The dorsolateral prefrontal cortex (DLPFC) is a crucial brain region with a unique executive attention role in actively maintaining access to stimulus representations and goals in interference-rich contexts (Kane and Engle, 2002).
Repetitive transcranial magnetic stimulation (rTMS) is a neuromodulation technique that uses electromagnetic coils applied to the scalp to produce a magnetic field, which either excites or inhibits cortical activity, according to the parameters of its delivery. There is a consensus that rTMS below 1 Hz at the motor cortex reduces cortical excitability, whereas rTMS above 5 Hz increases the excitability of the cerebral cortex (Fitzgerald et al., 2006). One study also reported an increase in intracortical inhibition and a decrease in pain when 10 Hz rTMS was applied (Lefaucheur et al., 2006). The modulation of cognitive function in various domains after rTMS in the prefrontal cortex has been undertaken in healthy individuals and patients with cognitive impairment. A systematic review determined that high-frequency rTMS was most likely to cause selective cognitive improvement when applied to the left DLPFC (Guse et al., 2010).
However, other research points to the following improvements: improved performance in attentional control during the Stroop task and positive effects of rTMS on attention in attention deficit hyperactivity disorder (ADHD) subjects (Bloch et al., 2010; Kim et al., 2012; Vanderhasselt et al., 2006), decreased reaction time (Evers et al., 2001) and commission errors in a continuous performance test (CPT) (Hwang et al., 2010), and improved working memory (Brunoni and Vanderhasselt, 2014). In contrast, several studies reported no significant cognitive changes after rTMS (Avery et al., 2006; Guse et al., 2013; Huang et al., 2004; Jorge et al., 2004) and even deterioration in cognitive function (Miniussi and Rossini, 2011; Pascual-Leone et al., 1996; Wagner et al., 2006). These results might be related to the study populations, different parameters of stimulation, timing of the assessments, and neuropsychological tasks.
Although the underlying neurobiological mechanisms of these rTMS effects have not been determined, it is generally agreed that the therapeutic effect is likely mediated by increased cortical excitability and neurochemical transmission (Ziemann, 2010). There are many reports on the positive effects of rTMS on attention in ADHD patients. These reports explain the mechanism of positive effects as rTMS triggers the modulation of neurotransmitters such as dopamine and its metabolites (Bloch et al., 2010; Pogarell et al., 2007; Uclés et al., 2000).
Many studies demonstrated that the activity changes of brain cortex elicited by rTMS occur not only in the directly stimulated area but also in the remote cortical and subcortical areas functionally connected (Cho and Strafella, 2009; Miniussi and Rossini, 2011; Paus et al., 2001; Shajahan et al., 2002). Many reports supported that some brain areas integrate both motor and cognitive functions. As well, Leisman et al. (2016) gives support that some motor and cognitive processes influence each other, dynamically.
There is no way to measure the activity of DLPFC directly after rTMS in a non-invasive way. We believe that the effect of rTMS can be elucidated indirectly by measuring the electrophysiological change of a functionally connected motor cortex. However, there is no apparent systematic evidence of interaction between the non-motor area, especially the DLPFC stimulated by rTMS, and motor cortex excitability measured by TMS (Nordmann et al., 2015). The changes in spatially distant motor cortex excitability after rTMS of the DLPFC represent a clue to the mechanism of the cognitive effects of rTMS. Therefore, we intend to test the assumption that non-invasive brain stimulation for the left DLPFC will also affect the excitability of the functionally connected motor cortex.
The excitability of the cerebral cortex can be measured by various methods using transcranial magnetic stimulation (TMS). These methods include resting and active motor threshold (RMT, AMT), motor evoked potential (MEP) amplitude, short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF), cortical silent period (CSP) and so on (Talelli et al., 2006). These tools are known to be useful in evaluating the motor cortex excitability and motor neurophysiology after non-invasive brain stimulation (Rothwell et al., 2009). We used these tools to identify changes in motor cortex excitability and see how it relates to changes in attentional functions.
