It has been previously established that total antioxidant capacity
concentrations of blood on the first day of ischemic stroke could predict
mortality. Therefore, our study objective was to determine whether total
antioxidant capacity concentrations in the blood during the first week of a
cerebral infarction could help predict mortality. We included severe and
malignant middle cerebral artery infarction patients (affecting 50% or more of
the territory in computed tomography and a score of nine or fewer points in the
Glasgow Coma Scale). Serum total antioxidant capacity concentrations were
determined on days first, fourth, and eighth of the diagnosis of a malignant
middle cerebral artery infarction. Higher serum total antioxidant capacity
concentrations at first (
A high number of disabilities, resource consumption, and deaths result from ischemic stroke (Powers et al., 2018). Oxidative stress can produce secondary brain injury in ischemic stroke, and antioxidants are generated to avoid cell damage due to reactive oxygen species (Manzanero et al., 2013; Olmez and Ozyurt, 2012; Pradeep et al., 2012; Radak et al., 2014; Rodrigo et al., 2013; Warner, 2004). The different antioxidant molecules act synergistically, and determining total antioxidant capacity (TAC) could provide better information on antioxidant status than the determination of each antioxidant compound separately (Ghiselli et al., 2000).
There is scarce data on blood TAC concentrations and prognosis of patients with
ischemic stroke (Fernández-Gajardo et al., 2019; Ghonimi et al., 2019; Guldiken et al., 2009; Lorente et al., 2016; Lorenzano et al., 2018, 2019). One study found lower blood TAC concentration measured on the first
day of ischemic stroke in patients with poor functional prognosis (Ghonimi et al., 2019). In contrast, higher blood TAC concentrations have been found on the
first day of ischemic stroke patients than in healthy subjects (Guldiken et al., 2009), and in non-surviving patients than in surviving ones (Lorente et al., 2016). Another study found no association between blood TAC concentrations
during the first week of ischemic stroke and the infarct volume
(Fernández-Gajardo et al., 2019). On the other hand, no significant
differences were found in plasma TAC concentrations within 9 hours of stroke
onset between patients with infarct growth and those without (Lorenzano et al., 2018), while statistically significant differences were observed in plasma TAC
concentrations within 9 hours of stroke onset between patients with ischemic
penumbra (perfusion-weighted/diffusion-weighted mismatch
Our perspective and observational study was developed in the Intensive Care Unit of six Spanish hospitals after the approval of the Institutional Ethical Board of all hospitals and with the written informed consent of a family member of each patient.
We included severe and malignant middle cerebral artery infarction (MMCAI)
patients, which affected 50% or more of the territory in computed tomography
and had
Previously, we had determined serum concentrations of TAC on the first day of severe MMCAI in 58 patients (Lorente et al., 2016). We then determined serum malondialdehyde concentrations on days one, four, and eight of a severe MMCAI in 68 patients (Lorente et al., 2019). For this research, we determined serum TAC concentrations on days one, four, and eight in those 68 severe MMCAI patients to determine its potential capability for mortality prediction and its potential association with lipid peroxidation, assessed by blood malondialdehyde concentrations (Dalle-Donne et al., 2006; Draper and Hadley, 1990).
Age, sex, body temperature, diabetes mellitus, sodium, arterial hypertension,
fraction inspired of oxygen (FI0
We obtained serum blood samples on days one, four, and eight of severe MMCAI
diagnosis and froze them at -80
We used frequencies (percentages) and medians (interquartile ranges) to describe categorical and continuous variables. We compared continuous and categorical variables between surviving patients and non-surviving ones by Wilcoxon-Mann-Whitney and chi-square tests.
We carried out receiver operating characteristic analyses to test the capacity
to predict mortality of serum TAC concentrations on days one, four, and eight of
MMCAI, and the area under the curve from serum TAC concentrations was
reported each day. The optimal cut-off values for each day were selected based on the
Youden J index. As deceased patients were 34, we performed two regression model
analyses with 4 variables in each model to test the association between serum TAC
levels and 30-day mortality controlling for different variables, in the first
model controlling for variables that had significant univariate associations with
30-day mortality (platelet count, GCS, and lactic acid) and in the second model
controlling for known risk factors of death after stroke (age, sex, baseline
blood glycemia). We have not included APACHE-II due to its inclusion of GCS and
age. Friedman test was used to test differences in serum TAC
concentrations at days one, four, and eight within the groups of surviving and
non-surviving patients.
We analyzed 34 non-surviving and 34 surviving patients. We found higher GCS and
platelets in surviving patients than in the non-survivors (Table 1). We found
no statistically significant differences in 30-day survival rate in patients
without decompressive craniectomy (48%, 25 of 52 patients) or with it (56%, 9
of 16 patients) (
| Survivors (n = 34) | Non-survivors (n = 34) | P-value | |
| Gender female | 14 (41.2) | 13 (38.2) | 0.99 |
| Arterial hypertension | 19 (55.9) | 16 (47.1) | 0.63 |