Twenty-seven right-handed healthy young adults (mean age: 27.26
The identification for right-handedness was conducted by verbally asking the subject. Participants were assigned randomly to either the active 10 Hz rTMS (n = 14) or the sham stimulation group (n = 13) by a centralized computer-generated randomization code. Participants, outcome assessors, and data analysts were kept blinded to the allocation. There were no significant differences in age, sex, and education years between the active and sham groups (Table 1).
| Characteristics | Active group (n = 14) | Sham group (n = 13) | P-value |
| Age, mean |
27 |
27 |
0.536 |
| Sex, n | 0.182 | ||
| Male | 10 | 6 | |
| Female | 4 | 7 | |
| Years of education, mean |
15.9 |
16.8 |
0.973 |
| - Abbreviations: SD, standard deviation. | |||
The rTMS screening standard questionnaires were administered to the subjects who were agreed to participate in the study (Wassermann, 1998). The procedure, possible risks, and side effects were explained, and signed consent was obtained from the subjects. The study was certified by the Institutional Review Board of Chungnam National University Hospital (IRB No. 2016-07-028).
The rTMS was delivered by using a Magstim
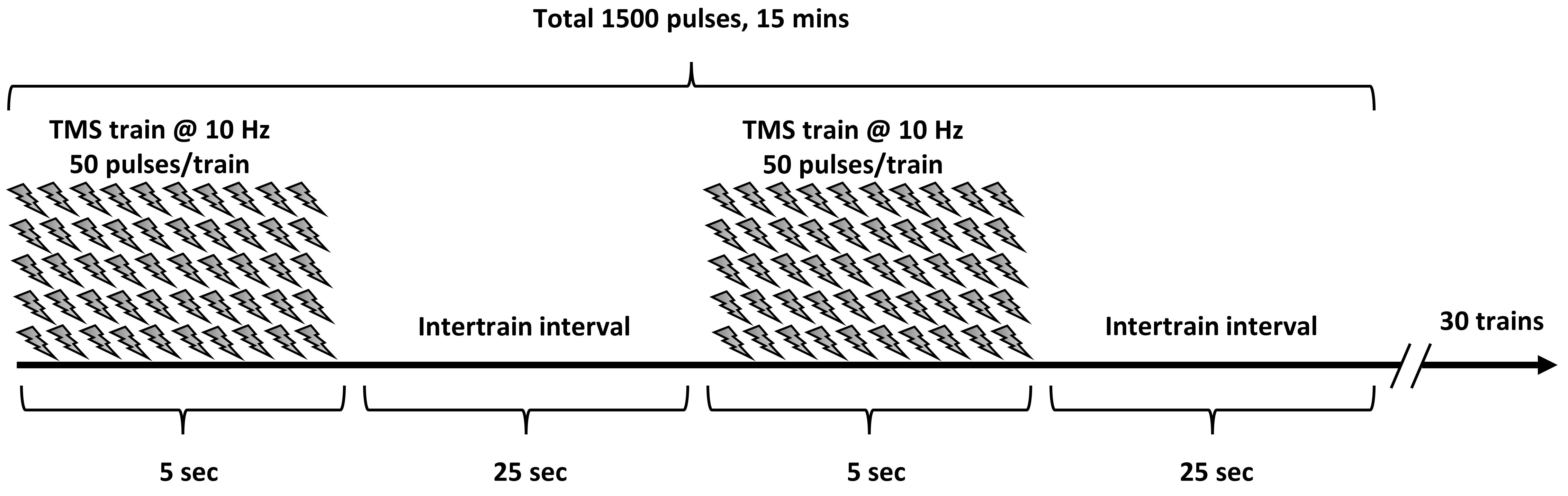 Fig. 1.
Fig. 1.The protocol of repetitive transcranial magnetic stimulation. The stimulation was performed with 10 Hz, 80% of the RMT of the right first dorsal interosseous muscle, 5 s of stimulation, and 25 s of rest, for a total of 1,500 pulses.
Before stimulation, we determined the optimal scalp position where the highest
amplitude of MEP was elicited in the right first dorsal interosseous. We used a
constant supra-threshold stimulus intensity and moved the coil systematically in
1-cm segments. The coil was positioned tangentially to the scalp and oriented so
that the induced electrical currents would flow approximately perpendicular to
the motor cortex. Single-pulse TMS was then delivered to the optimal location,
starting at the supra-threshold intensity and decreasing in steps of 1% of the
stimulator output. RMT was defined as the lowest stimulus intensity needed for
the induction of MEPs of 50
Subject assessments were performed immediately before and after the stimulation session. The order of assessments was the auditory CPT, the visual CPT, and the MEP recording, sequentially.
Attentional control was assessed using a CPT. During testing, participants were seated in a quiet room. A practice session was conducted before the presentation of each stimulus condition. Auditory and visual CPTs were measured using the Computerized Neuro-cognitive function Test Ver. 4.0 (CNT 40; Maxmedica Inc., Seoul, Korea) (Ha et al., 2002; Kim et al., 2006; Lee et al., 2003). In the test, the subjects were asked to press a response button as quickly as possible when a target number was presented among a random series of single numbers for 5 minutes. The target number was set to the number 3, and a random series of numbers was presented from the number 0 to the number 9 using the speaker or the screen at a rate of approximately 1 per second. The target: non-target stimuli ratio was 1 : 3 (Fig. 2). We recorded the reaction time of the correct response, omission error, and commission error.
The calculation was as follow:
For the assessment of motor cortical excitability, MagPro X100 stimulators
(MagVenture A/S, Denmark) were used for the magnetic stimulation. The focal TMS
with monophasic pulses was applied with a 90-mm figure-of-eight coil (MC-B70,
MagVenture A/S, Denmark) at the same motor cortex point for determining RMT with
the subject in a comfortable seated position. The coil was held tangentially over
the head with the handle pointing backward and laterally at approximately
45
The calculation was as follow:
 Fig. 2.
Fig. 2.Continuous performance test (CPT) protocol. The subjects were asked to press a response button as quickly as possible when a target number was presented among a random series of single numbers over a period of 5 minutes. The target number was set to the number 3, and a random series of numbers was presented from the number 0 to the number 9 using the speaker or the screen at a rate of approximately 1 per second.
SPSS version 23.0 (SPSS Inc., Chicago, IL, USA) was used for statistical
analysis. The statistical significance level was set at P
There was a statistically significant longer reaction time, higher omission, and
commission error in the auditory CPT than those of the visual CPT in the baseline
evaluation (P
After the active rTMS, the mean reaction time and commission error decreased
significantly from 600.7
| Variables | Active | Sham | P-value (Δ) | Power (1- |
Effect size | ||||
| Pre | Post | Δ | Pre | Post | Δ | ||||
| Auditory CPT | |||||||||
| Reaction time (ms) | 600.7 |
568.5 |
-32.14 |
589.2 |
586.9 |
-2.31 |
0.011 |
0.62 | 0.93 |
| Omission error (%) | 2.0 |
1.51 |
-0.49 |
2.43 |
2.58 |
0.15 |
0.512 |
0.12 | 0.31 |
| Commission error (%) | 0.79 |
0.53 |
-0.26 |
0.71 |
0.82 |
0.1 |
0.013 |
0.67 | 0.99 |
| Visual CPT | |||||||||
| Reaction time (ms) | 379.3 |
365.0 |
-14.29 |
380.4 |
376.2 |
-3.85 |
0.323 |
0.16 | 0.38 |
| Omission error (%) | 0.76 |
0.39 |
-0.38 |
1.19 |
1.31 |
0.12 |
0.616 |
0.13 | 0.34 |
| Commission error (%) | 0.38 |
0.28 |
-0.1 |
0.34 |
0.44 |
0.1 |
0.091 |
0.78 | 1.12 |
| Values are given as mean | |||||||||
The RMT showed a significant decrease only in the active rTMS group (
In the paired-pulse paradigm, each percentage of the amplitude of the SICI 2 ms
and 3 ms were significantly increased between time points only after the active
rTMS (
| Variables | Active | Sham | P-value (Δ) | Power (1- |
Effect size | ||||
| Pre | Post | Δ | Pre | Post | Δ | ||||
| RMT (%) | 36.8 |
35.0 |
-1.79 |
35.6 |
35.5 |
-0.08 |
0.039 |
0.56 | 0.85 |
| MEP amplitude (mV) | 0.67 |
0.60 |
-0.07 |
0.74 |
0.73 |
-0.01 |
0.216 |
0.06 | 0.12 |
| MEP latency (ms) | 19.04 |
18.95 |
-0.09 |
19.67 |
19.75 |
0.08 |
0.643 |
0.07 | 0.17 |
| SICI 2 ms (%) | 33.8 |
46.5 |
12.70 |
33.9 |
37.6 |
3.72 |
0.221 |
0.23 | 0.49 |
| SICI 3 ms (%) | 48.7 |
64.8 |
16.09 |
52.5 |
49.3 |
-3.22 |
0.034 |
0.59 | 0.88 |
| ICF 10 ms (%) | 490.2 |
473.9 |
-16.29 |
455.2 |
411.1 |
-44.13 |
0.769 |
0.06 | 0.12 |
| ICF 15 ms (%) | 567.5 |
565.4 |
-2.06 |
529.3 |
466.7 |
-62.62 |
0.923 |
0.09 | 0.24 |
| RMT, resting motor threshold; SICI, short-interval intracortical inhibition;
ICF, intracortical facilitation. | |||||||||