| Diabetes mellitus | 4 (11.8) | 9 (26.5) | 0.22 |
| Age (years) | 59 (47-68) | 63 (53-70) | 0.36 |
| Temperature ( |
36.4 (36.0-37.0) | 36.9 (36.0-37.3) | 0.15 |
| GCS score | 7 (6-8) | 6 (3-7) | 0.01 |
| Sodium (mEq/L) | 139 (136-145) | 140 (139-145) | 0.38 |
| Bilirubin (mg/dl) | 0.60 (0.40-0.83) | 0.60 (0.33-1.10) | 0.95 |
| Lactic acid (mmol/L) | 1.20 (0.90-1.70) | 1.55 (1.00-2.70) | 0.05 |
| Creatinine (mg/dl) | 0.80 (0.60-1.13) | 1.00 (0.70-1.25) | 0.19 |
| Glycemia (g/dL) | 127 (100-170) | 136 (118-162) | 0.40 |
| PaO2 (mmHg) | 156 (105-293) | 115 (94-267) | 0.26 |
| PaO2/FI0 |
300 (198-369) | 254 (192-325) | 0.24 |
| Platelets x10 |
202 (171-265) | 175 (136-216) | 0.02 |
| Hemoglobin (g/dL) | 12.1 (11.4-14.0) | 12.5 (11.0-14.8) | 0.81 |
| Leukocytes x 10 |
12.4 (9.6-16.9) | 13.9 (9.7-20.1) | 0.32 |
| INR | 1.06 (1.00-1.20) | 1.20 (1.01-1.31) | 0.07 |
| Fibrinogen (mg/dl) | 443 (416-489) | 419 (337-631) | 0.90 |
| aPTT (seconds) | 28 (25-30) | 27 (26-32) | 0.91 |
| APACHE-II score | 20 (16-25) | 22 (19-27) | 0.06 |
| Thrombolysis | 11 (32.4) | 10 (29.4) | 0.99 |
| Decompressive craniectomy | 9 (26.5) | 7 (20.6) | 0.78 |
| Haemorrhagic transformation | 7 (20.6) | 6 (17.6) | 0.99 |
| Volume infarction (ml) | 173 (100-231) | 180 (60-277) | 0.64 |
| Midline shift (mm) | 6.0 (2.5-11.5) | 9.0 (3.5-15.0) | 0.43 |
| TAC (mmol/mL) | 2.30 (1.89-3.29) | 6.10 (3.56-12.08) | < 0.001 |
| GCS = Glasgow Coma Scale; PaO | |||
Higher serum TAC concentrations on days one (
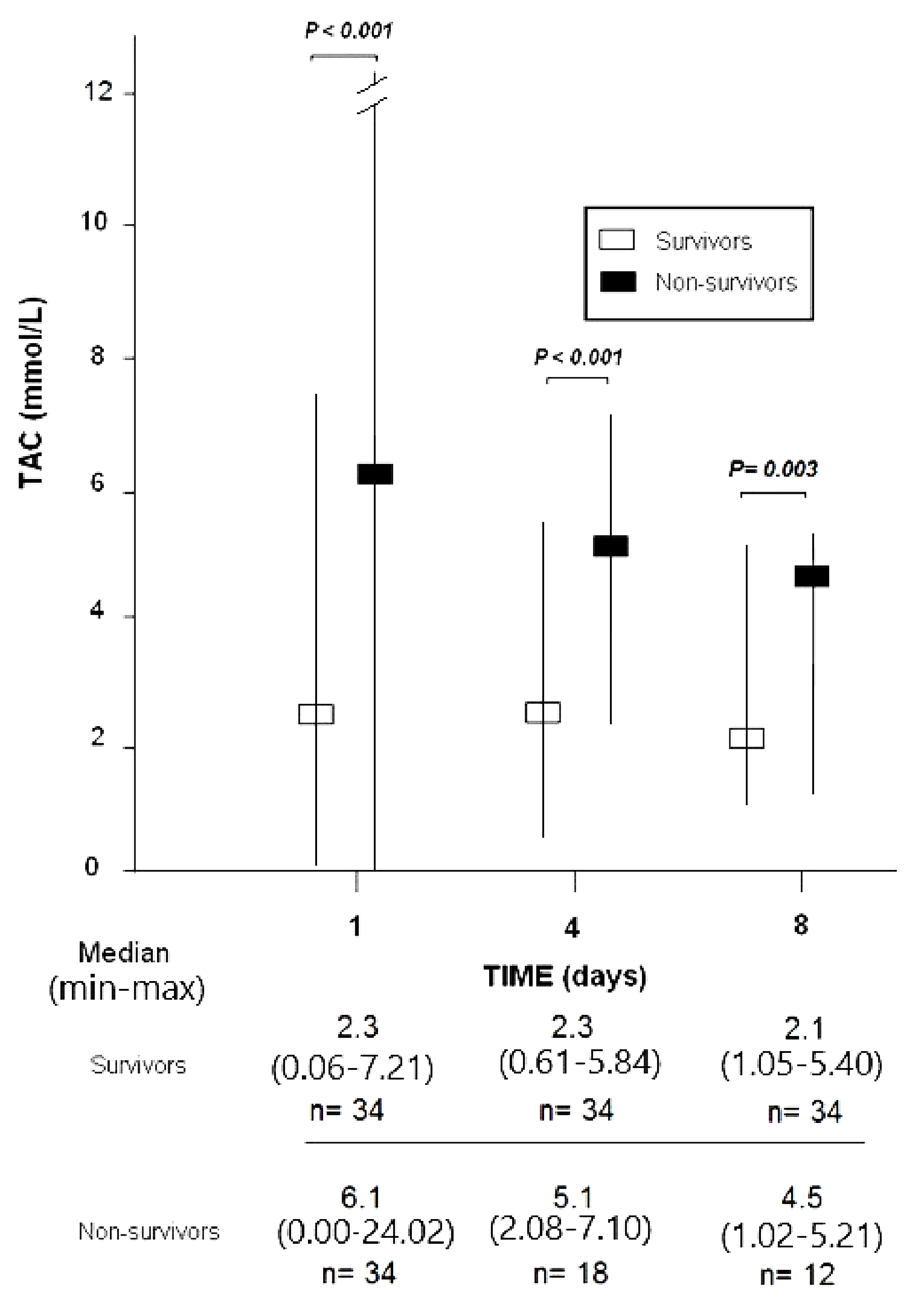 Fig. 1.
Fig. 1.