The significant finding of this paper was that 10 Hz rTMS to the DLPFC improved performance in terms of the reaction time and commission errors of the auditory CPT, with a significantly decreased RMT and SICI (increased amplitude ratio) of the ipsilateral motor cortex. CPT is a widely used computerized test for evaluating neurocognitive impairments in attention and vigilance (Zarghi et al., 2011). Typically, the subjects are asked to respond, as quickly as possible, to a rare stimulus that is embedded in a stream of similar ongoing stimuli. The tests typically measure reaction time, omission errors (missed target), which correlate with inattention, and commission errors (response to non-target), which correlate with impulsivity.
Changes in the neurocognitive profile after high-frequency rTMS on the left DLPFC reported in patients with depression (Brunoni and Vanderhasselt, 2014; Moser et al., 2002; Serafini et al., 2015; Shajahan et al., 2002; Triggs et al., 1999; Vanderhasselt et al., 2009), stroke (D’Agata et al., 2016; Jorge et al., 2004; Miniussi and Rossini, 2011), ADHD (Bloch et al., 2010; Cao et al., 2018), and progressive neurodegenerative disease (Ahmed et al., 2012; Anderkova and Rektorova, 2014; Hsu et al., 2015). In a systematic review, rTMS to the DLPFC significantly improved all measures of working memory performance (Brunoni and Vanderhasselt, 2014). Overall, high-frequency rTMS has been associated with some positive effects on cognition in both healthy subjects and those with cognitive impairment. However, the effects on a specific cognitive domain have varied widely depending on the study protocol. In the present study, the reaction time and commission errors of the auditory CPT were improved after 10 Hz rTMS to the left DLPFC, with no significant effects on the omission error of the auditory CPT and any of the visual CPT parameters. Roebuck et al. (2016) also reported that the error propensity of CPT was associated with the ceiling effect in adults.
In our work, the auditory reaction time and commission errors improved after rTMS with no effect on the visual CPT. There are several possible reasons for this. The auditory CPT reaction time was significantly longer than that in the visual CPT, in line with the results reported by Roebuck et al. (2016). Indeed, a higher omission and commission errors in the auditory CPT compared with those in the visual CPT were observed in the baseline evaluation. These findings suggest that the auditory CPT represents a more difficult task than visual CPT. Kim et al. (2012) demonstrated a reaction time reduction for incongruent trials only, which have a longer latency compared to the congruent trials of the Stroop task after rTMS. Recently, 5 Hz rTMS delivered to left DLPFC in healthy subjects significantly enhanced memory recall accuracy, but only for the most challenging task (Beynel et al., 2019). First, the evidence supports that the easier the test, the worse the ceiling effect. One study reported that 3-digit CPT, rather than relatively easy 2-digit CPT, reflects the performance improvement of healthy subjects without ceiling effect (Kahn et al., 2012). Second, is that the auditory CPT was performed before the visual CPT. There is a report in which rTMS induced a region-specific increase in resting regional cerebral blood flow in the stimulated area lasting approximately 8 min (Takano et al., 2004). This means that the visual CPT was inevitably performed at the point when the effect of rTMS begins to decline. This may have influenced the visual CPT results. Third, the neural resources of the auditory CPT may be relatively closer to the stimulation site, the DLPFC, than those of visual reaction tasks anatomically. This is because the auditory cortex is located roughly at the upper sides of the temporal lobes (Brӧadmann areas 41 and 42) (Nakai et al., 2017; Pickles, 2013), and the visual cortex is located in the posterior pole of the occipital lobe (Brodmann area 17, 18 and 19) (Bear et al., 2020). We believe that these distance differences may have influenced the performance of each task. Lastly, we used a single session of stimulation. Generally, multiple sessions of stimulation are more effective than a single session (Guse et al., 2010). If the multiple sessions of stimulation were performed, the visual CPT might have a more significant change.
It is challenging to determine how rTMS-induced neurobiological changes affect cognitive function (Guse et al., 2010). According to the accumulated evidence, it is known that rTMS causes neurophysiological and neurochemical changes in the stimulated area as well as changes in interconnected areas (Cho and Strafella, 2009; Paus et al., 2001; Strafella, 2003). Cognitive improvement may be the result of the facilitation of a neural network that supports the target functions or the suppression of a competing network that inhibits the target functions.
To assess the effect of DLPFC rTMS on the activity of the interconnected area, we measured ipsilateral motor cortical excitability. The RMT and the amplitude and latency of the MEPs were measured, along with the SICI and ICF by paired-pulse stimulation. RMT is associated with the membrane excitability of cortical motor neurons dependent on ion channel conductivity (Ziemann et al., 1996). Supporting evidence of a change in the RMT over a treatment course of rTMS to the prefrontal cortex is scarce. Triggs et al. (1999) demonstrated that left prefrontal rTMS was associated with small but significant decreases in RMT in patients with medication-resistant major depression. Another study also found that high-frequency rTMS to the left DLPFC decreased the RMT (Croarkin et al., 2012). Also, Pallanti et al. (2012) reported a decrease in RMT measured on the left hemisphere when low-frequency rTMS was performed on the right DLPFC. However, the majority of studies did not reveal any significant changes in RMTs (see Ahmed et al., 2012; Bajbouj et al., 2005; Daskalakis et al., 2006; Zarkowski et al., 2009). In the present study, the RMT was decreased after 10 Hz rTMS to the DLPFC, which indicates increased motor cortex excitability. The change in the MEP amplitude after rTMS has varied in previous studies, showing increases in patients with depression with marked clinical improvement (Chistyakov et al., 2005), decreases or no changes according to the stimulation frequency in patients with depression (Shajahan et al., 2002), and no changes at all (Daskalakis et al., 2006). The latency and amplitude of the MEPs showed no changes in the present study. The reason for the different results is that the consensus of the rTMS protocol is still lacking. The number of sessions, the stimulation frequency, and the stimulation intensity seem to be not sufficient to cause changes in the MEPs in this study.
In the paired-pulse method, a sub-threshold conditioning TMS stimulus precedes a supra-threshold test stimulus by a programmable ISI. The amplitude modulation of the test stimulus allows for the evaluation of inhibitory (at shorter intervals) and facilitatory (at longer ISIs) phenomena taking place intra-cortically. The SICI reflects the integrity and excitability of inhibitory intraneuronal circuits upstream from corticospinal neurons (Kujirai et al., 1994) and is predominantly mediated by GABAergic interneurons (Di Lazzaro et al., 2006; Ziemann et al., 1996). The main findings of our study were significantly decreased inhibition SICI with no change of ICF. Bajbouj et al. (2005) reported that the intracortical inhibition (ICI) was significantly enhanced after 10 sessions of rTMS at 20 Hz to the left DLPFC in depressive patients, with no significant changes in the RMT and ICF. They found that GABAergic changes in the motor cortex caused this inhibitory effect of rTMS. In contrast, non-motor rTMS was reported to have no effects on SICI values in healthy controls (Daskalakis et al., 2006). The majority of studies demonstrated that ICF is not sensitive to detect the effect of non-motor rTMS (Daskalakis et al., 2006; Nordmann et al., 2015).