Serum total antioxidant capacity (TAC) at
days 1
To predict the mortality at 30 days serum TAC concentrations on days one, four and eight of the MMCAI had an area
under the curve (95% CI = confidence intervals) of
0.86 (0.75-0.93;
| 2 Day 1 |
3 Day 4 |
4 Day 8 |
|
| Cut-off of TAC (mmol/mL) | > 3.31 | > 2.81 | > 3.13 |
| Sensitivity (95% CI) | 79 (62-91) | 89 (65-98) | 75 (43-95) |
| Specificity (95% CI) | 79 (62-91) | 82 (66-93) | 91 (76-98) |
| Negative likelihood ratio (95% CI) | 0.3 (0.1-0.5) | 0.2 (0.1-0.5) | 0.3 (0.1-0.7) |
| Negative predicted value (95% CI) | 79 (66-84) | 93 (79-98) | 91 (79-97) |
| Positive likelihood ratio (95% CI) | 3.9 (2.0-7.6) | 5.0 (2.4-10.6) | 8.5 (2.7-26.3) |
| Positive predicted value (95% CI) | 79 (66-84) | 73 (56-85) | 75 (49-90) |
| CI: confidence intervals | |||
| Variable | Odds Ratio | 95% Confidence Interval | P |
| First model | |||
| TAC (mmol/mL) | 2.672 | 1.489-4.796 | 0.001 |
| Platelet count (each 1,000/mm |
0.986 | 0.974-0.998 | 0.03 |
| Glasgow Coma Scale (points) | 0.721 | 0.487-1.066 | 0.10 |
| Lactic acid (mmol/L) | 1.205 | 0.628-2.312 | 0.57 |
| Second model | |||
| TAC (mmol/mL) | 1.969 | 1.288-3.011 | 0.002 |
| Sex (male vs. female) | 1.533 | 0.386-6.088 | 0.54 |
| Age (years) | 1.017 | 0.967-1.070 | 0.51 |
| Glycemia (g/dL) | 1.004 | 0.994-1.014 | 0.42 |
| TAC: total antioxidant capacity | |||
Regression analyses showed an association of serum TAC levels with 30-day
mortality controlling for platelet count, GCS and lactic acid (Odds ratio =
2.672; 95% CI = 1.489-4.796;
We found an association between serum concentrations of TAC and malondialdehyde
at days one (
The potential use of serum concentrations of TAC during the first week of severe MMCAI for predicting mortality was the main novel result in our study. On the one hand, no association was found between infarct growth and plasma TAC concentrations within 9 hours of stroke onset (Lorenzano et al., 2018), and between infarct size and blood TAC concentrations during the first week of ischemic stroke (Fernández-Gajardo et al., 2019); however, the association between blood TAC concentrations and mortality was not studied. Also, another study found lower blood TAC concentration on day 1 of ischemic stroke in patients with poor functional prognostic (Ghonimi et al., 2019). On the other hand, an association between high plasma TAC concentrations within 9 hours of stroke onset and brain ischemic penumbra has been found (Lorenzano et al., 2019). Previously, we had determined blood TAC concentration on the first day of an MMCAI, and we found higher concentrations in 30-day non-surviving patients (Lorente et al., 2016). Therefore, the determination of serum TAC concentrations at fourth and eighth day of MMCAI is a new aspect in our study.
Furthermore, higher serum TAC concentrations also at days four and eight of
severe MMCAI in non-surviving patients were novel findings in our study. Another
interesting and new finding in our study for clinicians was that serum TAC
concentrations in the first week of severe MMCAI could be used to predict
mortality. These novel findings from our study may be due to our inclusion of patients only with an acute infarction
Another interesting and new finding in our study was the correlation between lipid peroxidation (estimated by serum concentrations of malondialdehyde) and serum TAC concentrations during the first week of severe MMCAI. A study has found higher plasma TAC concentrations and higher oxidative stress, assessed by plasma concentrations of nitric oxide metabolite levels (nitrite and nitrate), in diabetic acute stroke patients than in healthy control subjects (Guldiken et al., 2009). We propose like Guldiken et al. (2009) that the high serum TAC concentrations in ischemic stroke patients and non-surviving patients and its correlation with lipid peroxidation may be due to high reactive oxygen species production, and an attempt to avoid those harmful effects.