What is notable from our results is that excitability increased in the M1 motor cortex despite non-invasive brain stimulation in the left DLPFC. These findings support the hypothesis that prefrontal rTMS may exert indirect effects on corticomotor excitability and improve cognitive function by inducing even more distant alterations in the cortical and subcortical systems. Leisman et al. (2016) reported that motor control integrates both cortical and subcortical structures, principally involving those connections between the basal ganglia and frontal lobes involved in the automaticity of motor function and its cognitive function and motor cognition encompasses the mental processes involved in the planning, preparation, and production of our actions, as well as the cognitive processes (Boring and Gibson, 1967). It is said that the frontal lobe is responsible for cognitive function, and the M1 cortex has a hierarchical relationship, anatomically. Mushiake et al. (1991) recorded single-cell activity from the M1 and cognitive area of the frontal lobe of monkeys, immediately before and during the execution of sequential motor activity. In that study, they demonstrated that the frontal lobe and M1 cortex are related physiologically and interact in complex ways. According to Friston (2002), the sensory, motor, or cognitive processes rely on context-dependent interactions between different brain regions based on precise anatomical and functional connectivities. This supports the hypothesis that the motor cortex is functionally and physiologically connected to the frontal lobe responsible for cognitive function.
Eventually, high-frequency rTMS for left DLPFC increases the excitability of the cerebral cortex, which causes priming of functionally recruited neurons, modulation of the natural oscillatory brain behavior, and induced neuroplasticity, thereby contributing to attentional function improvement (Luber et al., 2007). It is known that an increase in neurotransmitters, such as dopamine, is involved in this process after brain stimulation (Sawaguchi et al., 1990). Also, as mentioned above, the improvement of cognitive function seems to be related to the increased activity of the primary motor cortex because the cognitive function and the motor area are functionally related.
The high variability in the changes of MEP, SICI, and ICF could be interpreted as different activation thresholds. The high heterogeneity of the investigated studies concerning the stimulation parameters (stimulation pattern, frequency, site, intensity, number of pulses, and number of sessions) and the intrinsic variability of brain function have contributed to the lack of a systematic pattern of results (Nordmann et al., 2015). Therefore, higher methodological standards in future rTMS studies (larger sample size, sham-controlled designs) are recommended. To further understand neural and cognitive mechanisms, rTMS studies using a combination of electrophysiological methods (EEG, evoked potentials) and advanced multimodal imaging techniques (fMRI, PET) or near-infrared spectroscopy are warranted.
The present study has several limitations that must be considered, some of which are described in the above discussion. The study subjects included only younger-aged participants. A large sample size study, which includes older-aged subjects, would facilitate a more comprehensive understanding of the effects of rTMS on cognitive function. Another major limitation was the small sample size and relatively large standard deviation of the outcome data. We did not perform a sample size calculation. Going forward, larger sample sizes and the collection of high-quality data by multiple conditions will yield more complete and meaningful results. Nevertheless, the effect sizes of the parameters with a significant difference between the delta values were 0.8 or more. This means that the effect of the experiment was high. Also, we did not assess the participants’ moods before and after stimulation to control for the possible confounding effects of mood on the task performance. Furthermore, the sequence of neuropsychological tests was not randomized. Thus, the time course of possible rTMS effects and the specificity of the neuropsychological tasks cannot be disentangled. Lastly, we did not perform testing the practice effect of the CPT. Yet, the order of the presentation of numbers in the CPT was randomized. The authors believe that the practice effect could be minimized.
In conclusion, high-frequency rTMS to the left DLPFC may be used to modulate attentional function in healthy individuals and for cognitive rehabilitation, and the mechanism of this effect can be assumed that the motor cortex excitability increase is somewhat involved.
Y.W.K., S.J.J., and M.K.S. conceptualization; J.X.C., S.L.J. data curation; M.K.S. methodology; J.X.C., S.L.J, and M.K.S. software; M.K.S. funding acquisition; S.J.J. and M.K.S. investigation; S.L.J. and J.X.C. visualization; J.X.C. and S.L.J. IRB approval; S.J.J. and S.L.J. data analysis; Y.W.K. wrote the original draft preparation, M.K.S. wrote and edited the paper.
The Institutional Review Board approved this study of Chungnam National University Hospital, Republic of Korea. The approval number was 2016-07-028.
This research was funded by grants from Chungnam National University (2016-1554-01) and the Korean government (MSIP) (NRF-2017R1A2B4006500). The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
The authors declare no conflict of interest.