The blood-brain barrier (BBB) is a selective semipermeable border that separates peripheral blood and neural brain tissue in the central nervous system. The BBB restricts the passage of pathogens and large or hydrophilic molecules and the impermeability of the BBB has hampered the use of antioxidants agents for neuroprotection (Rashid et al., 2014). However, some murine model studies have shown that after the oral administration of tea, different substances of tea are brain permeable and have neuroprotective effects (by increasing antioxidant capacity in brain samples) (Pervin et al., 2019; Rashid et al., 2014). Oxidative stress and antioxidant capacity in brain samples have been reduced in cerebral ischemia animal models with melatonin administration (Blanco et al., 2017; Kryl’skii et al., 2019). Also, oxidative stress measured in plasma has been reduced by different antioxidant vitamins administered orally in patients with acute ischemic stroke (E, C, B2, B6, B12) (Ullegaddi et al., 2004, 2005, 2006). It is possible that the BBB damage that occurs in cerebral ischemia (and that could contribute to hemorrhagic transformation) (Li et al., 2019) could favor the passage of those substances through BBB and reduce oxidative stress in cerebral ischemia. We found high serum concentrations of TAC and malondialdehyde concentrations in non-survivor patients; however, we have not assessed antioxidant capacity and oxidative stress in brain samples in our study.
The 2007 American guidelines on ischaemic strokes (Adams et al., 2007)
suggested the use of intravenous thrombolysis therapy in patients without
multilobar infarction in CT (hypodensity in a third of cerebral hemisphere). The improved outcome with thrombectomy is unclear, and in severely
affected patients due to malignant edema, decompressive surgery is
recommended. The American guidelines of 2018 (Powers et al., 2018) suggested
the use of intravenous thrombolysis therapy in patients with an ischemic injury
involving less than one-third of the middle cerebral artery territory; the use of
thrombectomy in patients with occlusion of the internal carotid artery or middle
cerebral artery infarction segment 1, pre-stroke modified Rankin scale
There are some limitations in our study. We have not recorded the time interval from the onset of stroke to blood draws for the TAC measurement, to surgical intervention, and to hemorrhagic transformation to evaluate whether those factors could modify serum TAC levels. We have not registered the number and causes of exclusion and the percentage of severe MMCAI about the total ischemic stroke patients. Patients with autoimmune diseases or sepsis were excluded from the study, and we did not find differences in renal or hepatic functions at inclusion between surviving and non-surviving patients; however, data on atrial fibrillation, coronary artery disease, smoking and dyslipidemia which could influence serum TAC levels were not provided.
We chose to include only patients with severe MMCAI because of the higher mortality in that group of patients (50% in our series). Serum TAC levels could have potential predictive capabilities within the population under study; however, the results of this study are not generalizable to all strokes due to exclusion criteria. Also, the results of this study need to be replicated by another team before using them in the prediction of mortality due to the scarce and contradictory data on blood TAC concentrations and prognosis of patients suffering from ischemic stroke. We think that the interest of this study is not only that serum TAC concentrations could potentially predict mortality, but also to explore the effect of new modalities of treatment in this subgroup of patients at high risk of mortatility.
The potential use of serum TAC concentrations at any time during the first 7 days of a severe MMCAI infarction without thrombectomy to predict mortality was the main novel finding of our study.
APACHE: Acute Physiology and Chronic Health Evaluation; aPTT: activated
partial thromboplastin time; FIO
LL coordinated and designed the project, and drafted the article. JJC, JSV, MA, MMM, LRG, VGM, and RS participated in data recollection. PAG, APC, and AFGR performed blood determinations. AJ participated in data interpretation. Finally, the manuscript was critically revised and approved by all authors.
Our perspective and observational study were developed in the Intensive Care Unit of six Spanish hospitals after the approval of the Institutional Ethical Board of all hospitals and with the written informed consent of a family member of each patient.
Grants of Fundación DISA a la Investigación Médica 2017 (Tenerife, Spain) (code: OA18/011) and Instituto de Salud Carlos III (Madrid, Spain) (code: PI18-00500). The funders had no influence in the design, analyses or preparation of the article.
The authors have not competing interests.
